Structure and Photoluminescence of WO3-x Aggregates Tuned by Surfactants
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Synthesis
2.3. Characterization and PL Measurement
3. Results
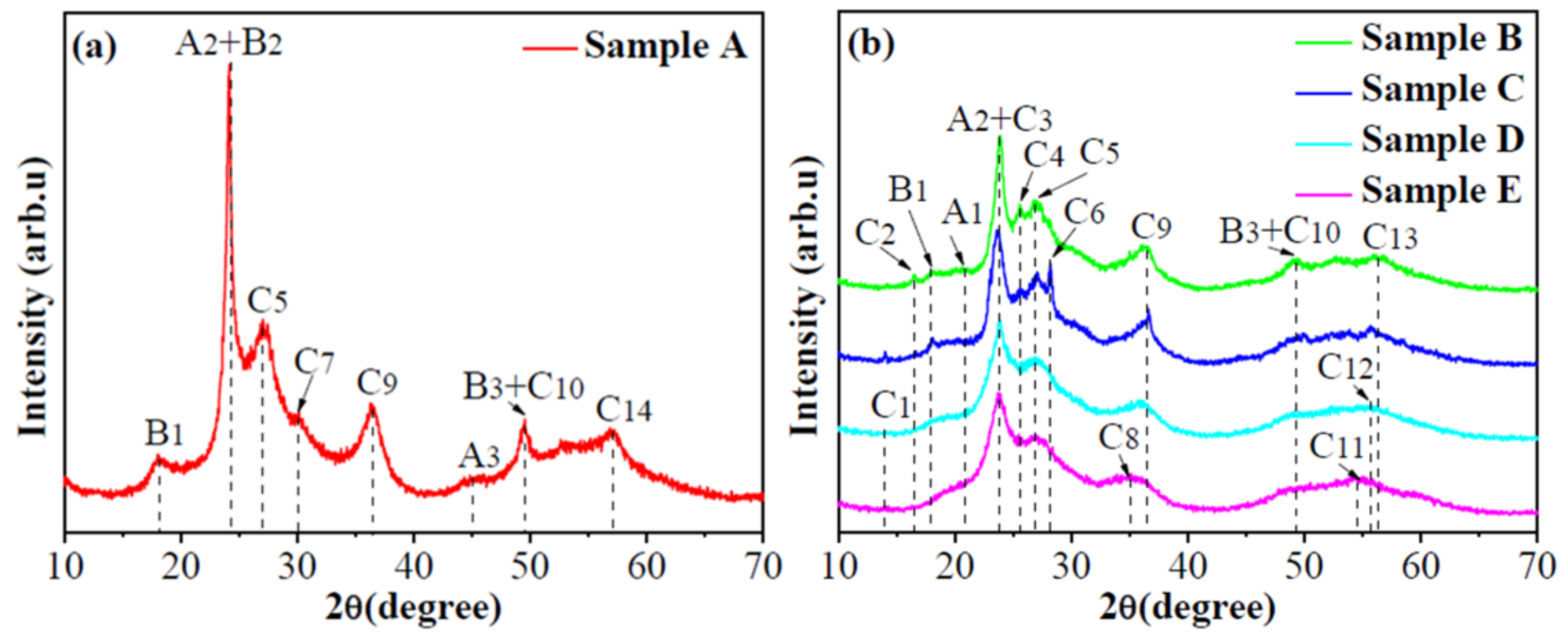
3.1. Phases and Composition
3.2. Binding States of Elements
3.3. Morphology and Microstructure
3.4. Aggregation of WO3-x Nanoparticles in Different Solutions
3.4.1. Aggregation of WO3-x Nanoparticles in Acetic Acid
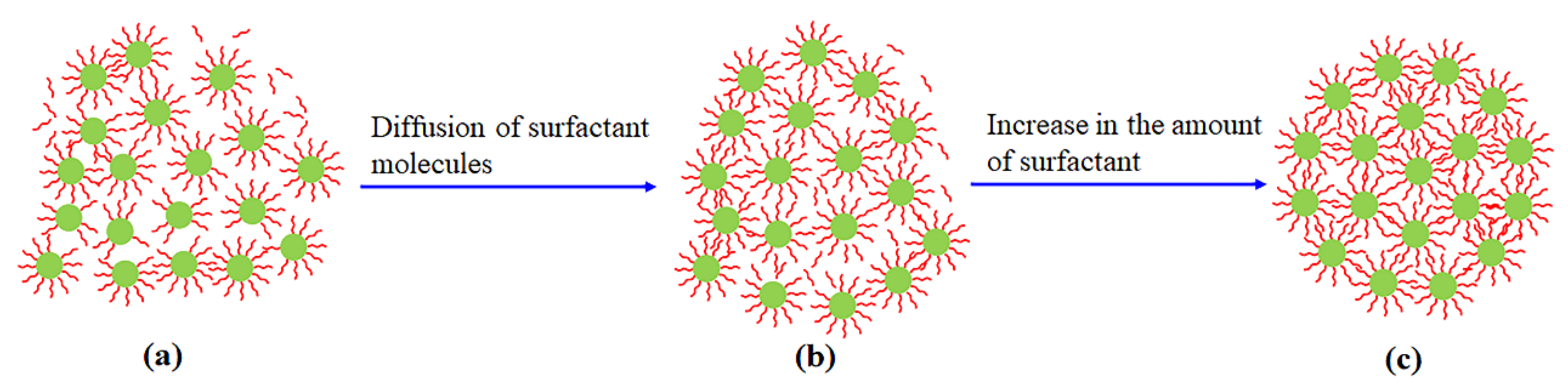
3.4.2. Aggregation under Surfactants
- Changes in size, motion and distribution of WO3-x nanoparticles
- 2.
