Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture
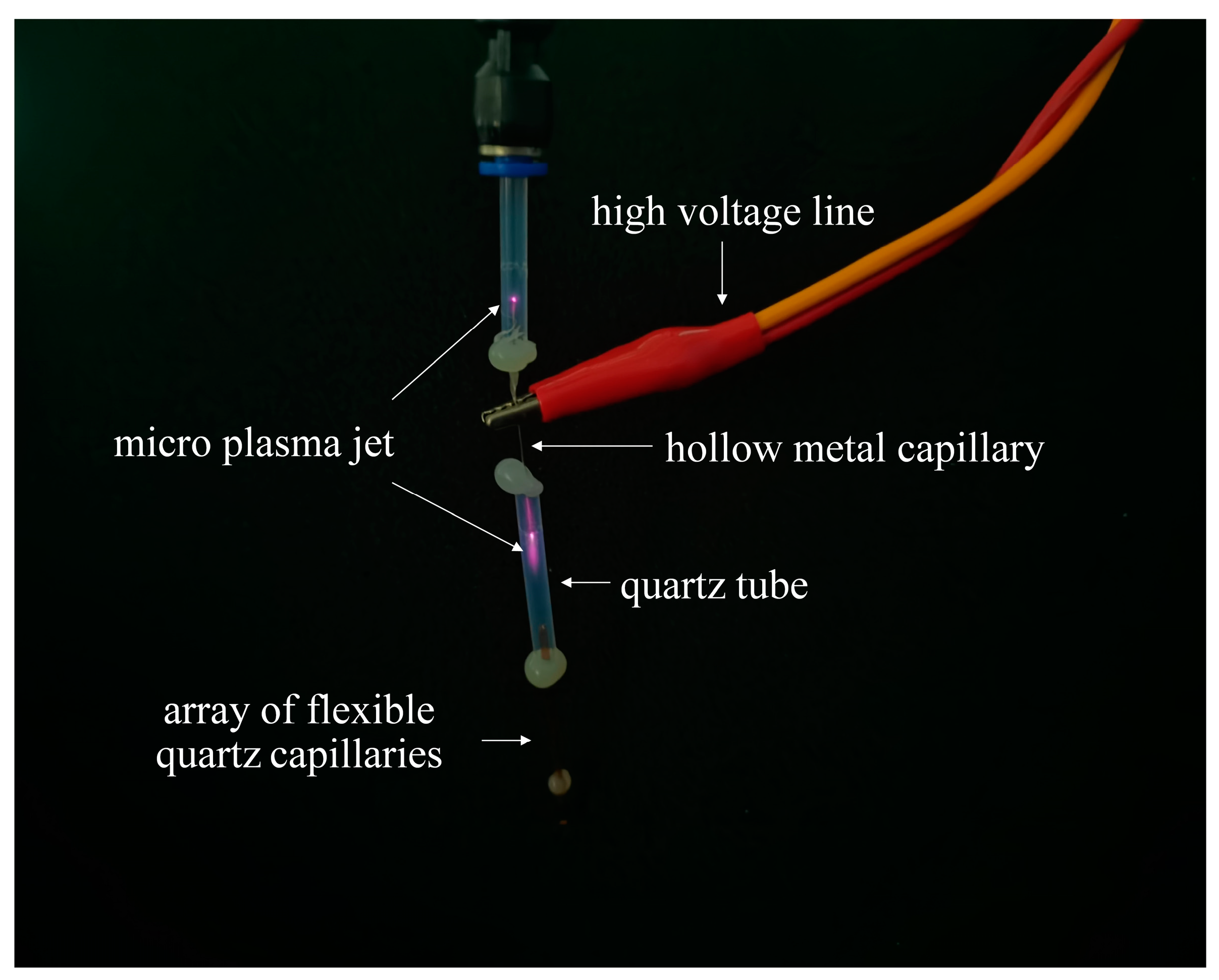
2.2. Experimental Setup
2.3. PAM Treatment
2.4. Determination of RONS, Temperature, and pH
2.5. Cellular Viability of μ-PAW-Treated Cells In Vitro
2.6. Cellular Apoptosis of μ-PAW-Treated Cells In Vitro
3. Results
3.1. Measurement of the Optical Emission Spectrum (OES)
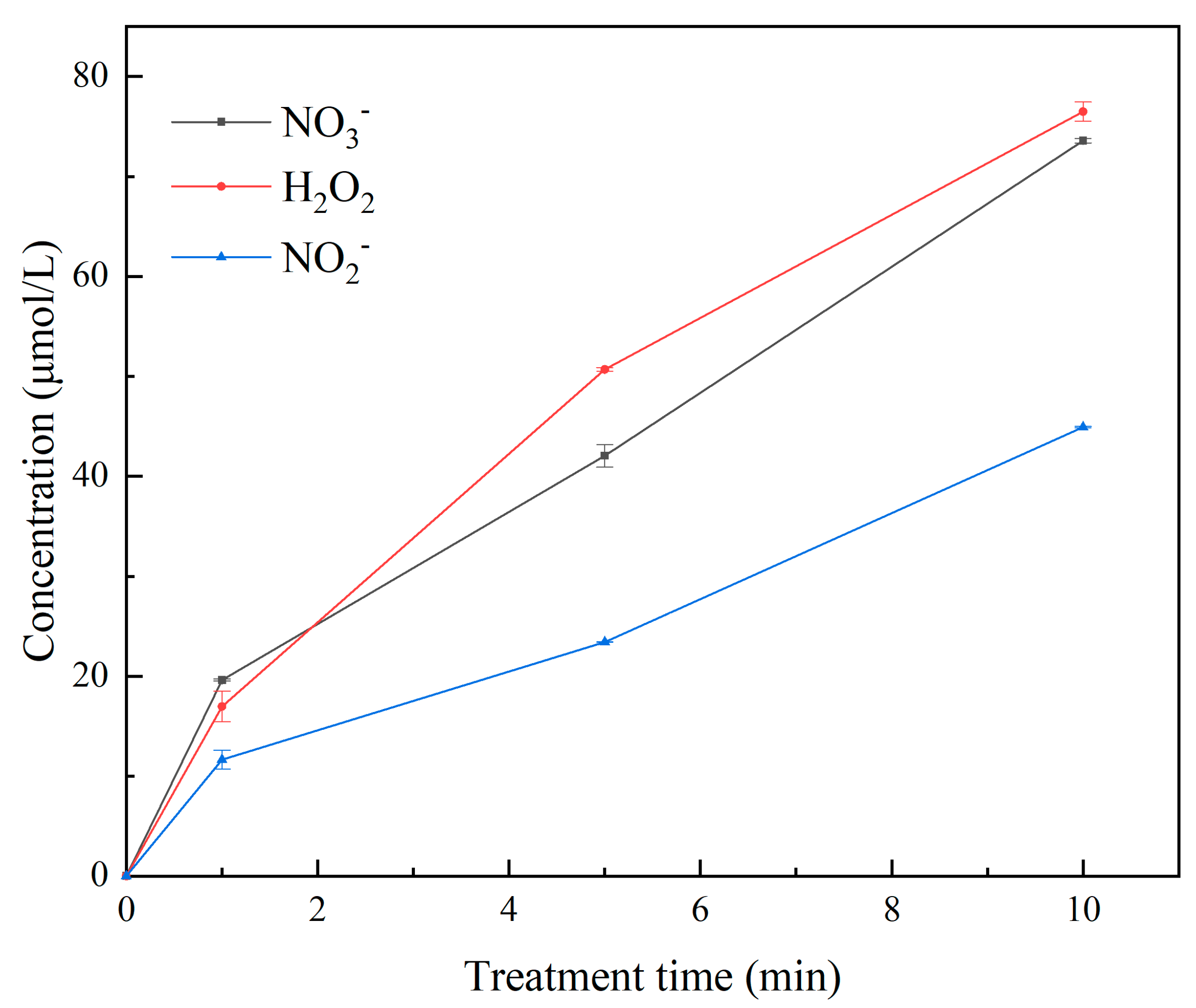
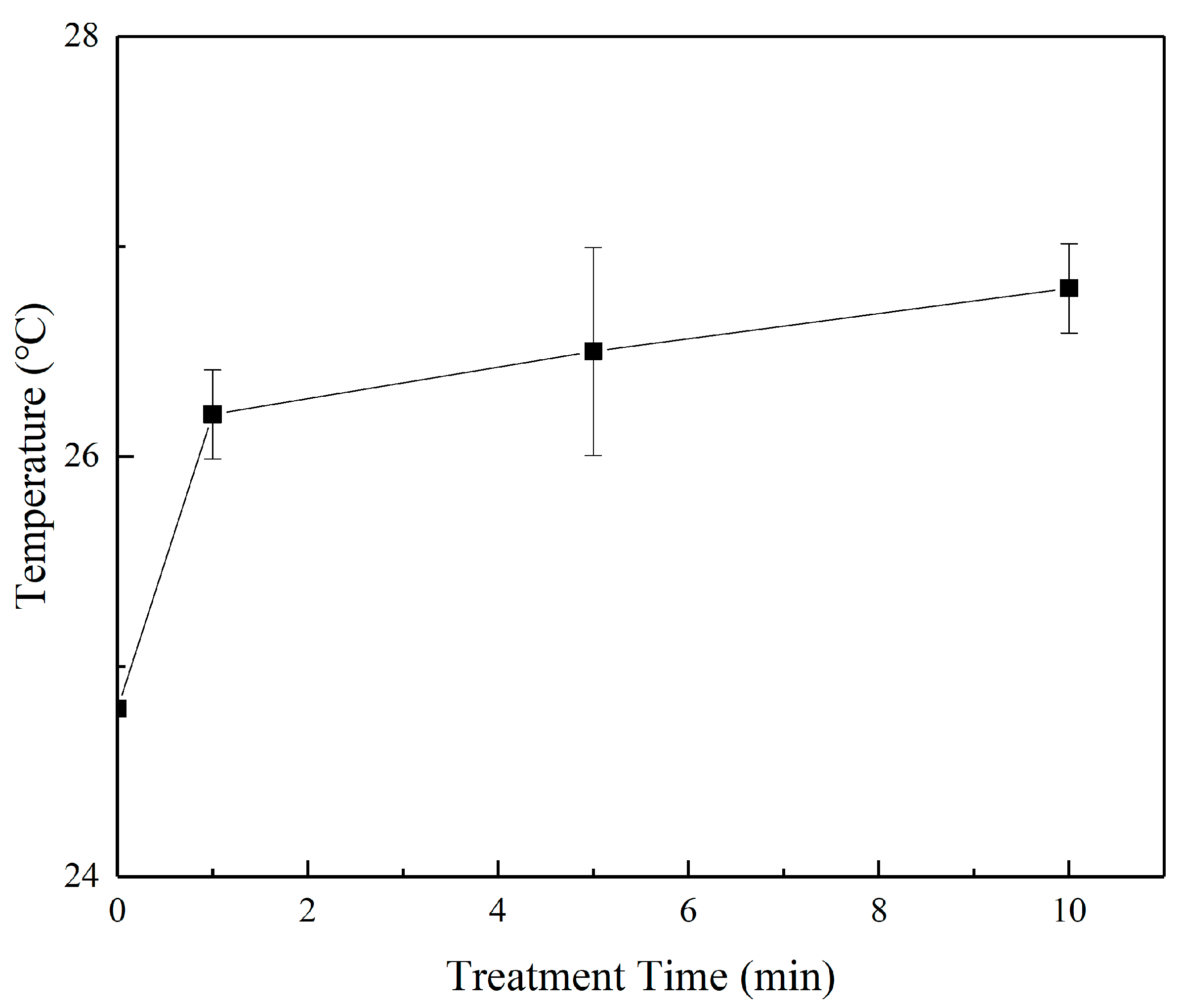
3.2. Measurement of RONS, Temperature, and pH
3.3. Detection of Cell Proliferation
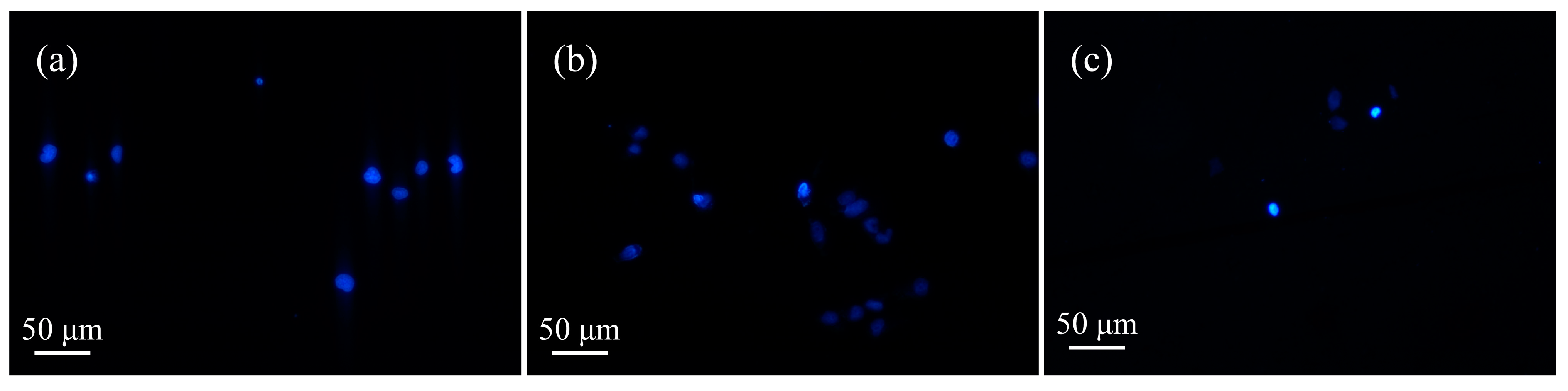
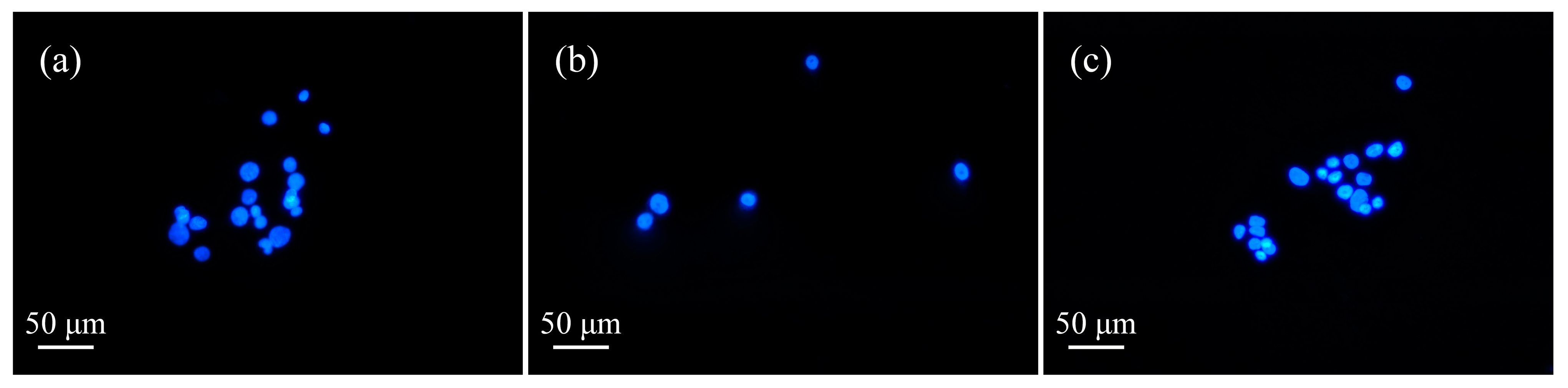
3.4. Detection of Cell Apoptosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Park, J.S.; Lee, J.H.; Jeong, B.H. Space Sterilization Effect Through High-Density Plasma Ozone Using DBD Device. J. Electr. Eng. Technol. 2022, 17, 2771–2778. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Xie, N.; Liu, F.; Li, Z.X.; Yun, N.; Ji, X.T.; Wang, D.Y. Application of atmospheric-pressure low-temperature plasma in stomatology: A review. Chin. J. Med. Phys. 2021, 38, 245–249. [Google Scholar]
- Karakas, E.; Munyanyi, A.; Greene, L.; Laroussi, M. Destruction of α-synuclein based amyloid fibrils by a low temperature plasma jet. Appl. Phys. Lett. 2010, 97, 143702. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Chen, Z.T.; Lin, L.; Cheng, X.Q.; Gjika, E.; Keidar, M. Effects of cold atmospheric plasma generated in deionized water in cell cancer therapy. Plasma Processes Polym. 2016, 13, 1151–1156. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, Y.S.; You, Y.S.; Huh, J.Y.; Kim, K.; Hong, Y.C.; Kim, C.H. Antimicrobial effects of microwave plasma-activated water with skin protective effect for novel disinfectants in pandemic era. Sci. Rep. 2022, 12, 5968. [Google Scholar] [CrossRef]
