Polishing Performance and Removal Mechanism of Core-Shell Structured Diamond/SiO2 Abrasives on Sapphire Wafer
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Chemicals and Materials
2.2. Synthesis of Diamond/SiO2 Composite Abrasives



2.3. Characterizations
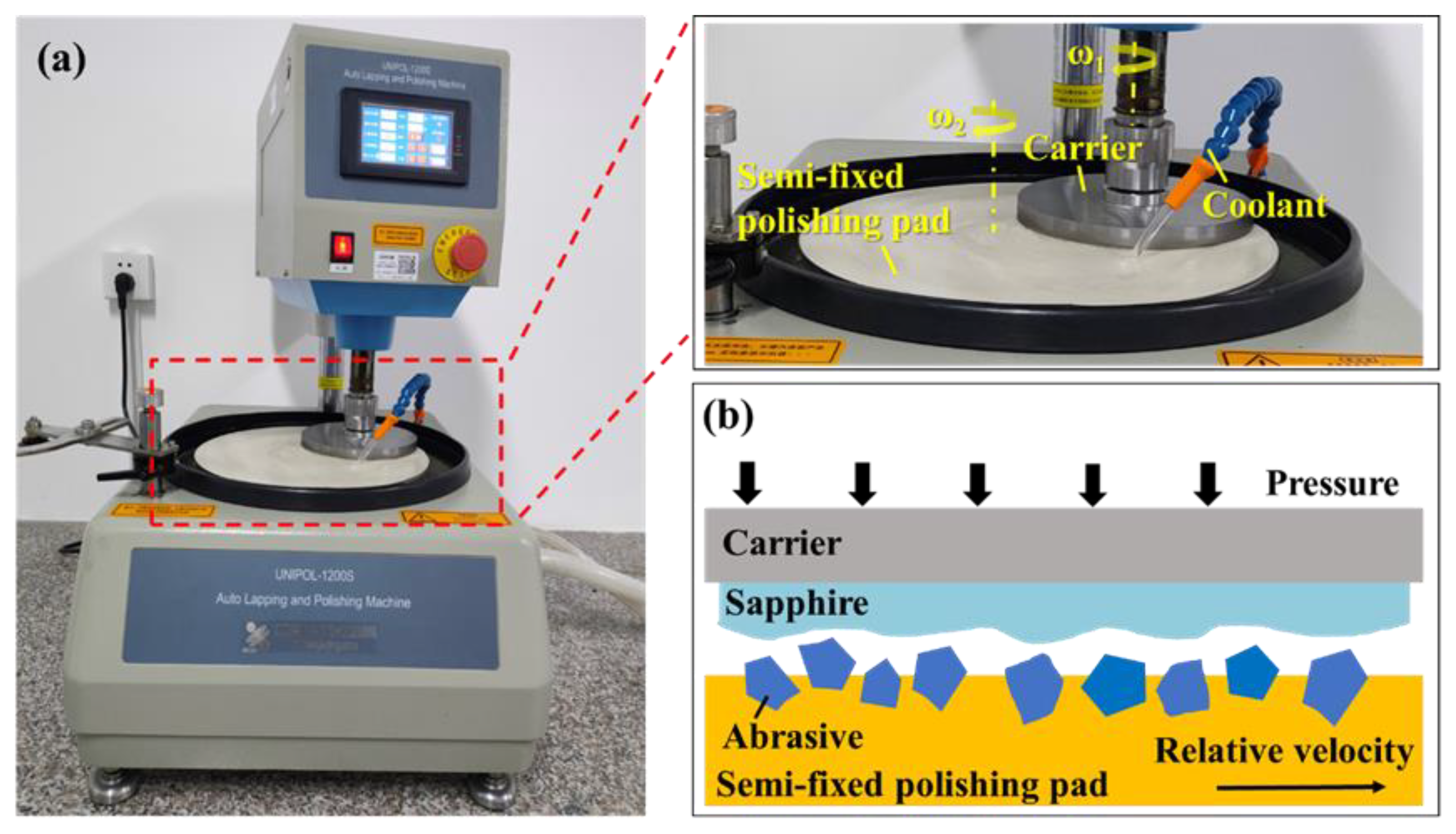
2.4. Polishing Tests
3. Results and Discussion
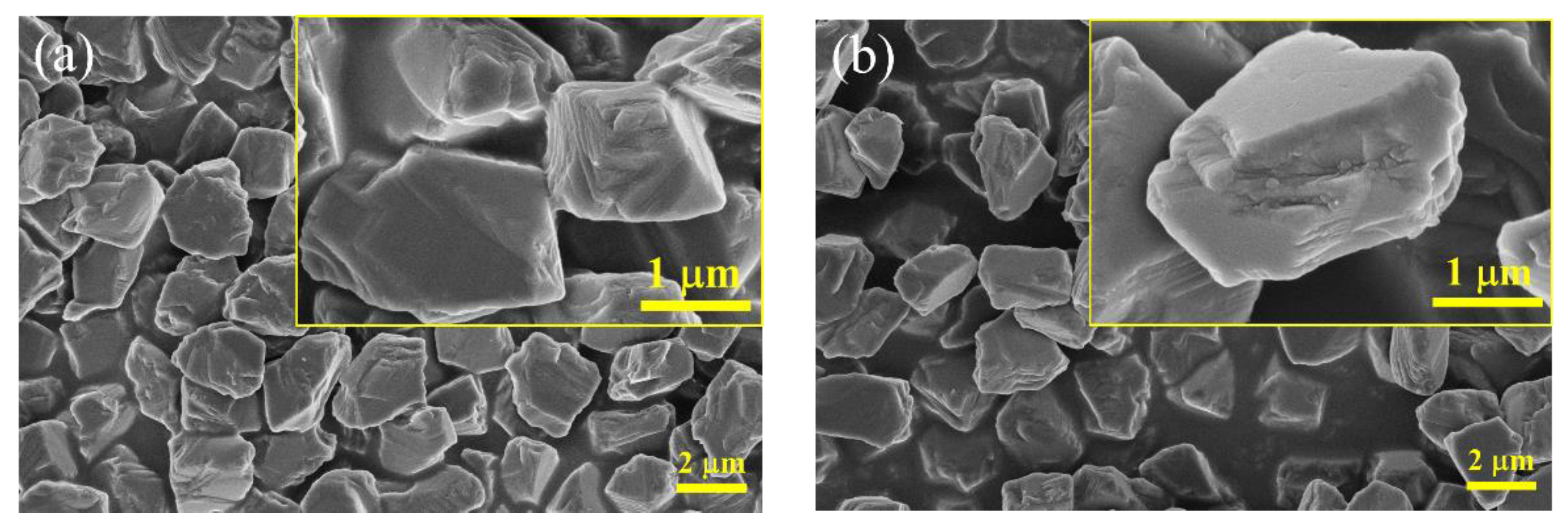
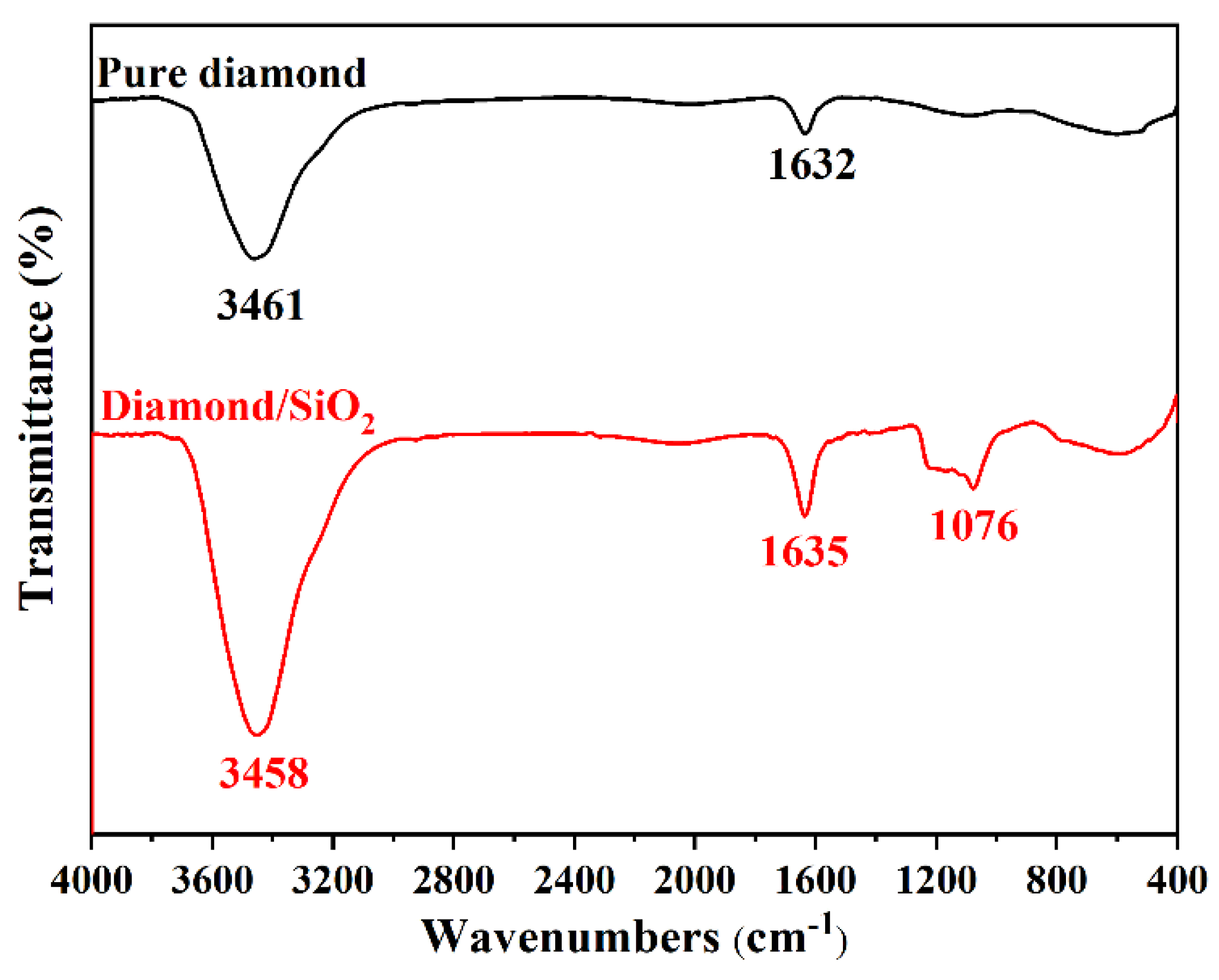
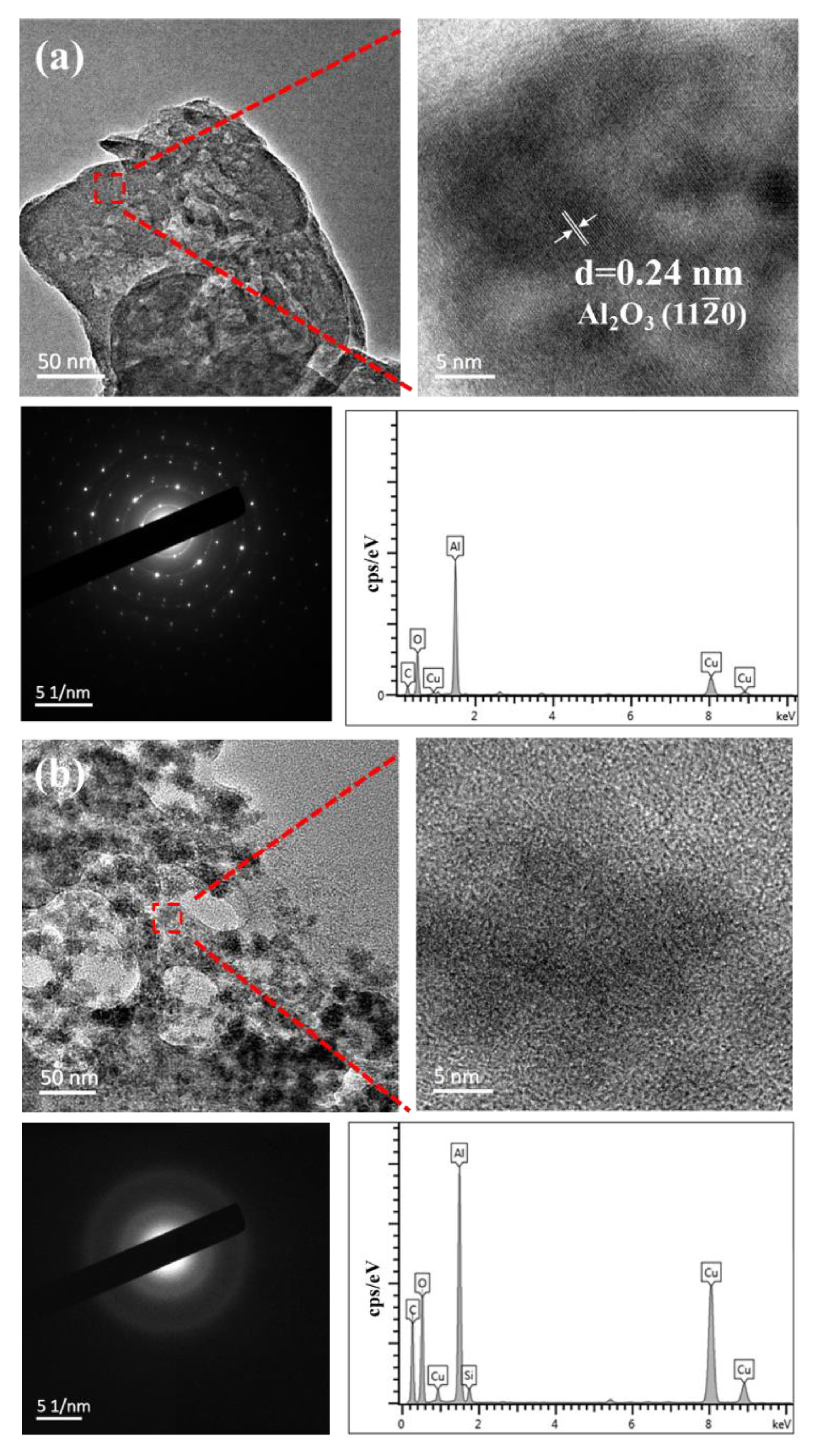
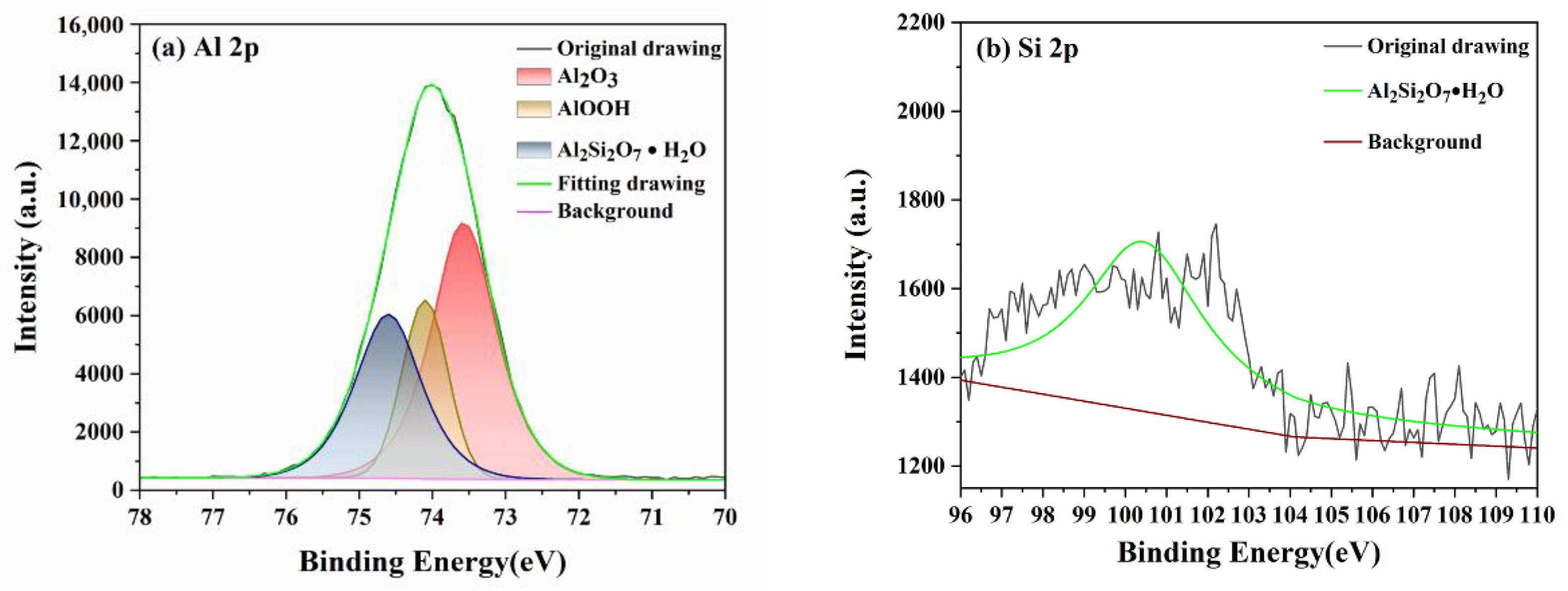
3.1. Characterizations of the Diamond/SiO2 Composite Abrasives
3.2. Polishing Test
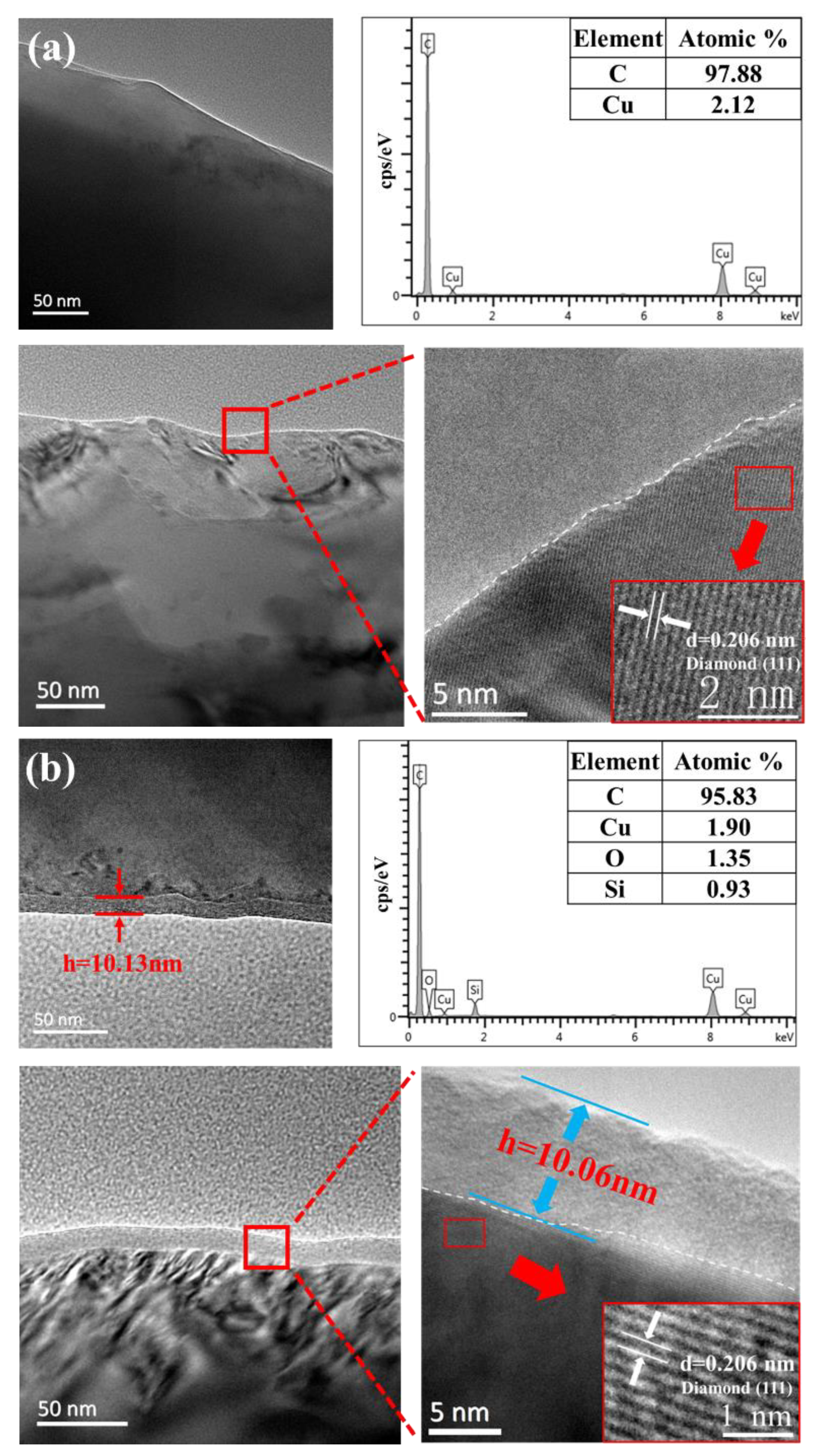
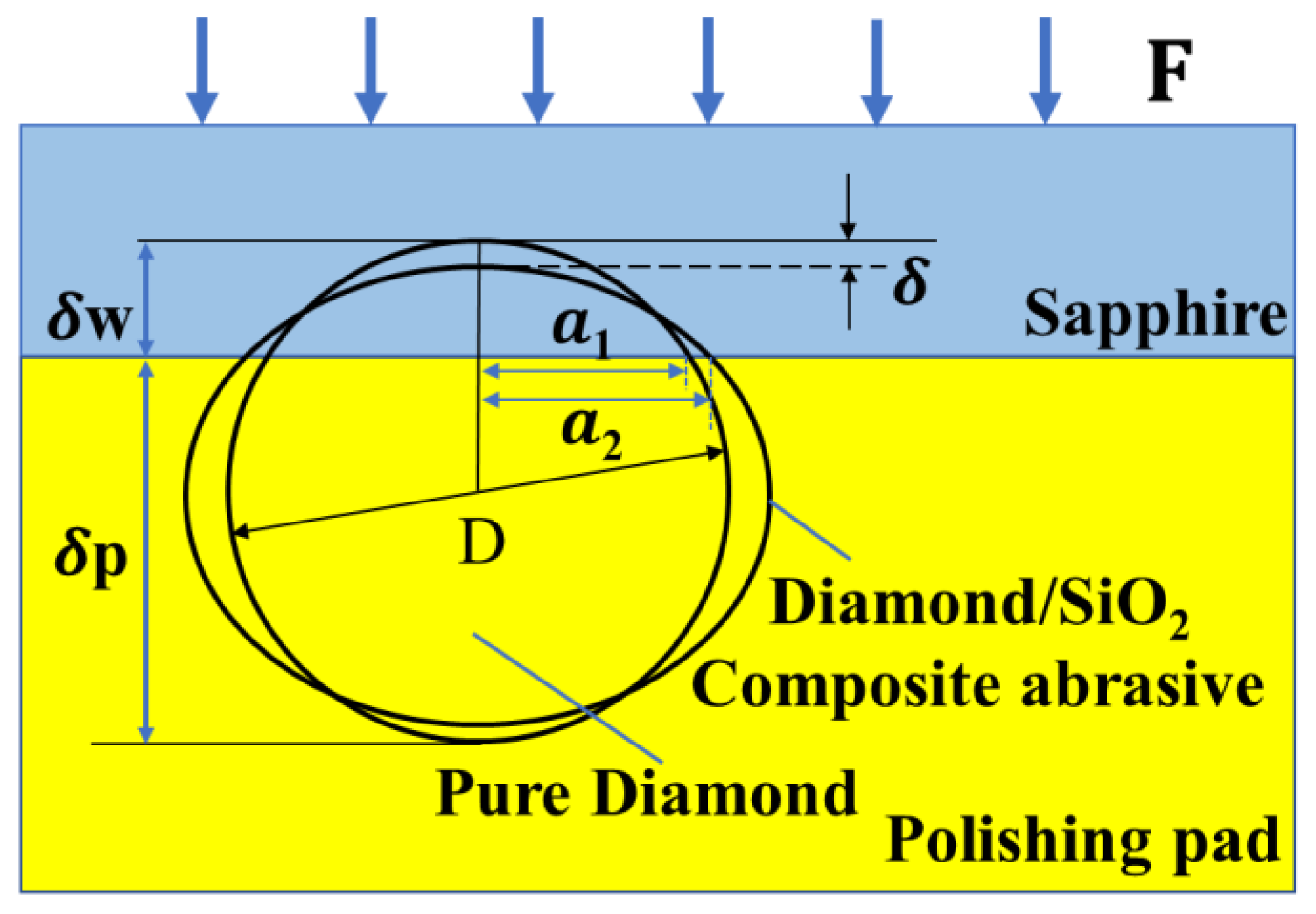
3.3. Polishing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhai, Q.; Zhai, W.; Gao, B. Investigation on the relationship between apparent viscosity of Fe3O4@SiO2 abrasive-based magneto-rheological fluid and material removal rate of sapphire in magneto-rheological polishing. Colloids Surf. A 2022, 640, 20. [Google Scholar] [CrossRef]