- Aggregation of WO3-x nanoparticles
3.5. PL Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, D.; Fang, Z.; Yang, E.; Ning, Z.; Zhou, Q.; Chen, K.; Zheng, Y.; Zhang, Y.; Shen, Y. Facile preparation of WO3-x dots with remarkably low toxicity and uncompromised activity as coreactants for clinic electrochemiluminescent diagnosis. Angew. Chem. Int. Ed. 2020, 59, 16747–16754. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, X.; Wang, R.; Liu, J.; Liu, L. Study on the gamma irradiation characteristics of a carbon nanotube sponge/polydimethylsiloxane/tungsten oxide flexible force-sensitive structure. Micromachines 2022, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Han, J.; Tang, Z. Formation of supraparticles and their application in catalysis. ACS Mater. Lett. 2020, 2, 95–106. [Google Scholar] [CrossRef]
- Zhao, Q.; Gouget, G.; Guo, J.; Yang, S.; Zhao, T.; Straus, D.B.; Qian, C.; Oh, N.; Wang, H.; Murray, C.B.; et al. Enhanced carrier transport in strongly coupled, epitaxially fused CdSe nanocrystal solids. Nano Lett. 2021, 21, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Qin, X.; Song, Q.; Qiao, X.; Zhang, Z.; Xue, Z.; Liu, C.; Mo, G.; Wang, T. Ordered superparticles with an enhanced photoelectric effect by sub-nanometer interparticle distance. Adv. Funct. Mater. 2017, 27, 1701982. [Google Scholar] [CrossRef]
- Narayanaswamy, A.; Xu, H.; Pradhan, N.; Peng, X. Crystalline nanoflowers with different chemical compositions and physical properties grown by limited ligand protection. Angew. Chem. 2006, 118, 5487–5490. [Google Scholar] [CrossRef]
- Evers, W.H.; Schins, J.M.; Aerts, M.; Kulkarni, A.; Capiod, P.; Berthe, M.; Grandidier, B.; Delerue, C.; van der Zant, H.S.J.; van Overbeek, C.; et al. High charge mobility in two-dimensional percolative networks of PbSe quantum dots connected by atomic bonds. Nat. Commun. 2015, 6, 8195. [Google Scholar] [CrossRef]
