Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams
Abstract
:1. Introduction
2. Methodology and Experimental Setup
2.1. Fabrication of Copper Foams Sample
- (1)
- The copper foam was first cleaned in an ultrasonic bath with deionized water for two intervals of 5 min and replaced with fresh deionized water in between the intervals.
- (2)
- The copper foam was then immersed in the acetic acid (99.9%) bath for 1 h at 40 °C and atmospheric conditions. The acid treatment was performed in a rectangular glass container using a stirrer at 250 rpm.
- (3)
- After that, the copper foams were flushed with nitrogen flow for 5 min and then dried in the vacuum oven operating at 1 kPa. The heating process was composed of a ramp from room temperature (20 °C) to 70 °C in 30 min and followed by a 30 min plateau at 70 °C.
- (4)
- Subsequently, the copper foams were placed into a solution of NaOH and NaClO2 at 90 °C for varying treatment concentrations. Since NaOH reacts with NaClO2 in a 1:1 molar stoichiometric ratio, the concentration of NaOH is equal to that of NaClO2. The blackening treatment was performed in a rectangular glass container using a stirrer at 250 rpm for 1 h.
- (5)
- After the blackening, the copper foams were flushed with nitrogen flow for 15 min and then dried at 250 °C in the vacuum oven at 1 kPa for 2 h. The heating process was composed of a ramp from room temperature (20 °C) to 250 °C in 1.2 h and followed by a 2 h plateau at 250 °C. The blackening copper foams were then stored in a vacuum desiccator until the next use.
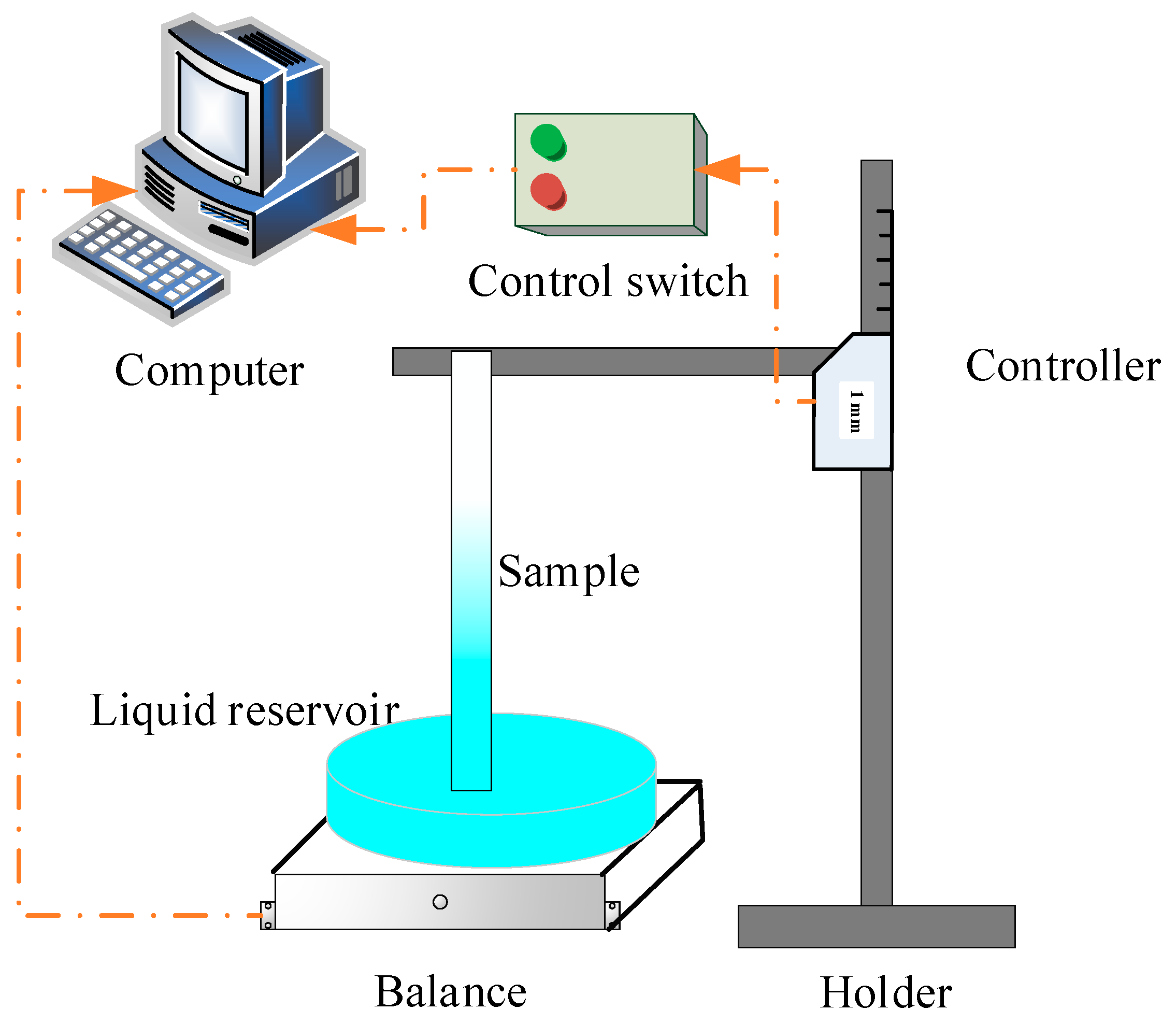
2.2. Setup for Rate-of-Rise Experiment
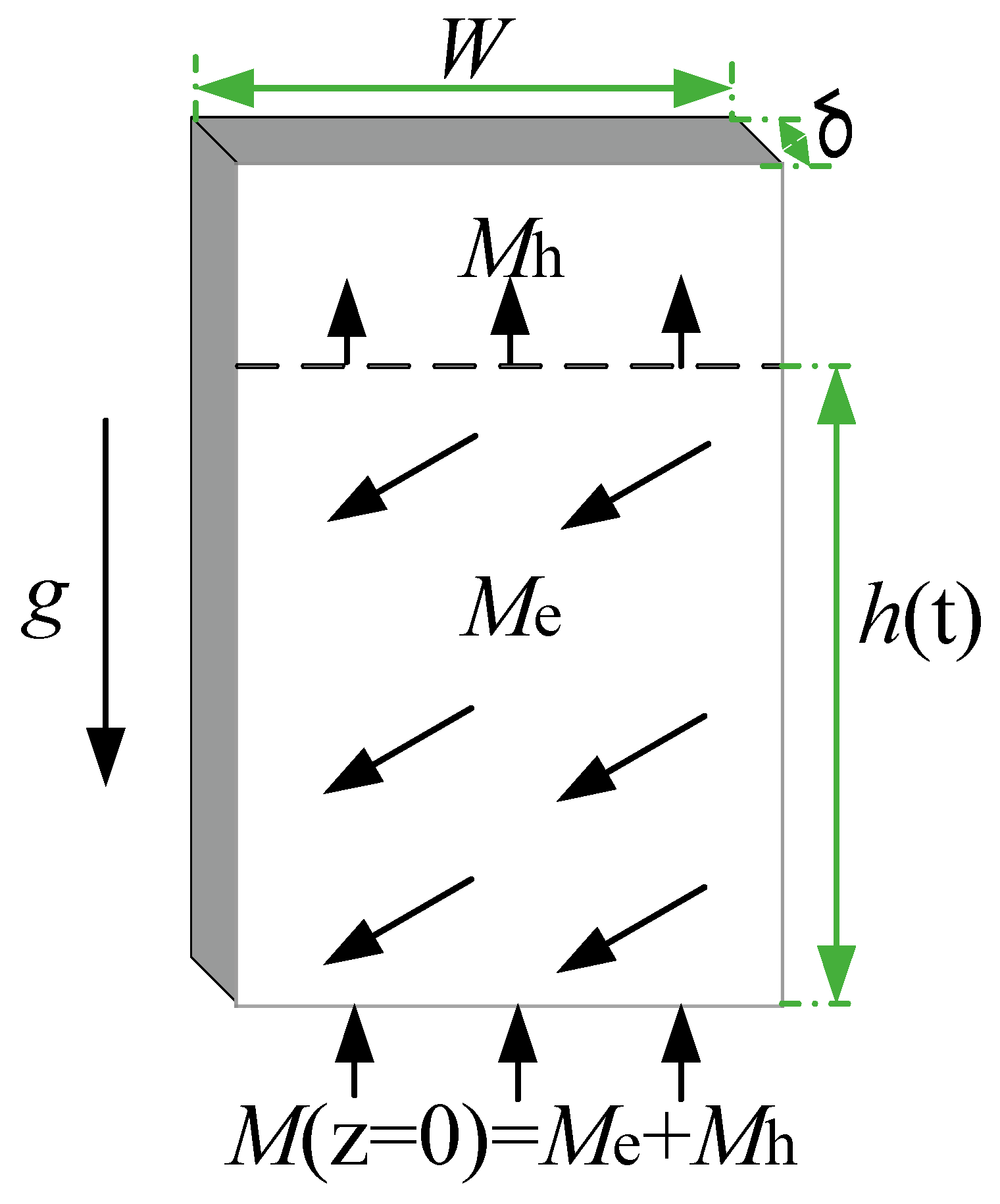
3. Theoretical Model
- (1)
- When the gravitational and evaporation effects are neglected in Equation (14), B = C = 0, it is the Lucas-Washburn equation as