- Giovanni, B.; Eric, R.; Nadira, C.H.; Jean-Michel, P.; Catherine, G. The emerging potential of cold atmospheric plasma in skin biology. Free Radical Biol. Med. 2020, 161, 290–304. [Google Scholar]
- Gay-Mimbrera, J.; García, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; García-Nieto, A.V.; Ruano, J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef]
- Angelou, V.; Psalla, D.; Dovas, C.I.; Kazakos, G.M.; Marouda, C.; Chatzimisios, K.; Kyrana, Z.; Moutou, E.; Karayannopoulou, M.; Papazoglou, L.G. Locally Injected Autologous Platelet-Rich Plasma Improves Cutaneous Wound Healing in Cats. Animal 2022, 12, 1993. [Google Scholar] [CrossRef]
- IbáñezMancera, N.; ToralRizo, V.H.; LaraCarrillo, E.; LópezCallejas, R. Non-thermal atmospheric plasma generated with helium gas as a promoter of wound healing by salivary gland biopsy in Sjögren’s syndrome. Presentation two cases. Reumatol. Clin. 2022, 18, 439–440. [Google Scholar]
- Liu, Y.X.; Zhao, C.; Huang, M.L.; Cui, S.; Cao, J.P.; Wang, J.Y.; Huang, L.X. Effect of cold atmospheric plasma on the gray mold rot of postharvest mulberry fruit. Food Control 2022, 137, 108906. [Google Scholar]
- Nagar, V.; Kar, R.; Pansare, G.L.; Chand, N.; Bute, A.; Bhale, D.; Rao, A.V.S.S.N.; Shashidhar, R.; Maiti, N. Evaluation of Virucidal Efficacy of Cold Plasma on Bacteriophage Inside a Three-Layered Sterilization Chamber. Plasma Chem. Plasma Process. 2022, 42, 1115–1126. [Google Scholar] [CrossRef]