- Pereira, R.N.; Coutinho, J.; Niesar, S.; Oliveira, T.A.; Aigner, W.; Wiggers, H.; Rayson, M.J.; Briddon, P.; Brandt, M.S.; Stutzmann, M. Resonant electronic coupling enabled by small molecules in nanocrystal solids. Nano Lett. 2014, 14, 3817–3826. [Google Scholar] [CrossRef]
- Liu, Y.; Gibbs, M.; Puthussery, J.; Gaik, S.; Ihly, R.; Hillhouse, H.W.; Law, M. Dependence of carrier mobility on nanocrystal size and ligand length in PbSe nanocrystal solids. Nano Lett. 2010, 10, 1960–1969. [Google Scholar] [CrossRef]
- Konstantatos, G.; Howard, I.; Fischer, A.; Hoogland, S.; Clifford, J.; Klem, E.; Levina, L.; Sargent, E.H. Ultrasensitive solution-cast quantum dot photodetectors. Nature 2006, 442, 180–183. [Google Scholar] [CrossRef]
- Tang, Z.; Kotov, N.A.; Giersig, M. Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 2002, 297, 237–240. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zietz, B.; Niklasson, G.A.; Österlund, L. Optical properties of nanocrystalline WO3 and WO3-x thin films prepared by DC magnetron sputtering. J. Appl. Phys. 2014, 115, 213510. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhong, X.X.; He, C.L.; Zhang, B.; Cvelbar, U.; Ostrikov, K. Solvent-dependent structures and photoluminescence of WO3-x nanomaterials grown in nonaqueous solutions. J. Alloys Compd. 2021, 854, 157249. [Google Scholar] [CrossRef]
- Paik, T.; Cargnello, M.; Gordon, T.R.; Zhang, S.; Yun, H.; Lee, J.D.; Woo, H.Y.; Oh, S.J.; Kagan, C.R.; Fornasiero, P.; et al. Photocatalytic hydrogen evolution from substoichiometric colloidal WO3−x nanowires. ACS Energy Lett. 2018, 3, 1904–1910. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Liao, W.; Zheng, M.; Xiao, L.; Zhu, H.; Zhang, M.; Du, M.; Yao, J. WO3−x nanoplates grown on carbon nanofibers for an efficient electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 18132–18139. [Google Scholar] [CrossRef]