- (2)
- When the evaporation effect is neglected in Equation (14), C = 0, it leads to the following equation
- (3)
- When the gravitational effect is neglected in Equation (14), B = 0, it can be rewritten as
- (4)
- As gravity and evaporation are considered, the solution have to satisfy that , the final solution is
4. Results and Discussion
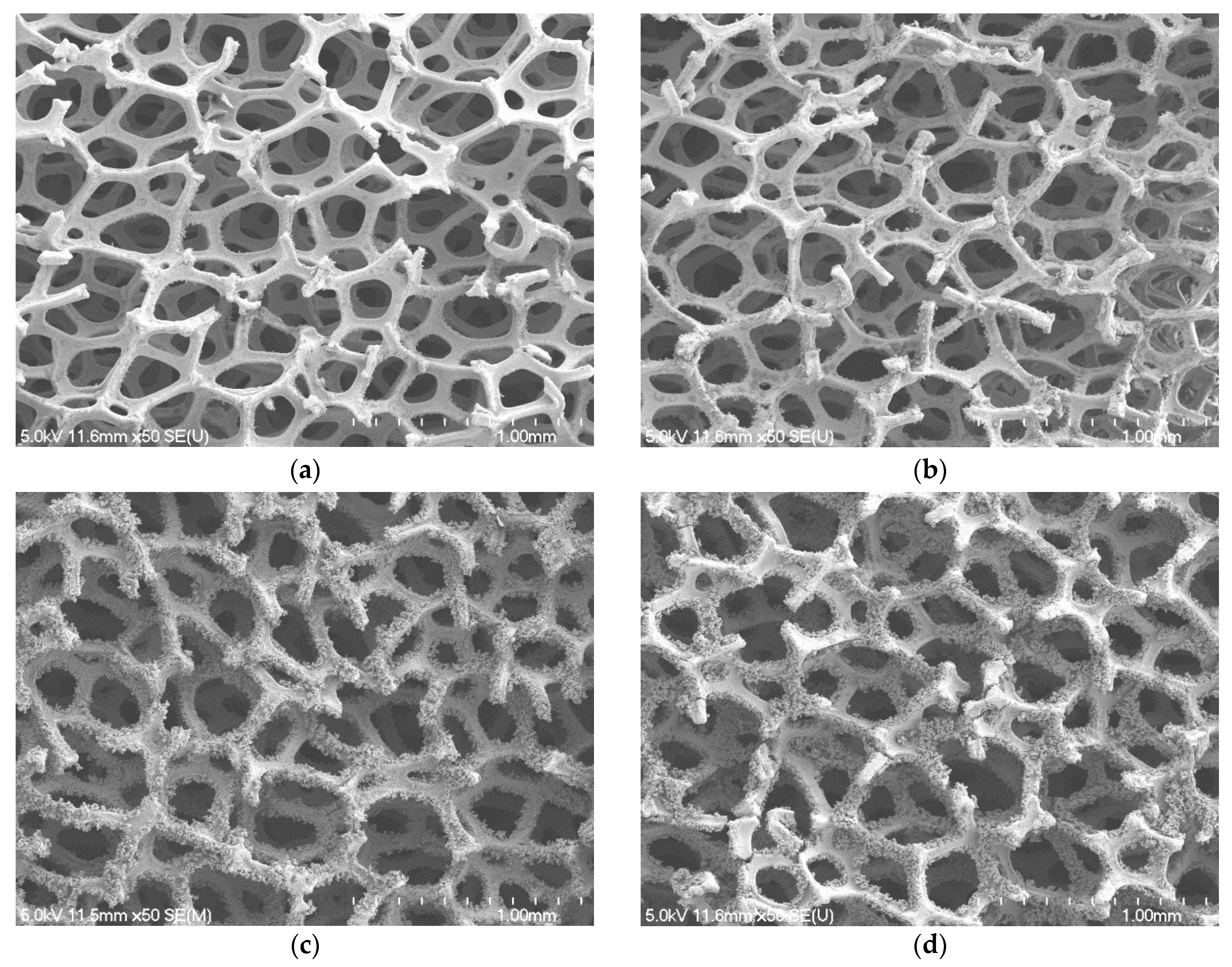
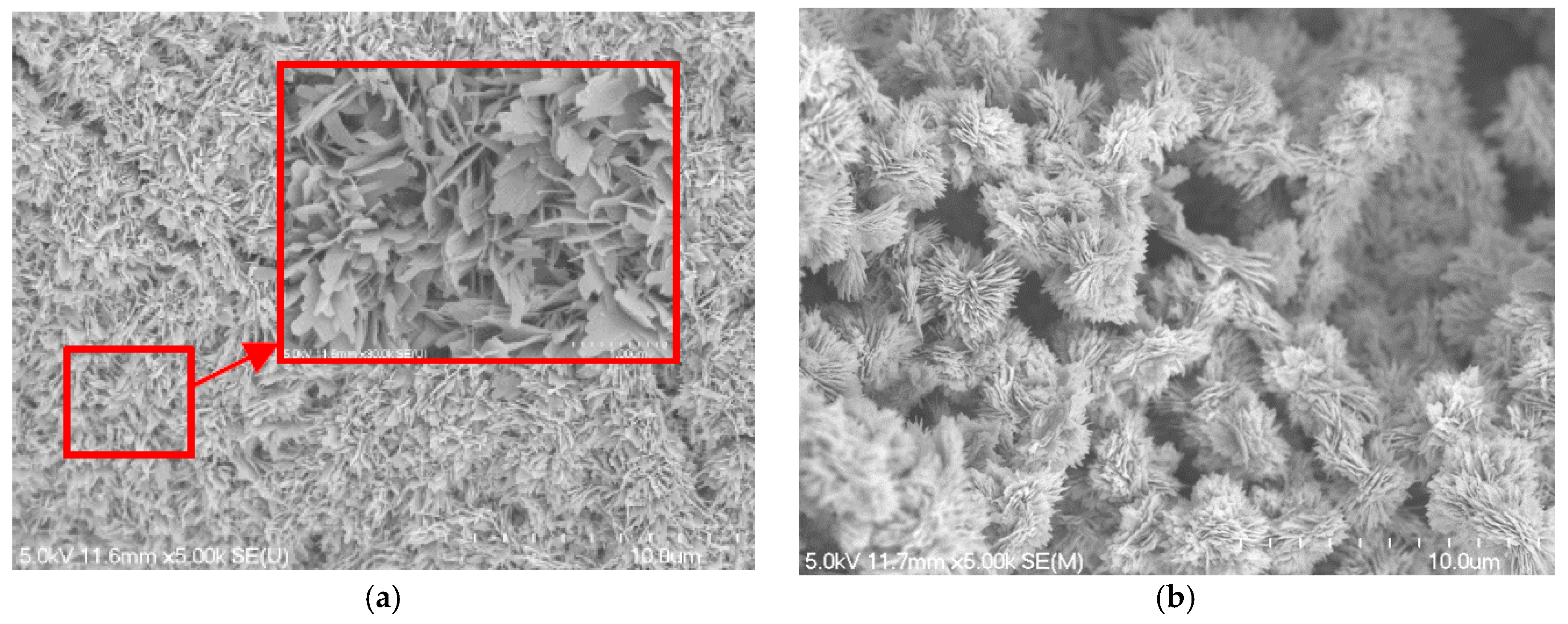
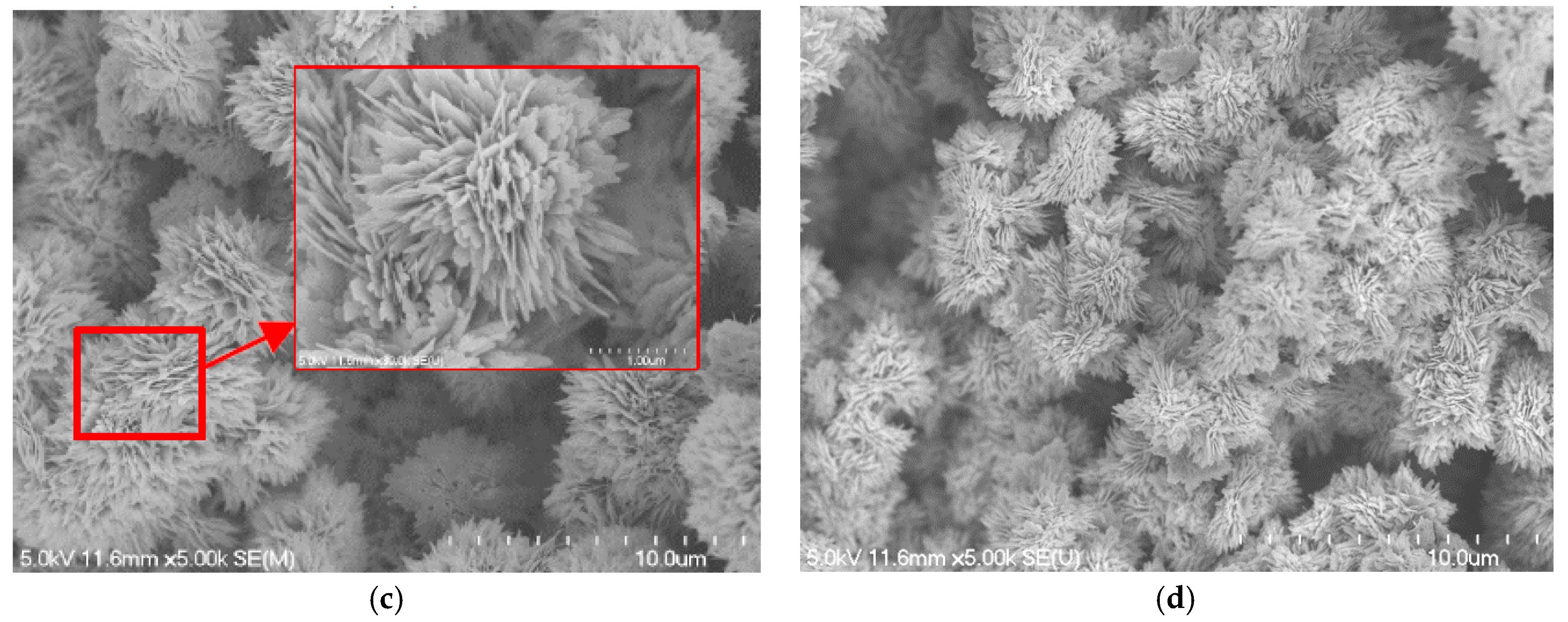
4.1. Surface Characteristics of Blackening Copper Foam
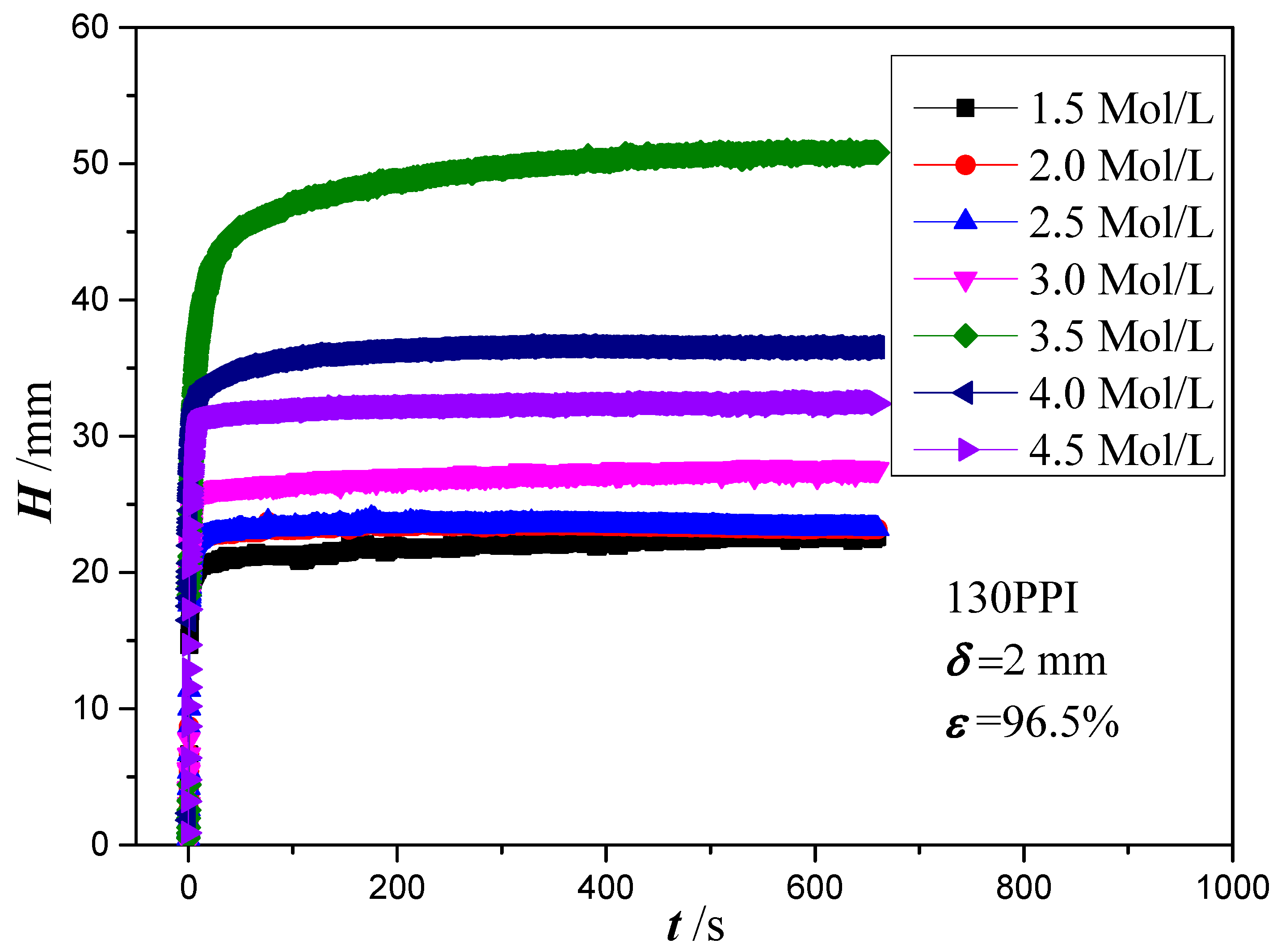
