Anchoring Mechanism for Capsule Endoscope: Mechanical Design, Fabrication and Experimental Evaluation
Abstract
:1. Introduction
- The design and development of a low footprint anchoring mechanism that fit within a capsule endoscope and can anchor at the target site in the gut;
- The design and selection of the custom-built SMA spring actuator to operate the anchoring mechanism;
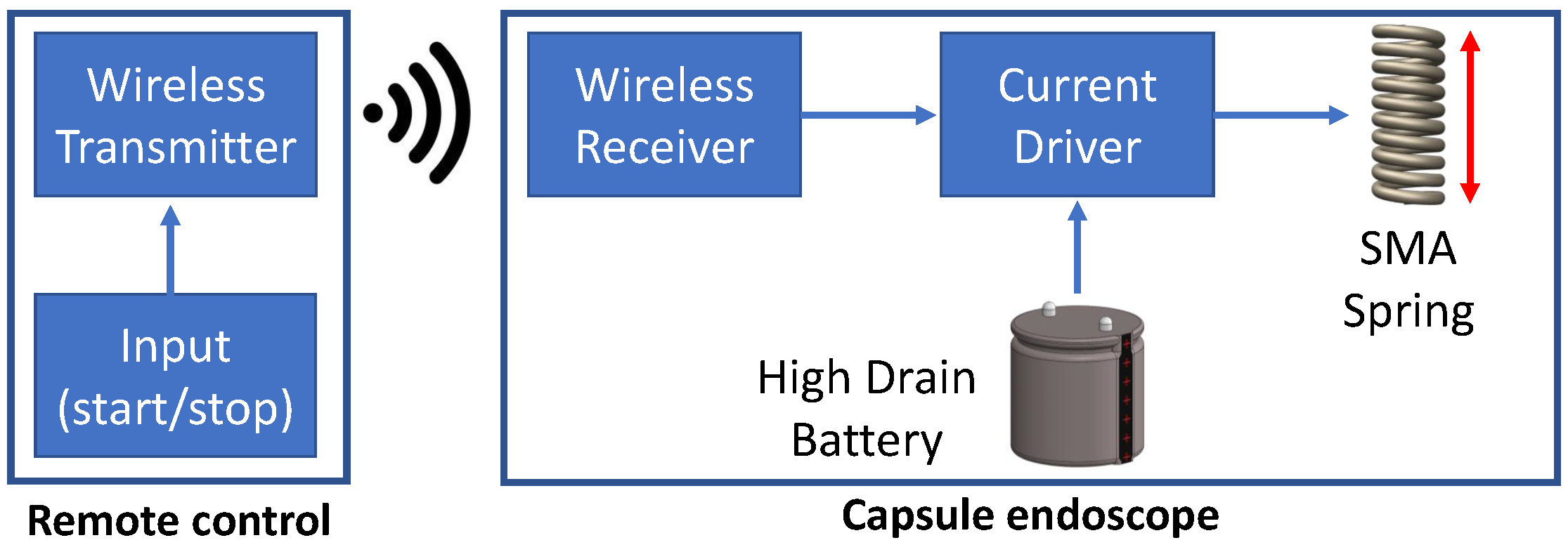
- The development of a miniaturised actuation system based on an SMA spring actuator by tackling the high-drain current requirements using a custom-built battery and a miniaturised wireless transceiver.