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Wang, F.; Valentin, C.D.; Pacchioni, G. Semiconductor-to-metal transition in WO3−x: Nature of the oxygen vacancy. Phys. Rev. B 2011, 84, 073103. [Google Scholar] [CrossRef]
- Gerosa, M.; Valentin, C.D.; Onida, G.; Bottani, C.E.; Pacchioni, G. Anisotropic effects of oxygen vacancies on electrochromic properties and conductivity of γ-monoclinic WO3. J. Phys. Chem. C 2016, 120, 11716–11726. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, X.; Ma, J.; Zhu, Y.; Cheng, X.; Luo, W.; Deng, Y. Rational synthesis and gas sensing performance of ordered mesoporous semiconducting WO3/NiO composites. ACS Appl. Mater. Interfaces 2019, 11, 26268–26276. [Google Scholar] [CrossRef]
- Duan, G.; Chen, L.; Jing, Z.; Luna, P.D.; Wen, L.; Zhang, L.; Zhao, L.; Xu, J.; Li, Z.; Yang, Z.; et al. Robust antibacterial activity of tungsten oxide (WO3-x) nanodots. Chem. Res. Toxicol. 2019, 32, 1357–1366. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhong, X.X.; He, C.L.; Zhang, B.; Shao, R.W.; Shvalya, V.; Cvelbar, U.; Ostrikov, K. Controlling oxygen vacancies of WOx suboxides by ZnWO4 nanophase hybridization. Mater. Sci. Eng. B 2020, 262, 114706. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhong, X.X.; He, C.L.; Zhang, B.; Cvelbar, U.; Ostrikov, K. Nanostructure conversion and enhanced photoluminescence of vacancy engineered substoichiometric tungsten oxide nanomaterials. Mater. Chem. Phys. 2021, 262, 124311. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhong, X.X.; Zhu, J.; Zhang, Y.; Cvelbar, U.; Ostrikov, K. Single-step synthesis of sub-stoichiometric tungsten oxide particles in mixed acetic and oleic acids: Structural conversion and photoluminescence enhancement. J. Alloys Compd. 2022, 899, 163265. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, X.; Zhang, Y.; Xu, H.; Zhang, Y. Microspheres assembled by WO3-x nanoparticles under action of dual surfactants: Structure and photoluminescence properties. Opt. Mater. 