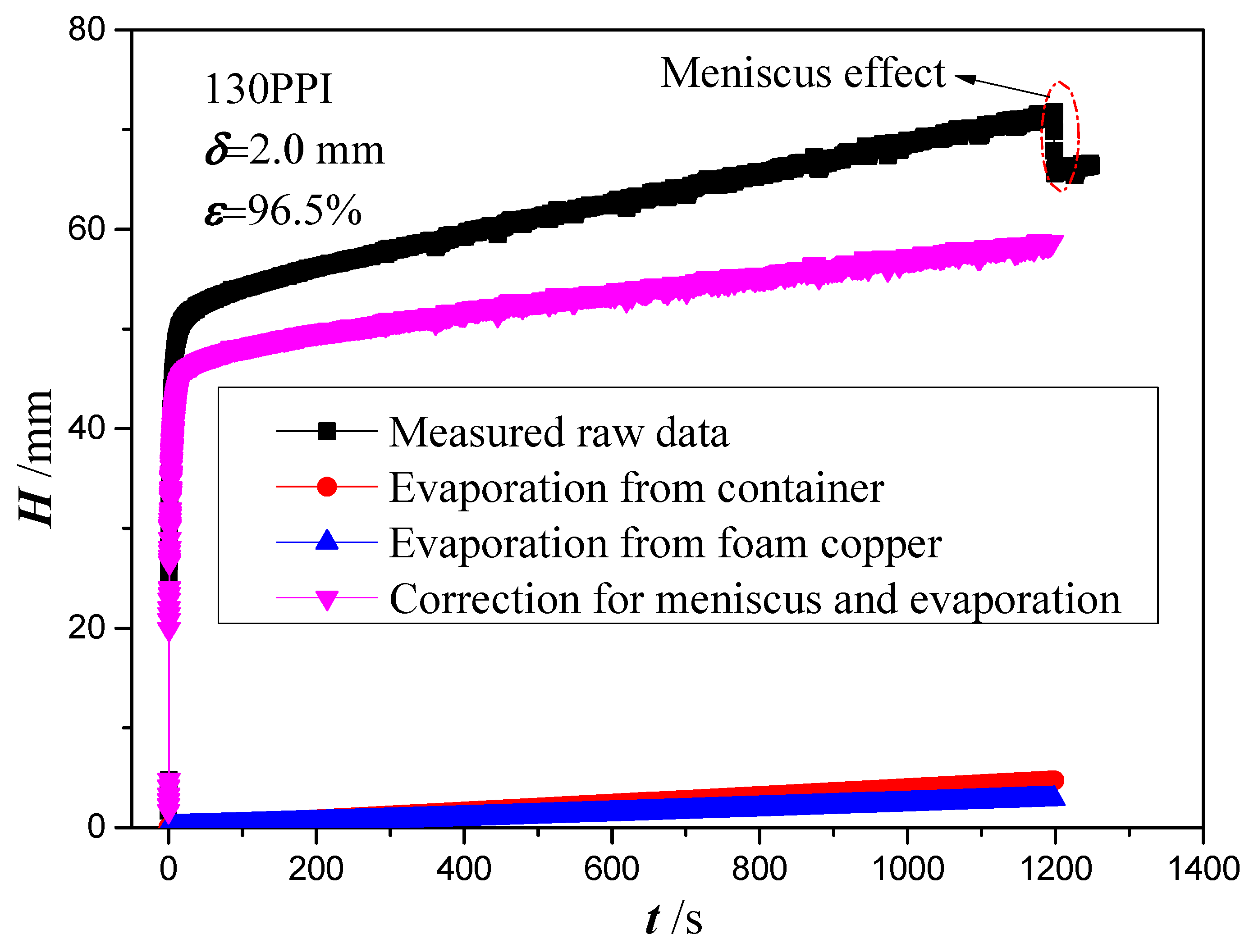
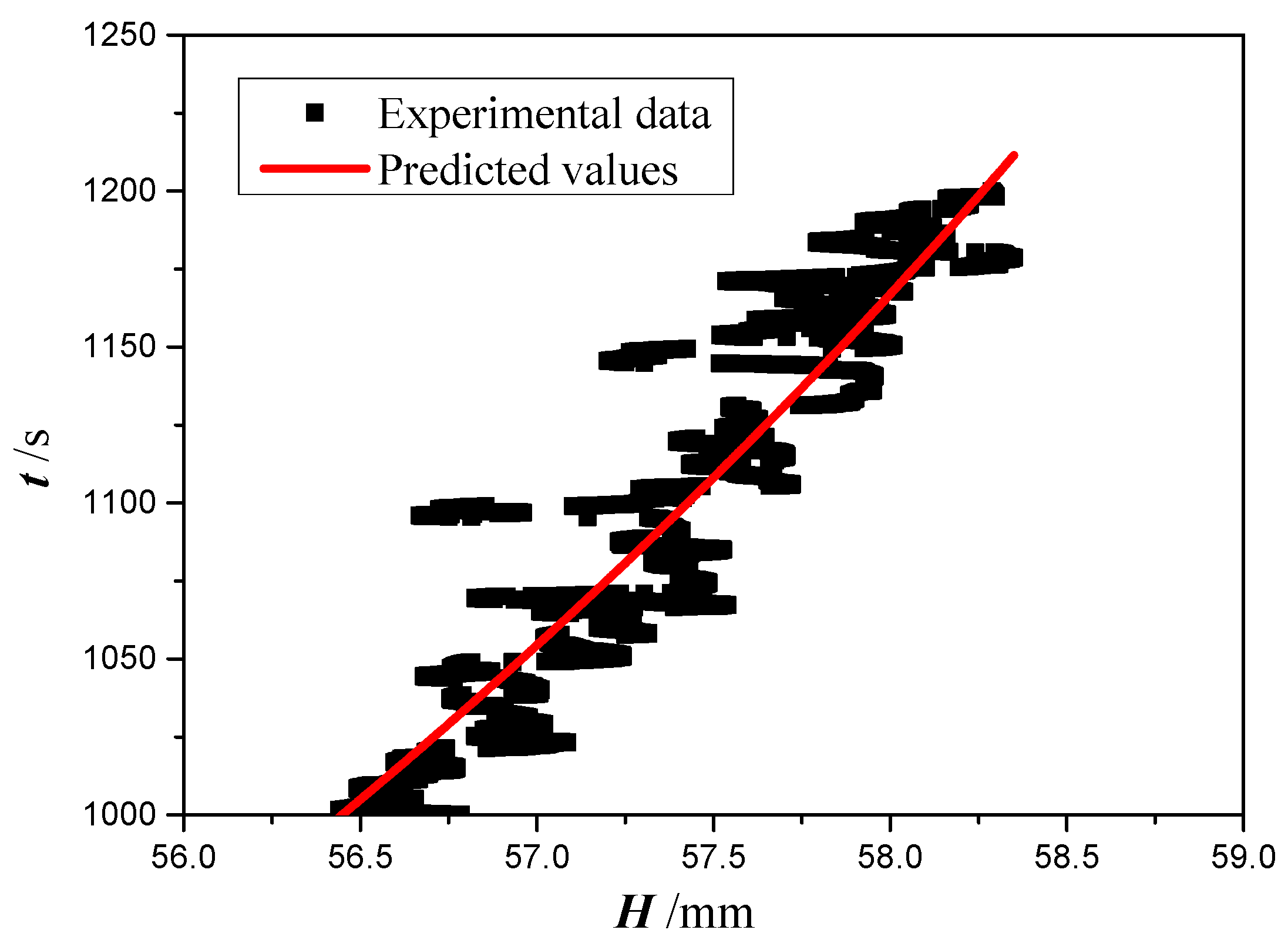
4.2. Analysis of Experiment Results
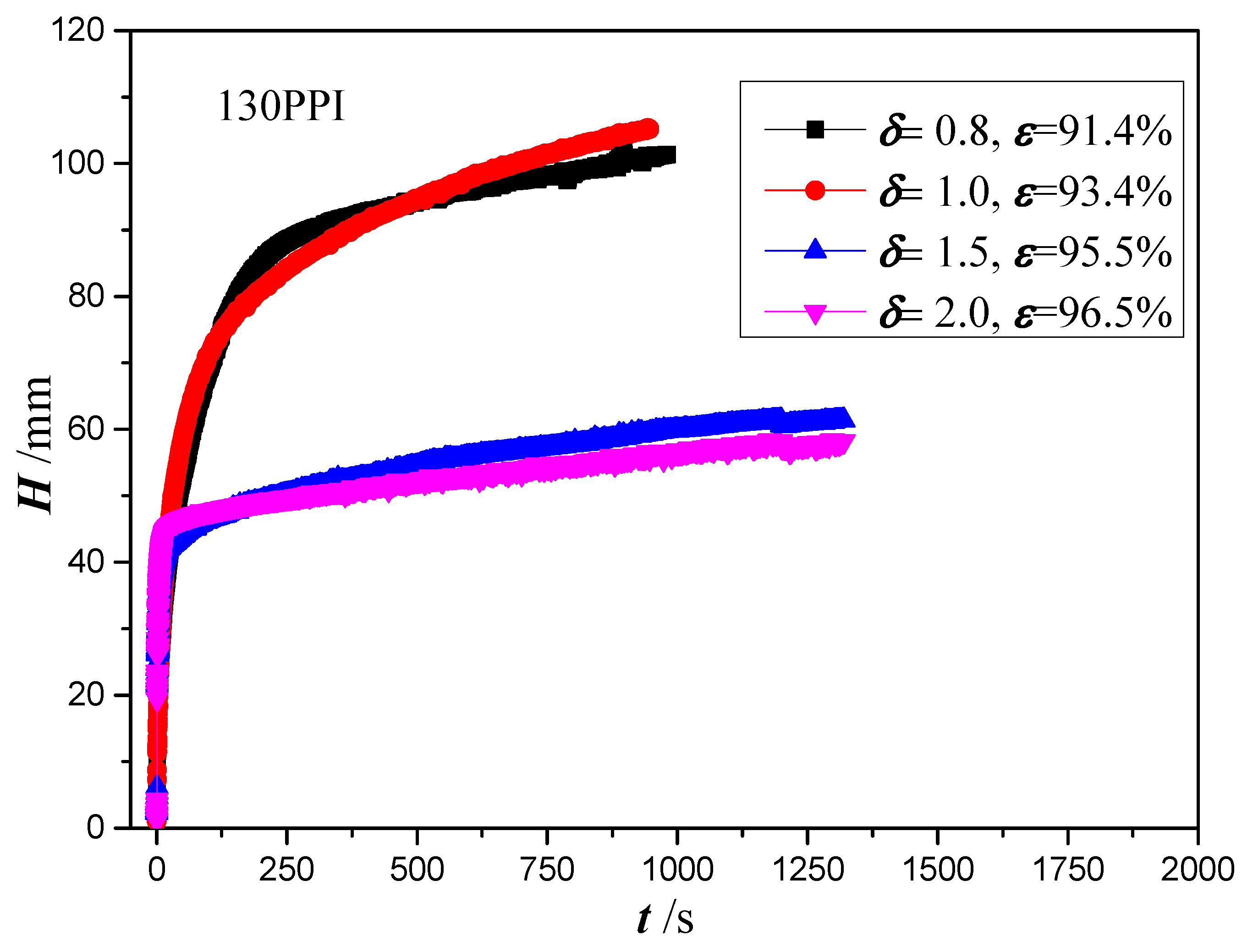
4.3. Effect of Copper Foam Thickness
5. Conclusions
- As the NaOH and NaClO2 solution concentration increases to 3.0 mol/L, the micro-scale ball-shape agglomerates are formed on the copper foam surface, which can significantly enhance the wettability performance of copper foam. The best wicking ability is obtained for oxidation of the copper foam using 3.5 mol/L of NaOH and NaClO2 solution.
- As the copper foam touches the liquid, the macroscopic meniscus is formed around the end and side surface of the copper foams. The meniscus applies a pulling force to the copper foam and results in a jump in wicking height at the initial stage using the measured mass method.
- Gravity plays a major role in defining the permeability and effective pore radius, while the effect of evaporation can be ignored. Permeability and effective pore radius of the four copper foams with PPI from 35 to 130 and thickness of 2.0 mm are obtained by fitting the theoretical model to the experimental data with an RMS of about 20%.