- Fukuhara, H.; Szili, E.J.; Oh, J.S.; Chiaki, K.; Yamamoto, S.; Kurabayashi, A.; Furihata, M.; Tsuda, M.; Furuta, H.; Lindsay, H.D.; et al. Oxidative Stress Pathways Linked to Apoptosis Induction by Low-Temperature Plasma Jet Activated Media in Bladder Cancer Cells: An In Vitro and In Vivo Study. Plasma 2022, 5, 233–246. [Google Scholar] [CrossRef]
- Jo, A.; Joh, H.M.; Bae, J.H.; Kim, S.J.; Chung, T.H.; Chung, J.W. Plasma activated medium prepared by a bipolar microsecond-pulsed atmospheric pressure plasma jet array induces mitochondria-mediated apoptosis in human cervical cancer cells. PLoS ONE 2022, 17, 0272805. [Google Scholar] [CrossRef] [PubMed]
- JiménezMorales, J.M.; HernándezCuenca, Y.E.; ReyesAbrahantes, A.; RuizGarcía, H.; BarajasOlmos, F.; GarcíaOrtiz, H.; Orozco, L.; QuiñonesHinojosa, A.; ReyesGonzález, J.; Del, C.A.M. MicroRNA delivery systems in glioma therapy and perspectives: A systematic review. J. Control. Release 2022, 349, 712–730. [Google Scholar] [CrossRef]
- Zhang, J.N.; Liu, C.W. Progress of Novel Treatment Options for Glioma. Cancer Res. Prev. Treat. 2022, 49, 505–513. [Google Scholar]
- Ye, X.B.; Schreck, K.C.; Ozer, B.H.; Grossman, S.A. High-grade glioma therapy: Adding flexibility in trial design to improve patient outcomes. Expert Rev. Anticancer Ther. 2022, 13, 275–287. [Google Scholar] [CrossRef]
- Eigenbrod, S.; Trabold, R.; Brucker, D.; Erös, C.; Egensperger, R.; Fougere, C.L.; Göbel, W.; Rühm, A.; Kretzschmar, H.A.; Tonn, J.C.; et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir. 2014, 156, 1427–1440. [Google Scholar] [CrossRef]
- Urso, G.; Boncu, A.G.; Carrar, N.; Zaman, D.; Malfassi, L.; Marcarini, S.; Minoli, L.; Pavesi, S.; Sala, M.; Scanziani, E.; et al. Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report. Vet. Sci. 2022, 9, 541. [Google Scholar] [CrossRef]