2022, 129, 112550. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Turner, R.J. Stability of biogenic metal(loid) nanomaterials related to the colloidal stabilization theory of chemical nanostructures. Crit. Rev. Biotechnol. 2018, 38, 1137–1156. [Google Scholar] [CrossRef]
- Fulton, G.; Lunev, A. Probing the correlation between phase evolution and growth kinetics in the oxide layers of tungsten using Raman spectroscopy and EBSD. Corros. Sci. 2020, 162, 108221. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide hydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Garcia-Sanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal effects associated with the Raman spectroscopy of WO3 gas-sensor materials. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef]
- Blackman, C.S.; Parkin, I.P. Atmospheric pressure chemical vapor deposition of crystalline monoclinic WO3 and WO3-x thin films from reaction of WCl6 with O-containing solvents and their photochromic and electrochromic properties. Chem. Mater. 2005, 17, 1583–1590. [Google Scholar] [CrossRef]
- Yu, W.; Shen, Z.; Peng, F.; Lu, Y.; Ge, M.; Fu, X.; Sun, Y.; Chen, X.; Dai, N. Improving gas sensing performance by oxygen vacancies in sub-stoichiometric WO3-x. RSC Adv. 2019, 9, 7723–7728. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MI, USA, 1979; Volume 38, pp. 147–184. [Google Scholar]
- He, Y.; Liang, A.; Zhu, D.; Hu, M.; Xu, L.; Chao, S.; Zhou, W.; Wu, Y.; Xu, J.; Zhao, F. Organic-inorganic hybrid electrode engineering for high-performance asymmetric supercapacitor based on WO3-CeO2 nanowires with oxygen vacancies. Appl. Surf. Sci. 2022, 573, 151624. [Google Scholar] [CrossRef]
- Zhang, N.; Li, F.; Yin, Y.; Han, J.; Li, X.; Liu, C.; Zhou, J.; Wen, S.; Adimi, S.; Chen, Y.; et al. Gas sensor based on TiO2 nanofibers decorated with monodispersed WO3 nanocubes for fast and selective xylene detection. Mater. Sci. Eng. B 2021, 263, 114901. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef]