- Under the assumption of uniform saturation inside the copper foams, the maximum wicking height is obtained as 110 mm for thicknesses of 0.8 and 1.0 mm, and 60 mm for thicknesses of 1.5 mm and 2.0 mm in copper foams with a PPI of 130. A bright interface between the unsaturated and saturated zone is formed as the thickness of copper foams increases to 1.5 mm, which is considered the reason for wicking performance degradation. However, the effect of unsaturation to determine the permeability and effective pore radius of the copper metal needs to be studied further.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Latin symbols | |
| A | cross-section area, m2 |
| g | gravitational acceleration, kg·m−2·s−1 |
| h | height, m |
| K | permeability, m2 |
| m | mass, kg |
| reff | effective capillary radius, m |
| Δp | pressure drop, Pa |
| t | time, s |
| W | width, m |
| Greek symbols | |
| δ | thickness, m |
| ρ | density, kg·m−3 |
| μ | viscosity, kg·m−1·s−1 |
| σ | surface tension, N·m−1 |
| ε | porosity |
| θ | angle, ° |
| Subscripts | |
| cap | capillary |
| f | viscous |
| e | evaporation |
| h | hydrostatic |
References
- Cardin, N.; Lips, S.; Siedel, S.; Davoust, L.; Bonjour, J. Two-phase electrohydrodynamics along a grooved fat heat pipe. Exp. Fluids 2020, 61, 170. [Google Scholar] [CrossRef]
- Launay, S.; Vallée, M. State-of-the-art experimental studies on loop heat pipes. Front. Heat Pipes 2010, 2, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Nahar, M.M.; Ma, B.; Guye, K.; Chau, Q.H.; Padilla, J.; Iyengar, M.; Agonafer, D. Microscale evaporative cooling technologies for high heat flux microelectronics devices: Background and recent advances. Appl. Therm. Eng. 2021, 194, 117109. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Z.H.; Qing, J.B.; Xie, Z.C.; Fu, T.; Chen, W.B. Experimental investigation on isothermal performance of the micro-grooved heat pipe. Exp. Therm. Fluid Sci. 2013, 47, 143–149. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Azizian, R.; Akhiani, A.R.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C. Effect of nitrogen-doped graphene nanofluid on the thermal performance of the grooved copper heat pipe. Energy Convers Manag. 2016, 118, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.; Liang, D.; Tang, Y.; Peng, J.; Han, X.; Pan, M. Evaluation of capillary performance of sintered porous wicks for loop heat pipe. Exp. Fluid Sci. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Ling, W.; Zhou, W.; Yu, W.; Liu, R.; Hui, K. Thermal performance of loop heat pipes with smooth and rough porous copper fiber sintered sheets. Energy Convers Manag. 2017, 153, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Dillig, M.; Leimert, J.; Karl, J. Planar high temperature heat pipes for SOFC/SOEC stack applications. Fuel Cells 2014, 14, 479–488. [Google Scholar] [CrossRef]
- Jafari, D.; Shamsi, H.; Filippeschi, S.; Marco, P.D.; Franco, A. An experimental investigation and optimization of screen mesh heat pipes for low-mid temperature applications. Exp. Therm. Fluid Sci. 2017, 84, 120–133. [Google Scholar] [CrossRef]
- Zhou, W.; Ling, W.S.; Duan, L.; Hui, K.S.; Hui, K.N. Development and tests of loop heat pipe with multi-layer metal foams as wick structure. Appl. Therm. Eng. 2016, 94, 324–330. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Zeng, Z. Evaporation and condensation heat transfer in a heat pipe with a sintered-grooved composite wick. Appl. Eng. 2013, 50, 342–351. [Google Scholar] [CrossRef]
- Dhanabal, S.; Annamalai, M.; Muthusamy, K. Experimental investigation of thermal performance of metal foam wicked flat heat pipe. Exp. Therm. Fluid Sci. 2017, 82, 482–492. [Google Scholar]
- Bao, K.L.; Hua, C.; Wang, X.H.; Han, X.H.; Chen, G.M. Experimental investigation on the heat transfer performance and evaporation temperature fluctuation of a new-type metal foam multichannel heat pipe. Int. J. Heat Mass Transf. 2020, 154, 119672. [Google Scholar] [CrossRef]
- Shen, B.B.; Yan, H.B.; Sunden, B.; Xue, H.Q.; Xie, G.N. Forced convection and heat transfer of water-cooled microchannel heat sinks with various structured metal foams. Int. J. Heat Mass Transf. 2017, 113, 1043–1053. [Google Scholar] [CrossRef]
- Tang, H.; Weng, C.; Tang, Y.; Lu, Y.; Fu, T. Effect of inclination angle on the thermal performance of an ultrathin heat pipe with multi-scale wick structure. Int. Commun. Heat Mass Transf. 2020, 118, 104908. [Google Scholar] [CrossRef]
- Rachedi, R.; Chikh, S. Enhancement of electronic cooling by insertion of foam materials. Heat Mass Transf. 2001, 37, 371–378. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Du, Y.P.; Shum, C.; Cai, B.Y.; Le, N.C.H.; Chen, X.; Duck, B.; Fell, C.; Zhu, Y.G.; Gu, M. Efficiently-cooled plasmonic amorphous silicon solar cells integrated with a nano-coated heat-pipe plate. Sci Rep. 2016, 6, 24972. [Google Scholar] [CrossRef] [Green Version]
- Xin, F.; Lyu, Q.; Tian, W. Visualization and Heat Transfer Performance of Mini-Grooved Flat Heat Pipe Filled with Different Working Fluids. Micromachines 2022, 13, 1341. [Google Scholar] [CrossRef]
- Wong, S.; Lin, Y. Effect of copper surface wettability on the evaporation performance: Tests in a flat-plate heat pipe with visualization. Int. J. Heat Mass Transf. 2011, 54, 3921–3926. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; He, X.; Sun, F. Hierarchical nanostructures of cupric oxide on a copper substrate: Controllable morphology and wettability. J. Mater. Chem. 2006, 16, 4427–4434. [Google Scholar] [CrossRef]
- Oishi, T.; Goto, M.; Kasahara, A.; Tosa, M. Low frictional copper oxide film prepared with sodium hydroxide solution. Surf. Interface Anal. 2004, 36, 1259–1261. [Google Scholar] [CrossRef]
- Tu, S.H.; Wu, H.C.; Wu, C.J.; Cheng, S.L.; Sheng, Y.J.; Tsao, H.K. Growing hydrophobicity on a smooth copper oxide thin film at room temperature and reversible wettability transition. Appl. Surf. Sci. 2014, 316, 88–92. [Google Scholar] [CrossRef]