- Pan, J.; Chen, Z.; Yan, Q. Study on the rheological properties and polishing properties of SiO2@CI composite particle for sapphire wafer. Smart Mater. Struct. 2020, 29, 114003. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhai, W.; Gao, B. Effects of quantities and pole-arrangements of magnets on the magneto-rheological polishing (MRP) performance of sapphire hemisphere. Appl. Surf. Sci. 2022, 584, 152589. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, X.; Dai, L.; Gong, H.; Lin, S. Chemical-mechanical polishing performance of core-shell structured polystyrene@ceria/nanodiamond ternary abrasives on sapphire wafer. Ceram. Int. 2021, 47, 31691–31701. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhai, W.; Gao, B. Modeling of forces and material removal rate in ultrasound assisted magnetorheological polishing (UAMP) of sapphire. Colloids Surf. A 2021, 628, 127272. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Liao, L.; Liu, J.; Su, H.; Wang, S.; Guo, D. Green chemical mechanical polishing of sapphire wafers using a novel slurry. Nanoscale 2020, 12, 22518–22526. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Hu, W.; Zhang, L.; Liao, L. Chemical mechanical polishing for sapphire wafers using a developed slurry. J. Manuf. Process. 2021, 62, 762–771. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Z.; Hu, Y. Effects of polishing parameters on surface quality in sapphire double-sided CMP. Ceram. Int. 2020, 46, 13356–13364. [Google Scholar] [CrossRef]
- Lei, H.; Liu, T.; Xu, L. Synthesis of Sm-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing performances on sapphire substrates. Mater. Chem. Phys. 2019, 237, 121819. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Jeong, H. Approaches to Sustainability in Chemical Mechanical Polishing (CMP): A Review. Int. J. Pract. Eng. Man-Gt. 2021, 9, 349–367. [Google Scholar] [CrossRef]
- Hu, G.; Lu, J.; Xu, X. Polishing Silicon Wafers with the Nanodiamond Abrasive Tools Prepared by Sol-Gel Technique. Key. Eng. Mater. 2012, 496, 1–6. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Xu, X. The effects of abrasive yielding on the polishing of SiC wafers using a semi-fixed flexible pad. Proc. Inst. Mech. Eng. Part B J. Process Mech. Eng. 2015, 229, 170–177. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Wu, B.; Hu, W.; Meng, F.; Li, Y. A review: Green chemical mechanical polishing for metals and brittle wafer. J. Phys. D Appl. Phys. 2021, 54, 373001. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X. Study on planarization machining of sapphire wafer with soft-hard mixed abrasive through mechanical chemical polishing. Appl. Surf. Sci. 2016, 389, 713–720. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X.; Chen, C.; Lin, Y. Study on high efficient sapphire wafer processing by coupling SG-mechanical polishing and GLA-CMP. Int. J. Mach. Tool. Manu. 2018, 130, 12–19. [Google Scholar] [CrossRef]
- Gao, B.; Zhai, W.; Zhai, Q.; Wang, C. Novel photoelectrochemically combined mechanical polishing technology for scratch-free 4H-SiC surface by using CeO2-TiO2 composite photocatalysts and PS/CeO2 core/shell abrasives. Appl. Surf. Sci. 2021, 570, 151141. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhai, W.; Gao, B.; Shi, Y.; Cheng, X. Effect of core-diameters and shell-thicknesses of Fe3O4/SiO2 composite abrasives on the performance of ultrasound-assisted magnetorheological polishing for sapphire. Colloids Surf. A 2021, 625, 126871. [Google Scholar] [CrossRef]
- Wang, X.; Lei, H.; Chen, R. CMP behavior of alumina/metatitanic acid core–shell abrasives on sapphire substrates. Precis. Eng. 2017, 50, 263–268. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, C.; Chen, A. Core/shell structured sSiO2/mSiO2 composite particles: The effect of the core size on oxide chemical mechanical polishing. Adv. Powder Technol. 2018, 29, 18–26. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Zhang, D.; Xu, X. The Synthesis of the Core/Shell Structured Diamond/Akageneite Hybrid Particles with Enhanced Polishing Performance. Materials 2017, 10, 673. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, G.; Wang, Q.; Zhan, Y.; Chen, B. Synthesis of Al2O3/SiO2 core–shell composite abrasives toward ultrasmooth and high-efficiency polishing for sapphire wafers. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022. [Google Scholar] [CrossRef]