- Chen, S.-H.; Yu, C.-F.; Wang, C.-J.; Chen, S.-H.; Chen, Y.-D.; Chen, T.-C.; Lin, C.-F. Light enhancement of plasmonic nano-structure for PLEDs at RGB wavelengths. Org. Electron. 2016, 38, 337–343. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hsu, P.-J. Luminous efficiency improvement of polymer light-emitting diodes with platinum nanolayer at the PEDOT:PSS–ITO interface. Opt. Lett. 2021, 46, 6039–6042. [Google Scholar] [CrossRef]
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.-F.; Hirsch, A.; Peukert, W. Experimental and theoretical studies of the colloidal stability of nanoparticles―A general interpretation based on stability maps. ACS Nano 2011, 5, 4658–4669. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Patil, V.B.; Adhyapak, P.V.; Suryavanshi, S.S.; Mulla, I.S. Oxalic acid induced hydrothermal synthesis of single crystalline tungsten oxide nanorods. J. Alloys Compd. 2014, 590, 283–288. [Google Scholar] [CrossRef]
- Vayssieres, L. On the design of advanced metal oxide nanomaterials. Int. J. Nanotechnol. 2004, 1, 1–41. [Google Scholar] [CrossRef]
- Oswal, S.L.; Desai, H.S. Studies of viscosity and excess molar volume of binary mixtures. 1. Propylamineq1-alkanol mixtures at 303.15 and 313.15 K. Fluid Phase Equilibria 1998, 149, 359–376. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, M. Viscosity of organic compounds. J. South-Cent. Coll. Natl. (Nat. Sci.) 1997, 16, 5–10. (In Chinese) [Google Scholar]
- Sagdeev, D.; Gabitov, I.; Isyanov, C.; Khairutdinov, V.; Farakhov, M.; Zaripov, Z.; Abdulagatov, I. Densities and viscosities of oleic acid at atmospheric pressure. J. Am. Oil Chem. Soc. 2019, 96, 647–662. [Google Scholar] [CrossRef]
- Johes, R.A.L. Soft Condensed Matter; Oxford University Press: Oxford, England, 2002; p. 60. [Google Scholar]
