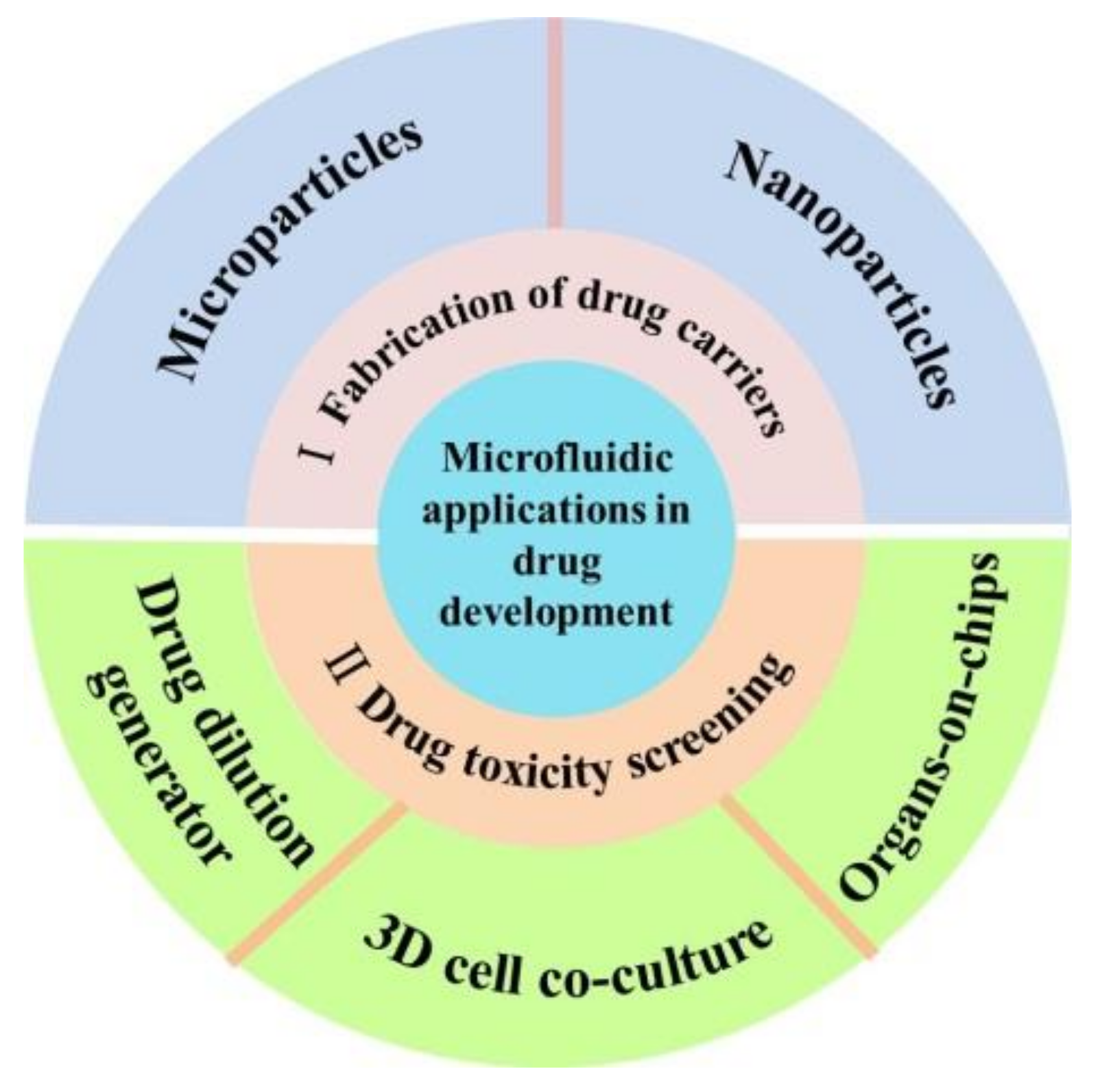
Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening
Abstract
:1. Introduction
2. Microfluidics for the Fabrication of Drug Carriers