- Mamani, R.; Jacobo, J.A.; Guinto-Nishimura, G.Y.; Hernández-Hernández, A.; Moreno-Jimenez, S. Motor outcome after resective surgery for the central lobe gliomas. Surg. Neurol. Int. 2022, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.Z.; Gareev, I.; Yuan, C.; Liang, Y.C.; Sun, J.X.; Chen, X.; Beylerli, O.; Sufiano, A.; Zhao, S.G.; Yang, G. The mechanisms of current platinum anticancer drug resistance in the glioma. Curr. Pharm. Des. 2022, 13, 1863–1869. [Google Scholar]
- Lin, S.Y.; Chuang, C.C.; Huang, Y.C.; Pai, P.C.; Lee, C.C.; Wei, K.C.; Tseng, C.K.; Yang, C.C. Neuropsychological performances in patients with infiltrative non-GBM gliomas after postoperative adjuvant photon or proton radiotherapy: A prospective and preliminary investigation. Appl. Neuropsych-Adul. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raschke, F.; Witzmann, K.; Seidlitz, A.; Wesemann, T.; Jentsch, C.; Platzek, I.; van den Hoff, J.; Kotzerke, J.; Beuthien-Baumann, B.; Baumann, M.; et al. Time- and dose-dependent volume decreases in subcortical grey matter structures of glioma patients after radio(chemo)therapy. Clin. Transl. Radiat. Oncol. 2022, 36, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.W.; Zhou, R.S.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K.K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.S.; Xu, S.D.; Liu, D.X.; Guo, L.; Li, H. Study on the anticancer effects of a 7 m sized helium plasma jet on micro-tumors. J. Phys. D Appl. Phys. 2021, 54, 385203. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Stepp, M.A.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapya. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 00636. [Google Scholar] [CrossRef]