- Fritz, G.; Schädler, V.; Willenbacher, N.; Wagner, N.J. Electrosteric stabilization of colloidal dispersions. Langmuir 2002, 18, 6381–6390. [Google Scholar] [CrossRef]
- Kotov, N.K. Practica l aspects of self-organization of nanoparticles: Experimental guide and future applications. J. Mater. Chem. 2011, 21, 16673–16674. [Google Scholar] [CrossRef]
- Sakohara, S.; Tickanen, L.D.; Anderson, M.A. Luminescence properties of thin zinc oxide membranes prepared by the Sol-Gel technique: Change in visible luminescence during firing. J. Phys. Chem. 1992, 96, 11086–11091. [Google Scholar] [CrossRef]


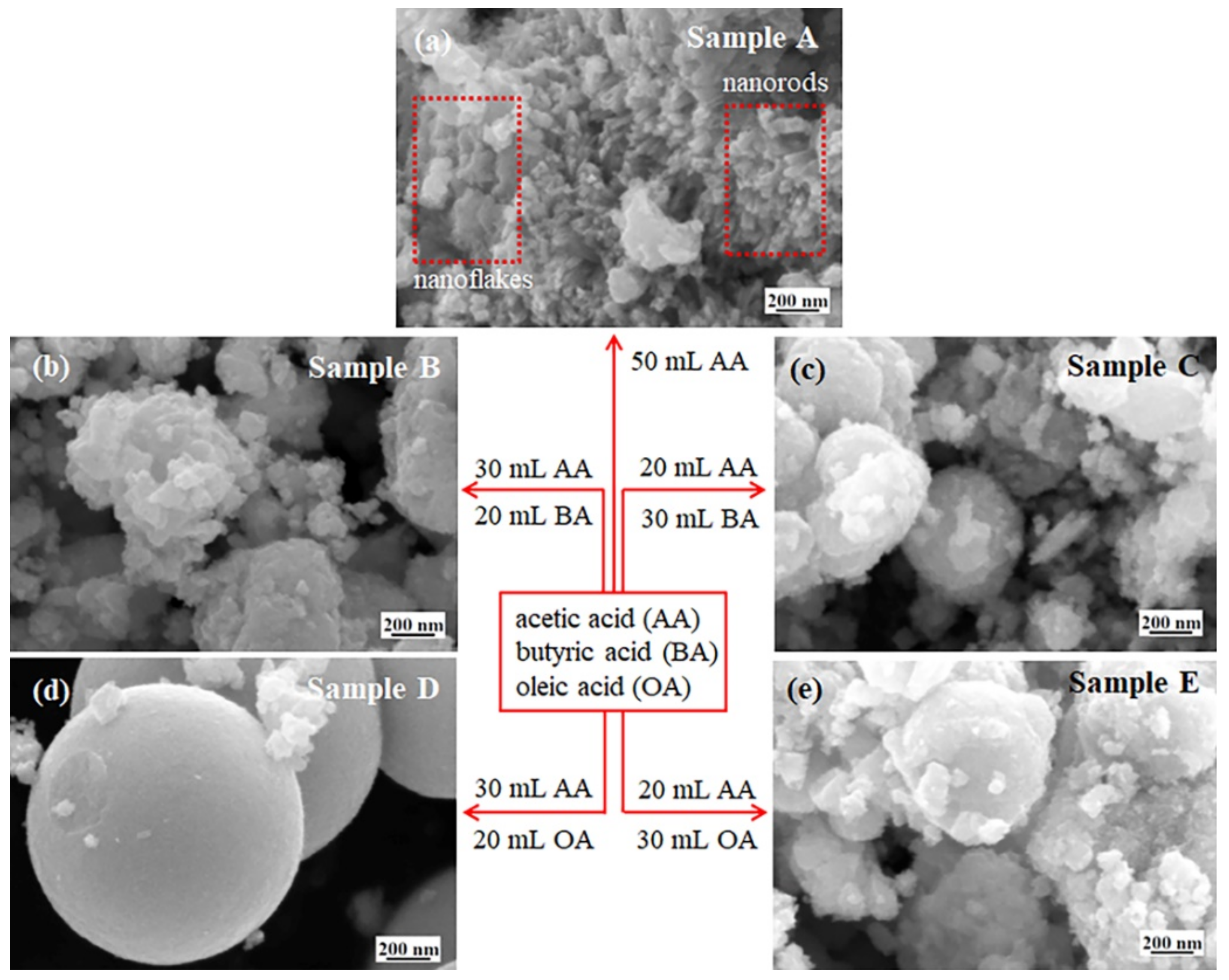

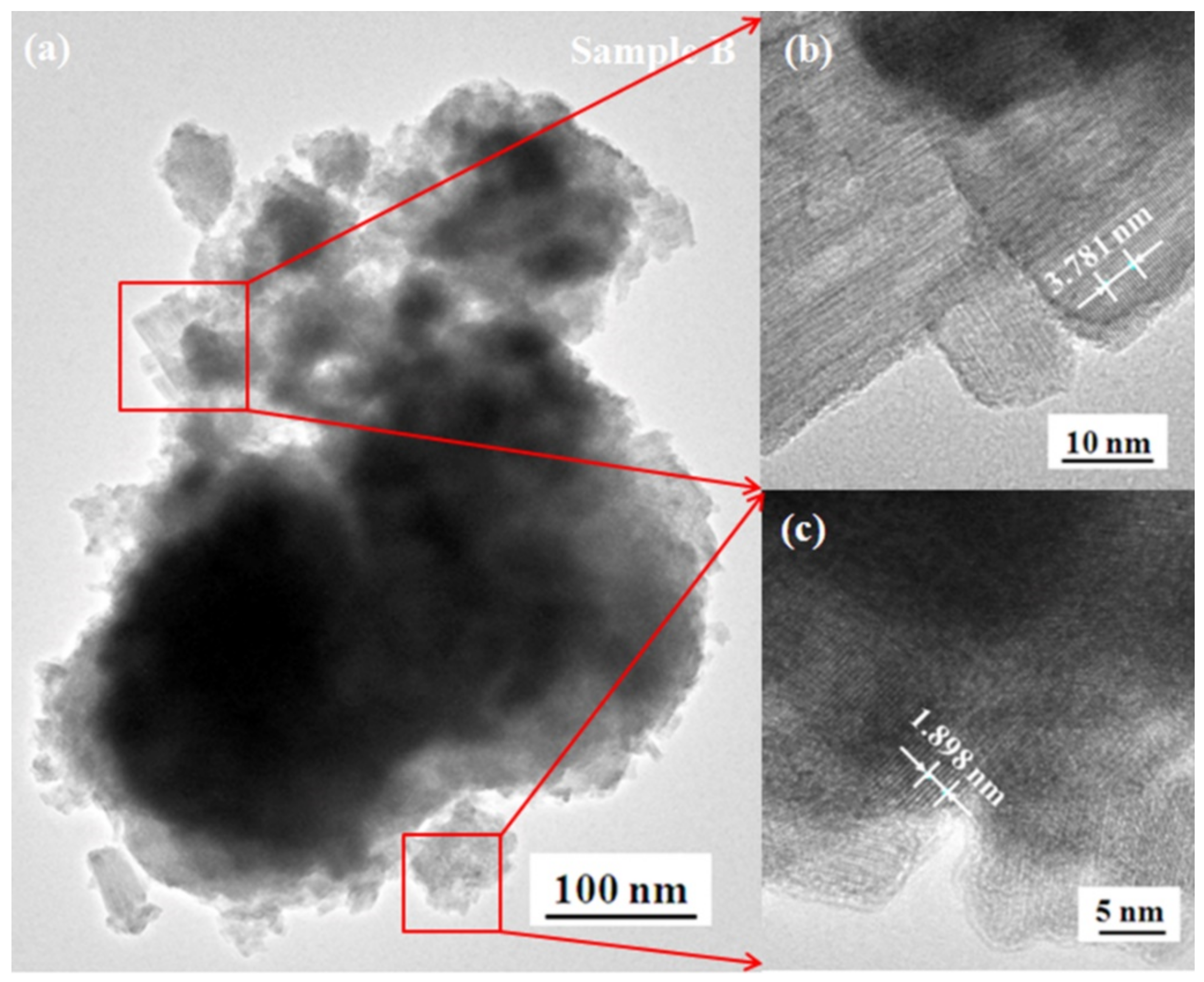
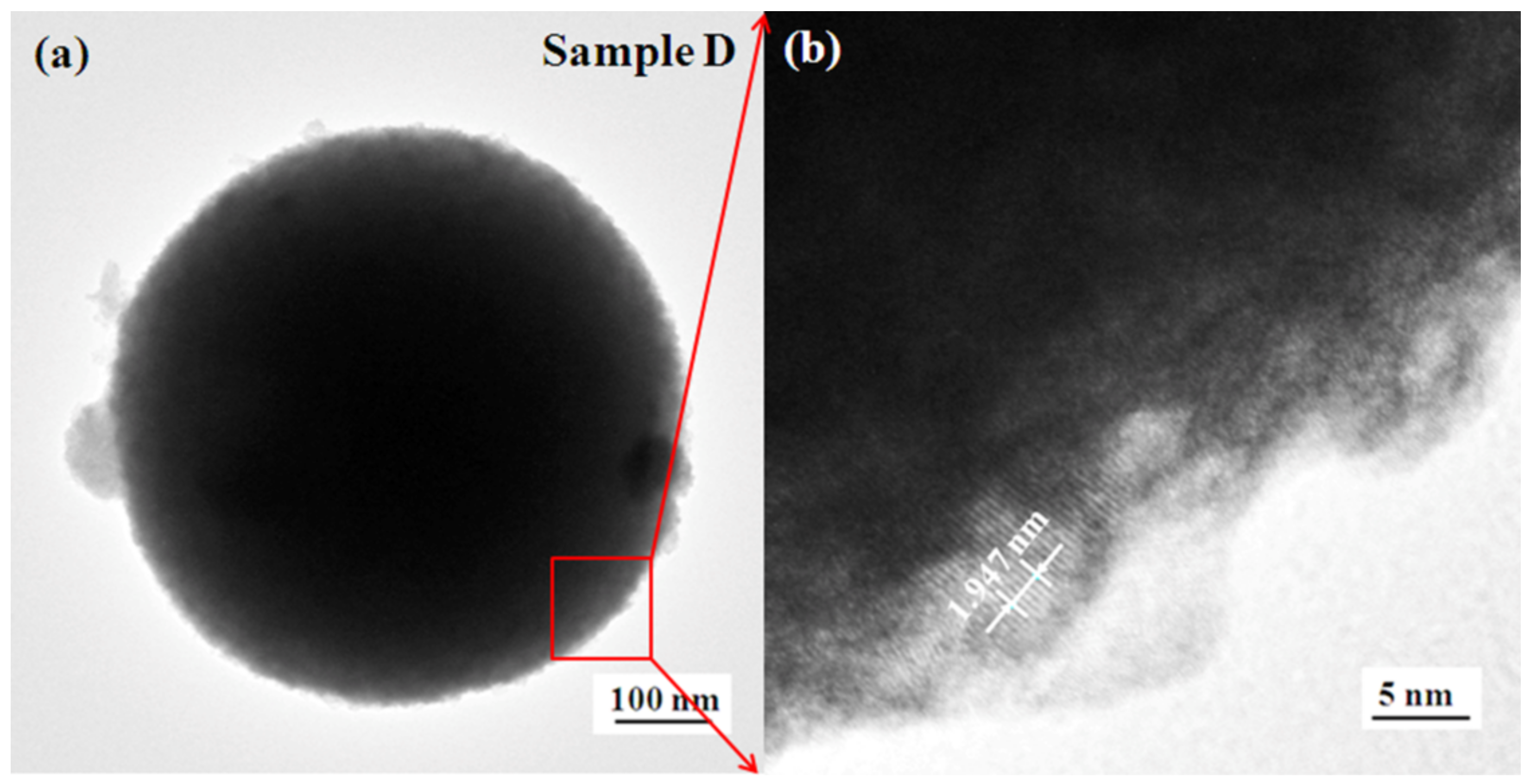

| Sample | WCl6 (g) | Acetic Acid (mL) | Butyric Acid (mL) | Oleic Acid (mL) | T (°C) | t (h) |
|---|---|---|---|---|---|---|
| A | 2.00 | 50 | - | - | 120 | 12 |
| B | 2.00 | 30 | 20 | - | 120 | 12 |
| C | 2.00 | 20 | 30 | - | 120 | 12 |
| D | 2.00 | 30 | - | 20 | 120 | 12 |
| E | 2.00 | 20 | - | 30 | 120 | 12 |
| Phase | Peak | JCPDS |
|---|---|---|
| A1: W19O55(-105); A2: W19O55(302); A3: W19O55(-2011) | 20.5°, 24.0°, 45.9° | 45-0167 |
| B1: WO2.92(-1012); B2: WO2.92(-1016); B3: WO2.92(-2128) | 18.5°, 24.0°, 49.8° | 30-1387 |
| C1: W18O49(-301); C2: W18O49(102); C3: W18O49(103) | 14.5°, 16.6°, 24.0° | 05-0392 |
| C4: W18O49(302); C5: W18O49(012); C6: W18O49(211) | 25.6°, 27.4°, 28.1° | |
| C7: W18O49(501); C8: W18O49(502); C9: W18O49(-704) | 30.6°, 35.8°, 36.6° | |
| C10: W18O49(021); C11: W18O49(123); C12: W18O49(-523) | 49.8°, 54.4°, 55.7° | |
| C13: W18O49(017); C14: W18O49(-1015) | 56.3°, 57.3° |
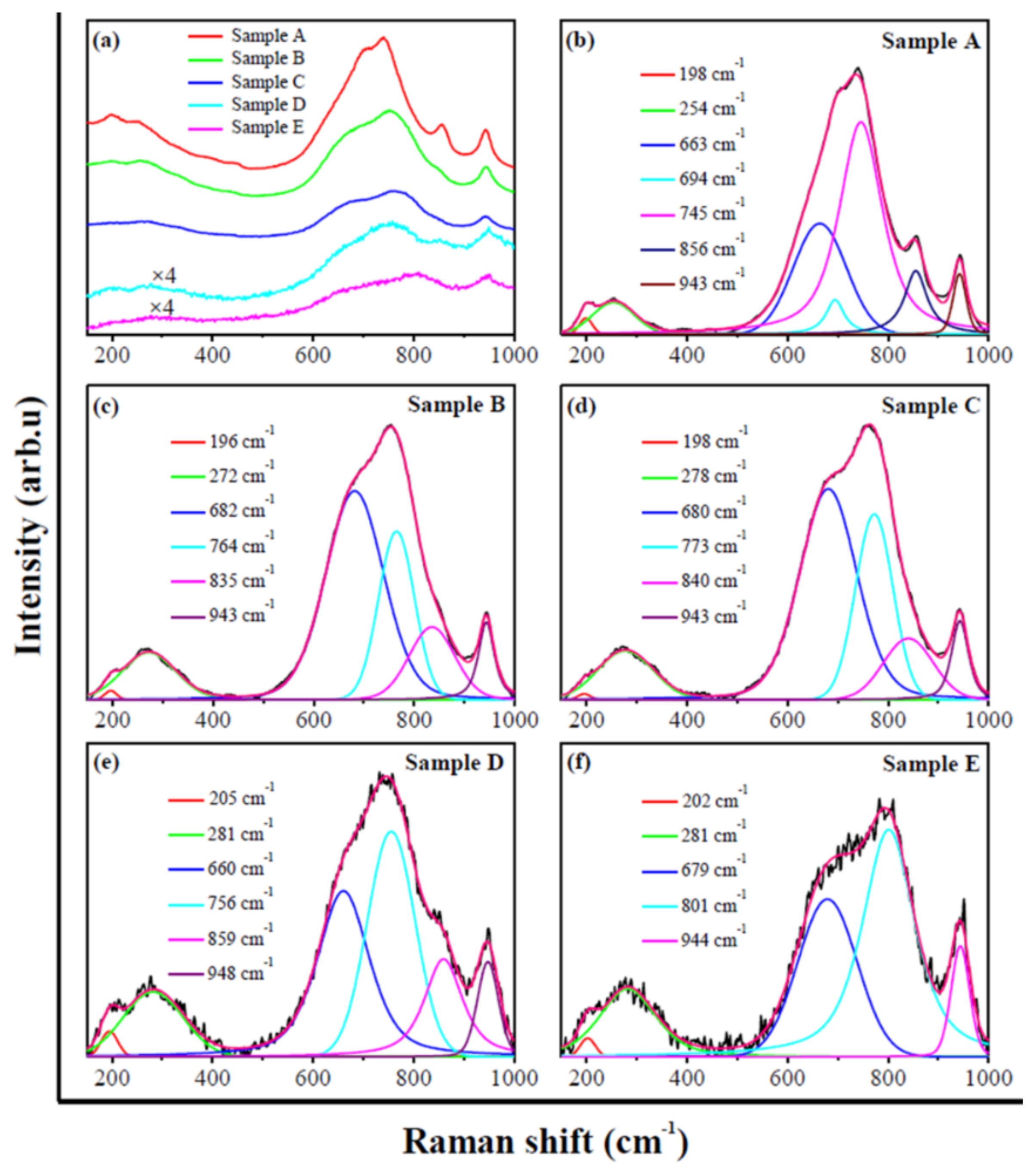
| Raman Peak (cm−1) | Assignment * | References |
|---|---|---|
| 196, 198 | Lattice vibration | [26] |
| 205, 254 | ν(W−O−W) | [27] |
| 272, 278, 281 | δ(O−W−O) | [27,28] |
| 660, 663, 679, 680, 682, 694 | ν(O−W−O) | [27] |