2.1. Microfluidics for the Fabrication of Microparticles
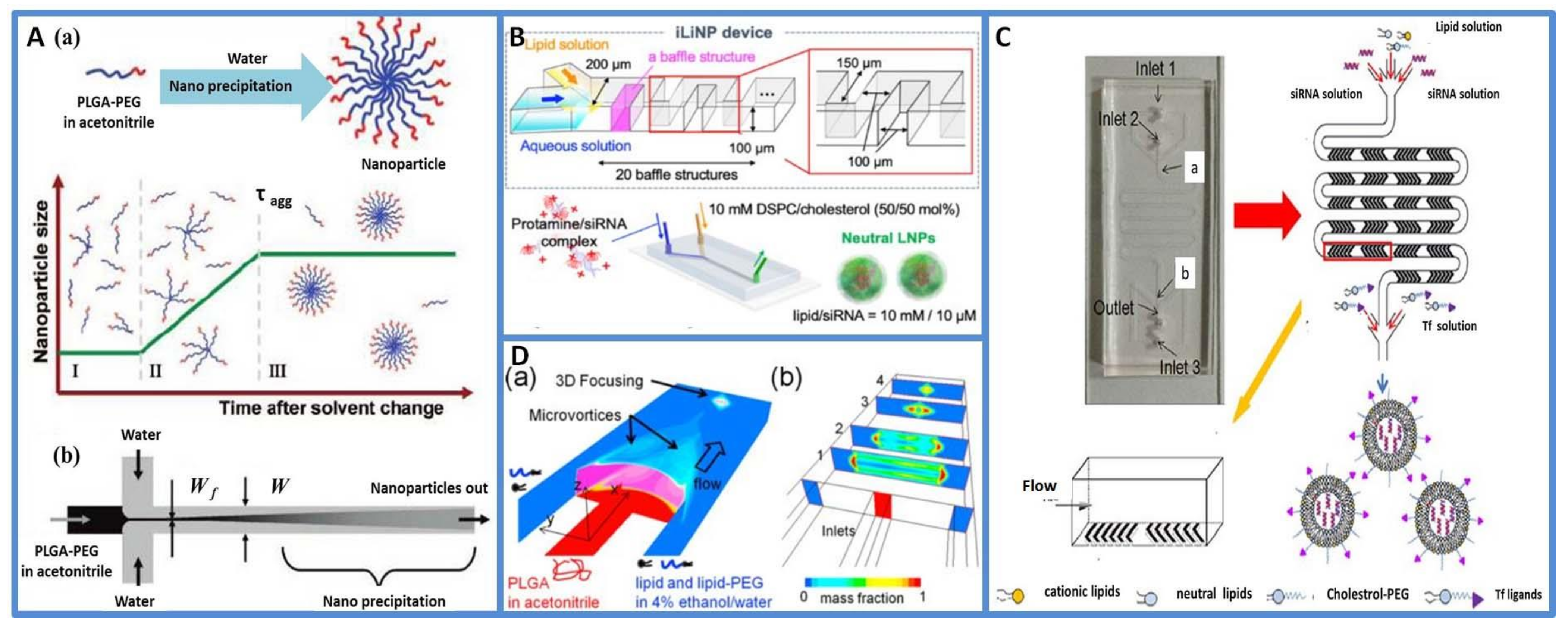
2.2. Microfluidics for the Fabrication of Nanoparticles
2.3. Outlook and Challenges
3. Microfluidics for Cell-Based Drug Toxicity Screening