2. Materials and Methods
2.1. Mechanical Design of Anchoring Mechanism
2.1.1. Capsule Size
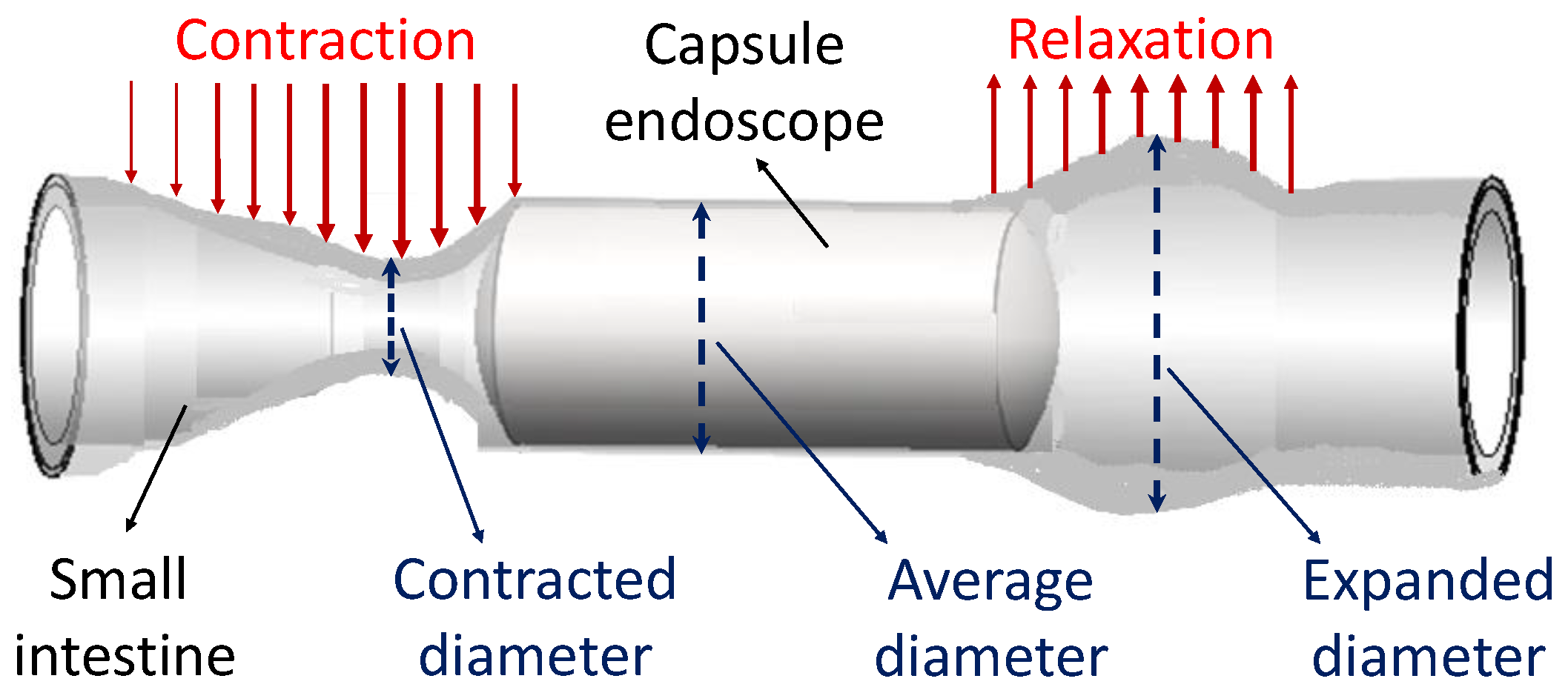
2.1.2. Parameters of Small Intestine
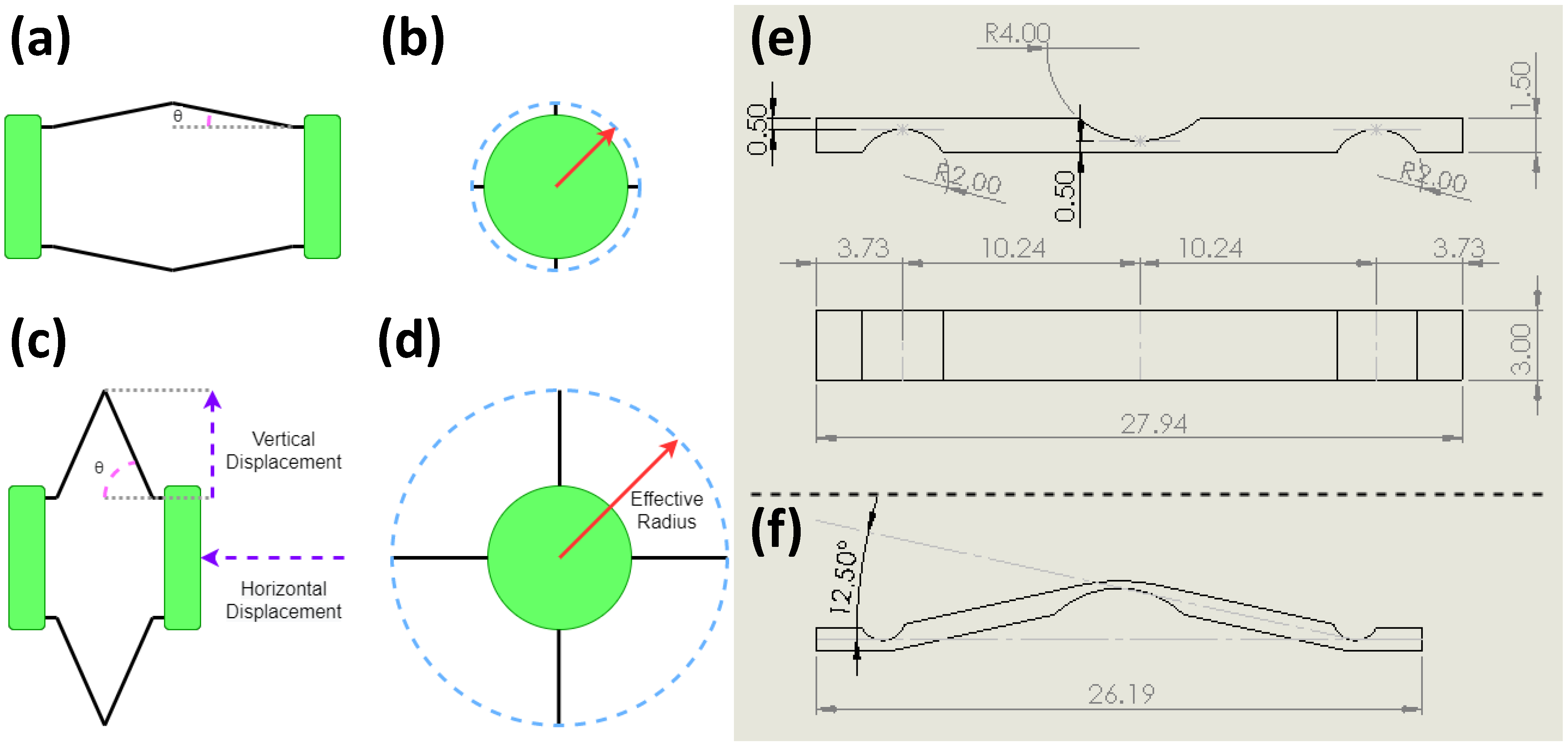
2.1.3. Development of Anchoring Mechanism
2.1.4. Capsule Fabrication
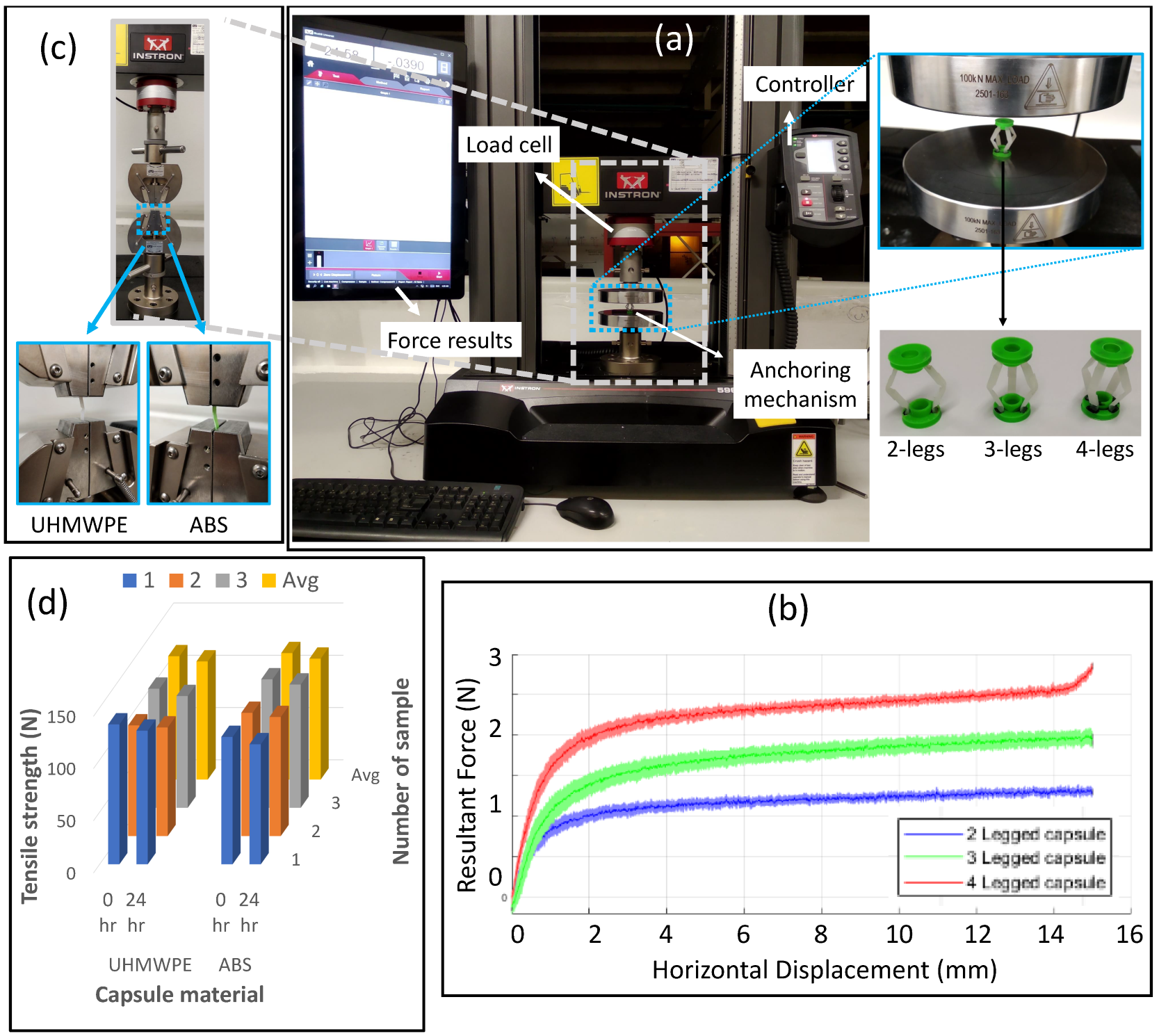
2.1.5. Biocompatibility and Strength of Capsule Material
2.2. Actuation System for Anchoring Mechanism
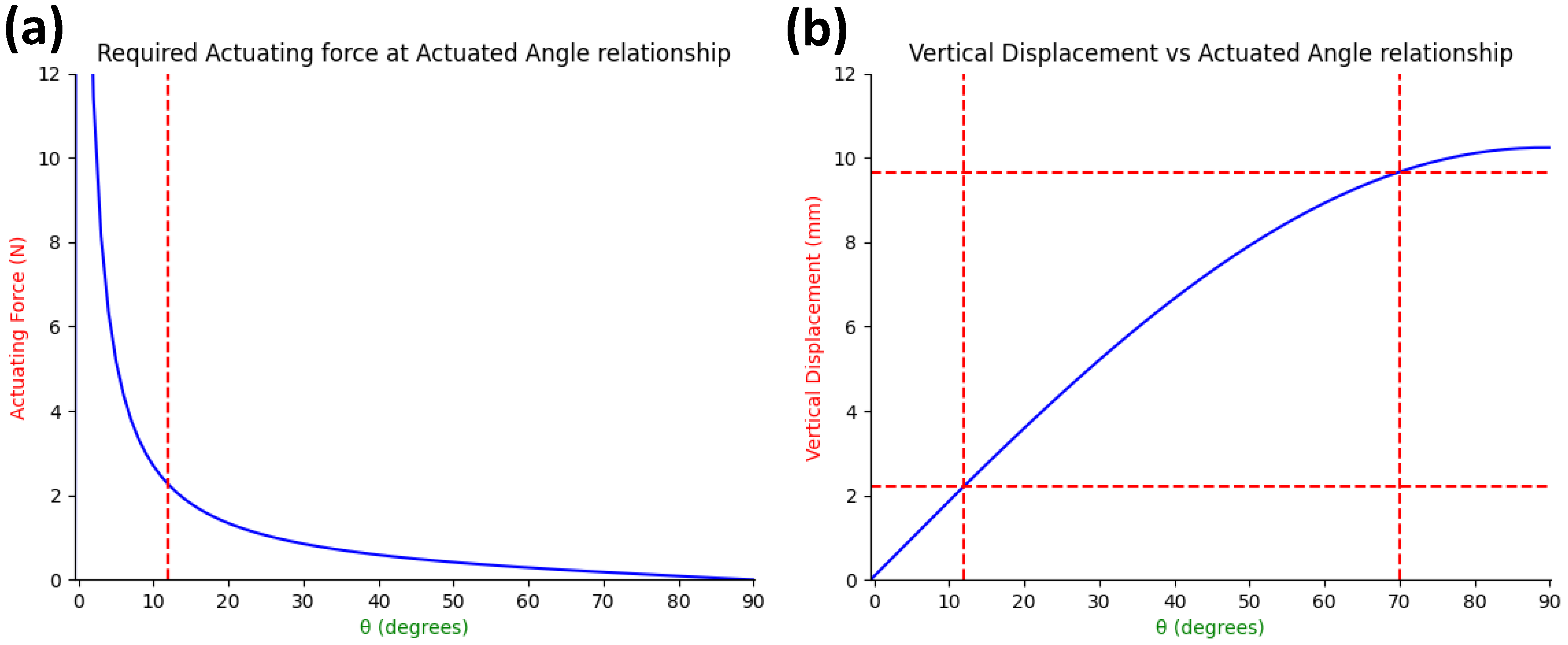
2.2.1. Force Analysis of Fabricated Anchoring Mechanism
2.2.2. Design Requirements for Actuator Selection
2.2.3. Design of SMA Spring Actuator
2.2.4. Development of Actuation System
2.2.5. Simulator for Anchoring Trials
2.2.6. Living Intestinal Trials
3. Results and Discussion
3.1. Anchoring Trials on a Simulator
3.2. Anchoring Trials on Ex-Vivo Intestine
3.3. Anchoring Trials on Living Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GI tract | Gastrointestinal tract |
| UHMWPE | Ultra-high-molecular-weight Polyethylene |
| SMA | Shape memory alloy |
References