- Ji, X.B.; Wang, Y.; Xu, J.L.; Huang, Y.P. Effects of oxidation processes and microstructures on the hydrophilicity of copper surface. Mater. Lett. 2017, 195, 71–75. [Google Scholar] [CrossRef]
- Köroğlu, B.; Lee, K.S.; Park, C. Nano/micro-scale surface modifications using copper oxidation for enhancement of surface wetting and falling-film heat transfer. Int. J. Heat Mass Transf. 2013, 62, 794–804. [Google Scholar] [CrossRef]
- Faghri, A. Heat Pipe Science and Technology, 1st ed.; Taylor & Francis: Washington, DC, USA, 1995. [Google Scholar]
- Popova, N.; Schaeffer, C.; Avenas, Y. Fabrication and thermal performance of a thin flat heat pipe with innovative sintered copper wick structure. In Proceedings of the 2006 IEEE Industry Applications, Tampa, FL, USA, Conference, 8–12 October 2006; Volume 2, pp. 791–796. [Google Scholar]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Yin, H.; Jin, Z.; Zhang, S.; Wang, S.; Zhang, Z. Reason for the loss of hydrophilicity of TiO2 film and its photocatalytic regeneration. Sci. China Ser. B Chem. 2002, 45, 625–632. [Google Scholar] [CrossRef]
- Shum, C.; Rosengarten, G.; Zhu, Y.G. Enhancing wicking microfows in metallic foams. Microfuid Nanofuid 2017, 21, 177. [Google Scholar] [CrossRef]
- Liu, J.F.; Wu, W.T.; Chiu, W.C.; Hsieh, W.H. Measurement and correlation of friction characteristic. Exp. Therm. Fluid Sci. 2006, 30, 329–336. [Google Scholar] [CrossRef]
- Dukhan, N. Correlations for the pressure drop for flow through metal foam. Exp. Fluids 2006, 41, 665–672. [Google Scholar] [CrossRef]
- Bağcı, Ö.; Dukhan, N. Experimental hydrodynamics of high-porosity metal foam: Effect of pore density. Int. J. Heat Mass Transf. 2016, 103, 879–885. [Google Scholar] [CrossRef]
- Onstad, A.J.; Elkins, C.J.; Medina, F.; Wicker, R.B.; Eaton, J.K. Full-field measurements of flow through a scaled metal foam replica. Exp. Fluids 2011, 50, 1571–1585. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Ma, B.; Zhu, Y. Review and a Theoretical Approach on Pressure Drop Correlations of Flow through Open-Cell Metal Foam. Materials 2021, 14, 3153. [Google Scholar] [CrossRef] [PubMed]
- Plessis, P.D. Pressure dorp prediction for flow through high porosity metallic foams. Chem. Eng. Sci. 1994, 49, 3545–3553. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, D.; Lu, L.; Pan, M.; Wang, Q. Experimental investigation on capillary force of composite wick structure by IR thermal imaging camera. Exp. Therm. Fluid Sci. 2010, 34, 190–196. [Google Scholar] [CrossRef]
- Deng, D.X.; Tang, Y.; Huang, G.H.; Lu, L.S.; Yuan, D. Characterization of capillary performance of composite wicks for two-phase heat transfer devices. Int. J. Heat Mass Transf. 2013, 56, 283–293. [Google Scholar] [CrossRef]
- Feng, C.; Yugeswaran, S.; Chandra, S. Capillary rise of liquids in thermally sprayed porous copper wicks. Exp. Therm. Fluid Sci. 2018, 98, 206–216. [Google Scholar] [CrossRef]
- Jafaria, D.; Wits, W.W.; Geurts, B.J. Metal 3D-printed wick structures for heat pipe application: Capillary performance analysis. Appl. Therm. Eng. 2018, 143, 403–414. [Google Scholar] [CrossRef]
- Schoelkopf, J.; Gane, P.A.C.; Ridgway, C.J.; Matthews, G.P. Practical observation of deviation from Lucas–Washburn scaling in porous media. Colloid Surf. A 2002, 206, 445–454. [Google Scholar] [CrossRef]
- Holley, B.; Faghri, A. Permeability and effective pore radius measurements for heat pipe and fuel cell applications. Appl. Therm. Eng. 2006, 26, 448–462. [Google Scholar] [CrossRef]
- Fries, N.; Odic, K.; Conrath, M.; Dreyer, M. The effect of evaporation on the wicking of liquids into a metallic weave. J. Colloid Interface Sci. 2008, 321, 118–129. [Google Scholar] [CrossRef]
- Ma, B. Analysis of Capillary Flow in a Parallel Microchannel-Based Wick Structure with Circular and Noncircular Geometries. Langmuir 2020, 36, 13485–13497. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, D.; Yang, H. Analytical and numerical study of capillary rise in sinusoidal wavy channel: Unveilling the role of interfacial wobbling. Phys. Fluids 2022, 34, 052114. [Google Scholar] [CrossRef]
- Moffat, R.J. Describing the uncertainties in experimental results. J. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Shirazy, M.R.S.; Fréchette, L.G. Capillary and wetting properties of copper metal foams in the presence of evaporation and sintered walls. Int. J. Heat Mass Transf. 2013, 58, 282–291. [Google Scholar] [CrossRef]




| PPI | Porosity ε | Thickness δ (mm) |
|---|---|---|
| 35 | 91.4% | 2 |
| 70 | 95.6% | 2 |
| 110 | 96.8% | 2 |
| 130 | 96.5% | 2 |
| 130 | 95.5% | 1.5 |
| 130 | 93.4% | 1.0 |
| 130 | 91.4% | 0.8 |
| Condition | K (μm2) | reff (μm) | K/reff (μm) | RMS |
|---|---|---|---|---|
| Ignore evaporation and gravity | 0.3342 | 27.2% | ||
| Ignore evaporation | 982.5 | 250.48 | 3.922 | 20.5% |
| Ignore gravity | 0.3343 | 27.3% | ||
| No restriction | 982.5 | 250.48 | 3.922 | 21.7% |
| Sample | ε | K (μm2) | reff (μm) | K/reff (μm) | RMS |
|---|---|---|---|---|---|
| 130 PPI | 91.4% | 982.5 | 250.48 | 3.922 | 20.5% |
| 110 PPI | 95.6% | 1421.1 | 302.26 | 4.702 | 15.9% |
| 70 PPI | 96.8% | 3403.5 | 371.64 | 9.158 | 18.6% |
| 35 PPI | 96.5% | 7535.5 | 791.58 | 9.520 | 15.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Yang, Y.; Ma, B.; Zhu, Y. Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams. Micromachines 2022, 13, 2052. https://doi.org/10.3390/mi13122052
Yang H, Yang Y, Ma B, Zhu Y. Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams. Micromachines. 2022; 13(12):2052. https://doi.org/10.3390/mi13122052
Chicago/Turabian StyleYang, Huizhu, Yue Yang, Binjian Ma, and Yonggang Zhu. 2022. "Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams" Micromachines 13, no. 12: 2052. https://doi.org/10.3390/mi13122052
APA StyleYang, H., Yang, Y., Ma, B., & Zhu, Y. (2022). Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams. Micromachines, 13(12), 2052. https://doi.org/10.3390/mi13122052