- Chen, Z.T.; Gjika, E.; Lin, L.; Cheng, X.Q.; Simonyan, H.; Young, C.N.; Keidar, M. Application of a Micro-Cold Atmospheric Plasma Device (CAP) in Vitro and Vivo for Brain Cancer Therapy. In Proceedings of the IEEE International Conference on Plasma Science (ICOPS), Atlantic City, NJ, USA, 21–25 May 2017. [Google Scholar]
- Zhang, Y.H.; Zhu, H.C.; Du, X.X.; Xiao, W.X.; Li, H. Micro plasma jet for micron sterilization range control. Opt. Precis. Eng. 2022, 30, 296–309. [Google Scholar]
- Kieft, I.E.; Broers, J.L.V.; Caubet-Hilloutou, V.; Slaaf, D.W.; Ramaekers, F.C.S.; Stoffels, E. Electric discharge plasmas influence attachment of cultured CHO K1 cells. Bioelectromagnetics 2004, 25, 362–368. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl. Phys. Lett. 2010, 97, 023702. [Google Scholar] [CrossRef]
- Yu, Y.L.; Zhuang, Y.T.; Wang, J.H. Advances in dielecric barrier discharge-optical emission spectrometry for the analysis of trace species. Anal. Methods 2015, 7, 1660–1666. [Google Scholar] [CrossRef]
- Zhao, J. Determination of Nitrate in Vegetables by Ultraviolet Spectrophotometry Method. J. Anhui Agric. Sci. 2014, 42, 9553–9554. [Google Scholar]
- Chai, X.S.; Hou, Q.X.; Luo, Q.; Zhu, J.Y. Rapid determination of hydrogen peroxide in the wood pulp bleaching streams by a dual-wavelength spectroscopic method. Anal. Chim. Acta. 2004, 507, 281–284. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Chen, P.C.; Li, C.; Wu, J.; Li, S.; Zheng, B. Determination of hydrogen peroxide residue in aqueous hair products by ammonium molybdate spectrophotometry. Mod. Prev. Med. 2008, 35, 1556–1558. [Google Scholar]