| 745, 756, 764, 773 | ν(W−O) | [13,28] |
| 801 | ν(O−W−O) | [28] |
| 943, 944, 948 | ν(W=O) | [13,28] |
| Sample | W5+ 4f7/2 | W6+ 4f7/2 | W5+ 4f5/2 | W6+ 4f5/2 | OL | OV | OC | (CH2)n | CH2O | O=C-O |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 34.7 | 35.5 | 36.1 | 37.8 | 530.4 | 531.5 | 532.8 | 284.7 | 285.8 | 289.0 |
| B | 34.8 | 35.6 | 36.3 | 37.8 | 530.4 | 531.7 | 533.1 | 284.7 | 285.6 | 288.9 |
| C | 34.7 | 35.6 | 36.3 | 37.8 | 530.4 | 531.7 | 533.1 | 284.7 | 285.5 | 288.9 |
| D | 34.9 | 35.6 | 36.2 | 37.8 | 530.5 | 531.7 | 533.0 | 284.7 | 285.6 | 288.9 |
| E | 34.7 | 35.6 | 36.2 | 37.8 | 530.3 | 531.3 | 532.7 | 284.7 | 285.5 | 288.9 |
| Sample | OL Peak Area | OV Peak Area | OC Peak Area | Ratio (%) |
|---|---|---|---|---|
| A | 50,970 | 17,025 | 11,230 | 25.0 |
| B | 39,685 | 16,637 | 12,062 | 29.5 |
| C | 52,013 | 17,025 | 10,649 | 24.7 |
| D | 54,684 | 16,901 | 11,866 | 23.6 |
| E | 47,576 | 20,926 | 14,301 | 30.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhong, X.; Xu, H.; Zhang, Y.; Cvelbar, U.; Ostrikov, K. Structure and Photoluminescence of WO3-x Aggregates Tuned by Surfactants. Micromachines 2022, 13, 2075. https://doi.org/10.3390/mi13122075
Wang B, Zhong X, Xu H, Zhang Y, Cvelbar U, Ostrikov K. Structure and Photoluminescence of WO3-x Aggregates Tuned by Surfactants. Micromachines. 2022; 13(12):2075. https://doi.org/10.3390/mi13122075
Chicago/Turabian StyleWang, Biben, Xiaoxia Zhong, Haiyan Xu, Yongcai Zhang, Uros Cvelbar, and Kostya (Ken) Ostrikov. 2022. "Structure and Photoluminescence of WO3-x Aggregates Tuned by Surfactants" Micromachines 13, no. 12: 2075. https://doi.org/10.3390/mi13122075
APA StyleWang, B., Zhong, X., Xu, H., Zhang, Y., Cvelbar, U., & Ostrikov, K. (2022). Structure and Photoluminescence of WO3-x Aggregates Tuned by Surfactants. Micromachines, 13(12), 2075. https://doi.org/10.3390/mi13122075







_Ostrikov.png)