- Mackay, R.S. Radio telemetering from within the human body. IRE Trans. Med. Electron. 1959, ME-6, 100–105. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Osawa, K.; Nakadate, R.; Arata, J.; Nagao, Y.; Akahoshi, T.; Eto, M.; Hashizume, M. Self-Propelled Colonoscopy Robot Using Flexible Paddles. IEEE Robot. Autom. Lett. 2020, 5, 6710–6716. [Google Scholar] [CrossRef]
- Meron, G.D. The development of the swallowable video capsule (M2A). Gastrointest. Endosc. 2000, 52, 817–819. [Google Scholar] [CrossRef]
- Cummins, G.; Cox, B.F.; Ciuti, G.; Anbarasan, T.; Desmulliez, M.P.; Cochran, S.; Steele, R.; Plevris, J.N.; Koulaouzidis, A. Gastrointestinal diagnosis using non-white light imaging capsule endoscopy. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 429–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Alici, G. A novel magnetic anchoring system for wireless capsule endoscopes operating within the gastrointestinal tract. IEEE/ASME Trans. Mechatronics 2019, 24, 1106–1116. [Google Scholar] [CrossRef]
- Mahoney, A.W.; Abbott, J.J. Five-degree-of-freedom manipulation of an untethered magnetic device in fluid using a single permanent magnet with application in stomach capsule endoscopy. Int. J. Robot. Res. 2016, 35, 129–147. [Google Scholar] [CrossRef]
- Lee, C.; Choi, H.; Go, G.; Jeong, S.; Ko, S.Y.; Park, J.O.; Park, S. Active locomotive intestinal capsule endoscope (ALICE) system: A prospective feasibility study. IEEE/ASME Trans. Mechatronics 2014, 20, 2067–2074. [Google Scholar] [CrossRef]
- Lucarini, G.; Ciuti, G.; Mura, M.; Rizzo, R.; Menciassi, A. A new concept for magnetic capsule colonoscopy based on an electromagnetic system. Int. J. Adv. Robot. Syst. 2015, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Carpi, F.; Pappone, C. Magnetic maneuvering of endoscopic capsules by means of a robotic navigation system. IEEE Trans. Biomed. Eng. 2009, 56, 1482–1490. [Google Scholar] [CrossRef]
- Liu, L.; Towfighian, S.; Hila, A. A review of locomotion systems for capsule endoscopy. IEEE Rev. Biomed. Eng. 2015, 8, 138–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagozler, M.E.; Cheung, E.; Kwon, J.; Sitti, M. Miniature endoscopic capsule robot using biomimetic micro-patterned adhesives. In Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, BioRob 2006, Pisa, Italy, 20–22 February 2006; pp. 105–111. [Google Scholar]
- Gao, J.; Zhang, Z.; Yan, G. Locomotion Analysis of a Clamper-based Capsule Robot in a Compliant Tube. IEEE/ASME Trans. Mechatronics 2021, 26, 55–65. [Google Scholar] [CrossRef]
- Jiang, C.; Xiang, L.; Miao, S.; Shi, L.; Xie, D.; Yan, J.; Zheng, Z.; Zhang, X.; Tang, Y. Flexible Interface Design for Stress Regulation of a Silicon Anode toward Highly Stable Dual-Ion Batteries. Adv. Mater. 2020, 32, 1908470. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Zhao, J.; Jiang, Z. Micromanufacturing of composite materials: A review. Int. J. Extrem. Manuf. 2019, 1, 012004. [Google Scholar] [CrossRef]
- Medtronic: PillCam UGI System. Available online: https://www.medtronic.com/covidien/en-us/products/capsule-endoscopy/pillcam-ugi-system.html (accessed on 5 November 2022).