- Liu, H.; Tian, Y.H.; Lao, W.M.; Ma, S.L.; Kan, X.R. Determination of nitrite in pickles by N(-1-naphthyl)-ethylenediamine dihydrochloride spectrophotometric method. China Brewing. 2010, 157–160+165. [Google Scholar] [CrossRef]
- Song, S.Q.; Xia, N. Discussion on Determination of NO2− in Natural Water with aminobenzene sulfonic acid—α-naphthylamine spectrophotometry. J. Qingdao Univ. 1997, 10, 102–103. [Google Scholar]
- Zhou, R.W.; Zhou, R.S.; Zhang, X.H.; Li, J.W.; Wang, X.Q.; Chen, Q.; Yang, S.; Chen, Z.; Bazaka, K.; Ostrikov, K.K. Synergistic effect of atmospheric-pressure plasma and TiO2 photocatalysis on inactivation of Escherichia coli cells in aqueous media. Sci. Rep. 2016, 13, 1151–1156. [Google Scholar] [CrossRef]
- Yan, X. The Mechanism of Anti-Proliferation Effect of Cold Atmospheric-Pressure Plasma on HepG2 Hepatoma Cells. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2011. [Google Scholar]
- Lu, X.P. Atmospheric Pressure Nonequilibrium Plasma Get II Biomedical Applications; Huazhong University of Science and Technology Press: Wuhan, China, 2021; pp. 92–93. [Google Scholar]
- Niu, J.B. Bacterial Inactivation in Water by Means of a Combined Process of Pulsed Dielectric Barrier Discharge and Silver-modified Natural Zeolite. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2018. [Google Scholar]
- Chen, Z.T.; Simonyan, H.; Cheng, X.Q.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A Novel Micro Cold Atmospheric Plasma Device for Glioblastoma Both In Vitro and In Vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. The selective effect of plasma activated medium in an in vitro co-culture of liver cancer and normal cells. J. Appl. Phys. 2017, 121, 013302. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Peng, S.S.; Xu, D.H.; Qi, M.; Liu, R.; Zhang, X.Y.; Zhang, H.Y.; Pang, B.L.; Zhang, X.; Zhang, H.; Liu, Z.J.; et al. Investigation of optimum discharge characteristics and chemical activity of AC driven air plasma jet and its anticancer effect. Plasma Sci. Technol. 2021, 23, 125401. [Google Scholar] [CrossRef]
- Du, H.L.; He, L.M.; Lan, Y.D.; Wang, F. Influence of reduced electric field on the evolvement characteristics of plasma under conditions of N2/O2 discharge. Acta Phys. Sin. 2011, 60, 115201. [Google Scholar]
- Du, H.L.; He, L.M.; Ding, W.; Yu, J.L.; Zuo, H. Evolution Law Analysis of Active Particles Density in Air Discharges Plasma. High Volt. Eng. 2010, 36, 2041–2046. [Google Scholar]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinová, Š. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Du, X.; Shi, Q.; Xiao, W.; Li, H. Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW). Micromachines 2022, 13, 2145. https://doi.org/10.3390/mi13122145
Zhang Y, Du X, Shi Q, Xiao W, Li H. Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW). Micromachines. 2022; 13(12):2145. https://doi.org/10.3390/mi13122145
Chicago/Turabian StyleZhang, Yuhan, Xiaoxia Du, Qihao Shi, Wenxiang Xiao, and Hua Li. 2022. "Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW)" Micromachines 13, no. 12: 2145. https://doi.org/10.3390/mi13122145
APA StyleZhang, Y., Du, X., Shi, Q., Xiao, W., & Li, H. (2022). Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW). Micromachines, 13(12), 2145. https://doi.org/10.3390/mi13122145