- Jeffrey, C. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 1990; pp. 2–8. [Google Scholar]
- Lismont, M.; Páez, C.; Dreesen, L. A one-step short-time synthesis of Ag@SiO2 core–shell nanoparticles. J. Colloid Interf. Sci. 2015, 447, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, G.; Zhou, L.; Wang, X.; Li, J. Highly sensitively detecting tetramethylthiuram disulfide based on synergistic contribution of metal and semiconductor in stable Ag/TiO2 core-shell SERS substrates. Appl. Surf. Sci. 2021, 539, 147744. [Google Scholar] [CrossRef]
- Zheltova, V.; Vlasova, A.; Bobrysheva, N.; Abdullin, I.; Osmolovskaya, O. Fe3O4@HAp core–shell nanoparticles as MRI contrast agent: Synthesis, characterization and theoretical and experimental study of shell impact on magnetic properties. Appl. Surf. Sci. 2020, 531, 147352. [Google Scholar] [CrossRef]
- Hsiang, H.; Wang, S.; Chen, C. Electromagnetic properties of FeSiCr alloy powders modified with amorphous SiO2. J. Magn. Magn. Mater. 2020, 514, 167151. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Tian, Z.; Jiang, F. Controllable material removal behavior of 6H-SiC wafer in nanoscale polishing. Appl. Surf. Sci. 2021, 562, 150219. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X. A comparative study on the material removal mechanisms of 6H-SiC polished by semi-fixed and fixed diamond abrasive tools. Wear 2016, 350, 99–106. [Google Scholar] [CrossRef]
- Remy, M.; Genet, M.; Poncelet, G.; Lardinois, P.; Notte, P. Investigation of dealuminated mordenites by X-ray photoelectron-spectroscopy. Cheminform 1992, 96, 2614–2617. [Google Scholar] [CrossRef]
- Pitts, J.; Thomas, T.; Czanderna, A.; Passler, M. XPS and ISS of submonolayer coverage of Ag on SiO2. Appl. Surf. Sci. 1986, 26, 107–120. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Wang, Y. Modeling the effects of particle deformation in chemical mechanical polishing. Appl. Surf. Sci. 2012, 258, 8469–8474. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhai, W.; Gao, B.; Shi, Y.; Chen, X. Synthesis and characterization of nanocomposite Fe3O4/SiO2 core–shell abrasives for high-efficiency ultrasound-assisted magneto-rheological polishing of sapphire. Ceram. Int. 2021, 47, 31681–31690. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Xu, Y.; Wang, Q.; Liu, J.; Zhan, Y.; Chen, B. Polishing Performance and Removal Mechanism of Core-Shell Structured Diamond/SiO2 Abrasives on Sapphire Wafer. Micromachines 2022, 13, 2160. https://doi.org/10.3390/mi13122160
Zhao G, Xu Y, Wang Q, Liu J, Zhan Y, Chen B. Polishing Performance and Removal Mechanism of Core-Shell Structured Diamond/SiO2 Abrasives on Sapphire Wafer. Micromachines. 2022; 13(12):2160. https://doi.org/10.3390/mi13122160
Chicago/Turabian StyleZhao, Guangen, Yongchao Xu, Qianting Wang, Jun Liu, Youji Zhan, and Bingsan Chen. 2022. "Polishing Performance and Removal Mechanism of Core-Shell Structured Diamond/SiO2 Abrasives on Sapphire Wafer" Micromachines 13, no. 12: 2160. https://doi.org/10.3390/mi13122160
APA StyleZhao, G., Xu, Y., Wang, Q., Liu, J., Zhan, Y., & Chen, B. (2022). Polishing Performance and Removal Mechanism of Core-Shell Structured Diamond/SiO2 Abrasives on Sapphire Wafer. Micromachines, 13(12), 2160. https://doi.org/10.3390/mi13122160