- Fuyuki, A.; Ohkubo, H.; Higurashi, T.; Iida, H.; Inoh, Y.; Inamori, M.; Nakajima, A. Clinical importance of cine-MRI assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction: A retrospective study of 33 patients. J. Gastroenterol. 2017, 52, 577–584. [Google Scholar] [CrossRef]
- Wakamiya, M.; Furukawa, A.; Kanasaki, S.; Murata, K. Assessment of small bowel motility function with cine-MRI using balanced steady-state free precession sequence. J. Magn. Reson. Imaging 2011, 33, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Al-Bahadly, I.; Thomas, D.G.; Avci, E. Measurement of Peristaltic Forces Exerted by Living Intestine on Robotic Capsule. IEEE/ASME Trans. Mechatron. 2021, 26, 1803–1811. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Bahadly, I.; Thomas, D.G.; Avci, E. Capsule robot for gut microbiota sampling using shape memory alloy spring. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. (1980–2015) 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Bahadly, I.; Thomas, D.G.; Avci, E. Towards Gut Microbiota Sampling Using an Untethered Sampling Device. IEEE Access 2021, 9, 127175–127184. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Bahadly, I.; Thomas, D.G.; Avci, E. Development of a Robotic Capsule for in vivo Sampling of Gut Microbiota. IEEE Robot. Autom. Lett. 2022, 7, 9517–9524. [Google Scholar] [CrossRef]
- Bülbring, E.; Crema, A.; Saxby, O. A method for recording peristalsis in isolated intestine. Br. J. Pharmacol. Chemother. 1958, 13, 440–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejati, S.; Wang, J.; Heredia-Rivera, U.; Sedaghat, S.; Woodhouse, I.; Johnson, J.S.; Verma, M.; Rahimi, R. Small intestinal sampling capsule for inflammatory bowel disease type detection and management. Lab Chip 2022, 22, 57–70. [Google Scholar] [CrossRef] [PubMed]



| Anchoring Force (mN) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diameter | 2-legs | 3-legs | 4-legs | |||||||
| Tube | 19 mm | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 |
| Intestine | 16 mm | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 | 2940 |
| 19 mm | 392 | 588 | 490 | 588 | 882 | 686 | 784 | 882 | 686 | |
| 21 mm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehan, M.; Yeo, A.G.; Yousuf, M.U.; Avci, E. Anchoring Mechanism for Capsule Endoscope: Mechanical Design, Fabrication and Experimental Evaluation. Micromachines 2022, 13, 2045. https://doi.org/10.3390/mi13122045
Rehan M, Yeo AG, Yousuf MU, Avci E. Anchoring Mechanism for Capsule Endoscope: Mechanical Design, Fabrication and Experimental Evaluation. Micromachines. 2022; 13(12):2045. https://doi.org/10.3390/mi13122045
Chicago/Turabian StyleRehan, Muhammad, Andrew G. Yeo, Muhammad Uzair Yousuf, and Ebubekir Avci. 2022. "Anchoring Mechanism for Capsule Endoscope: Mechanical Design, Fabrication and Experimental Evaluation" Micromachines 13, no. 12: 2045. https://doi.org/10.3390/mi13122045