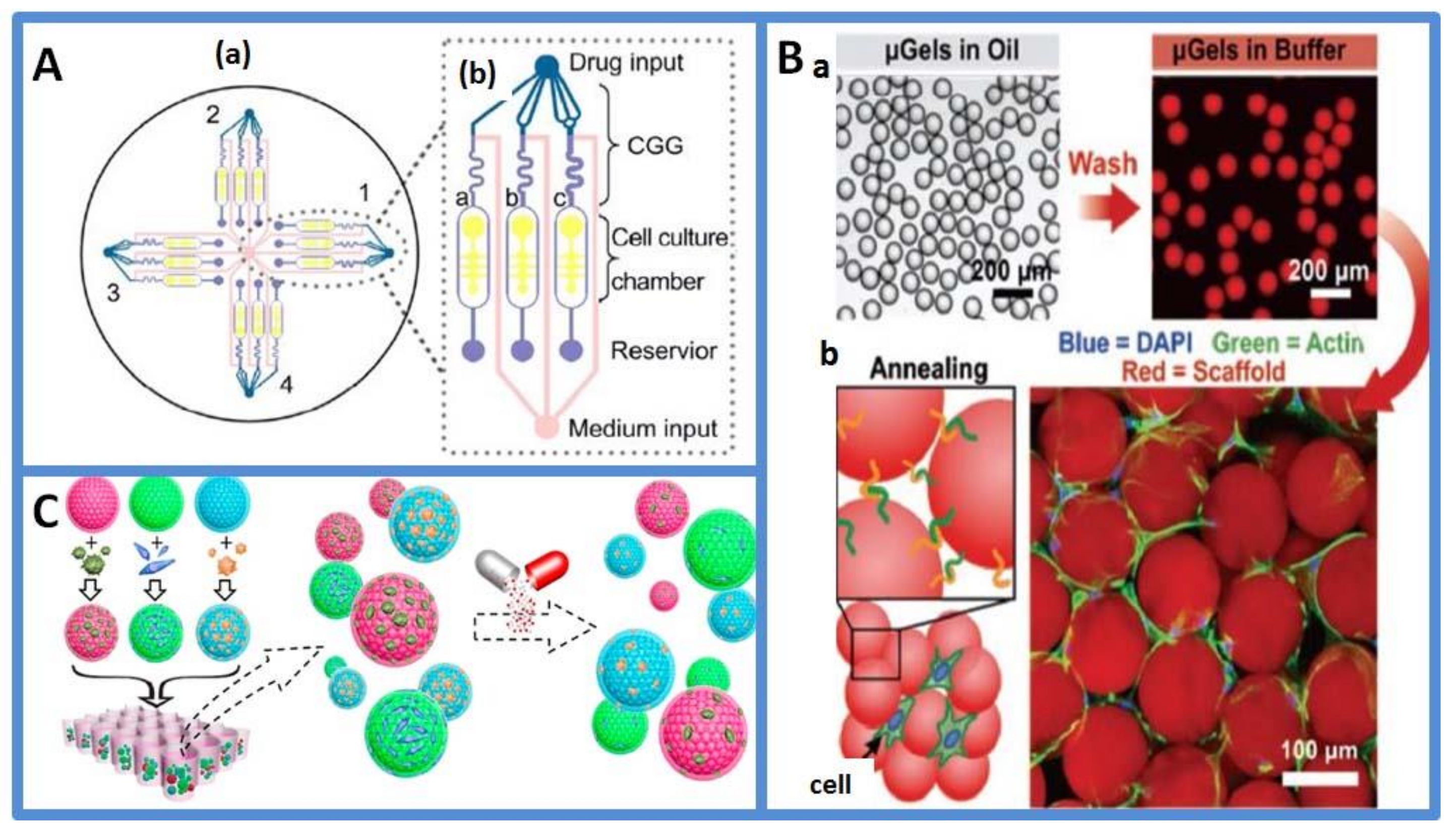
3.1. Drug Dilution Generator Based on Continuous-Flow Based Microfluidics
3.2. Three-Dimensional Cell Coculture on Droplet-Based Microfluidics
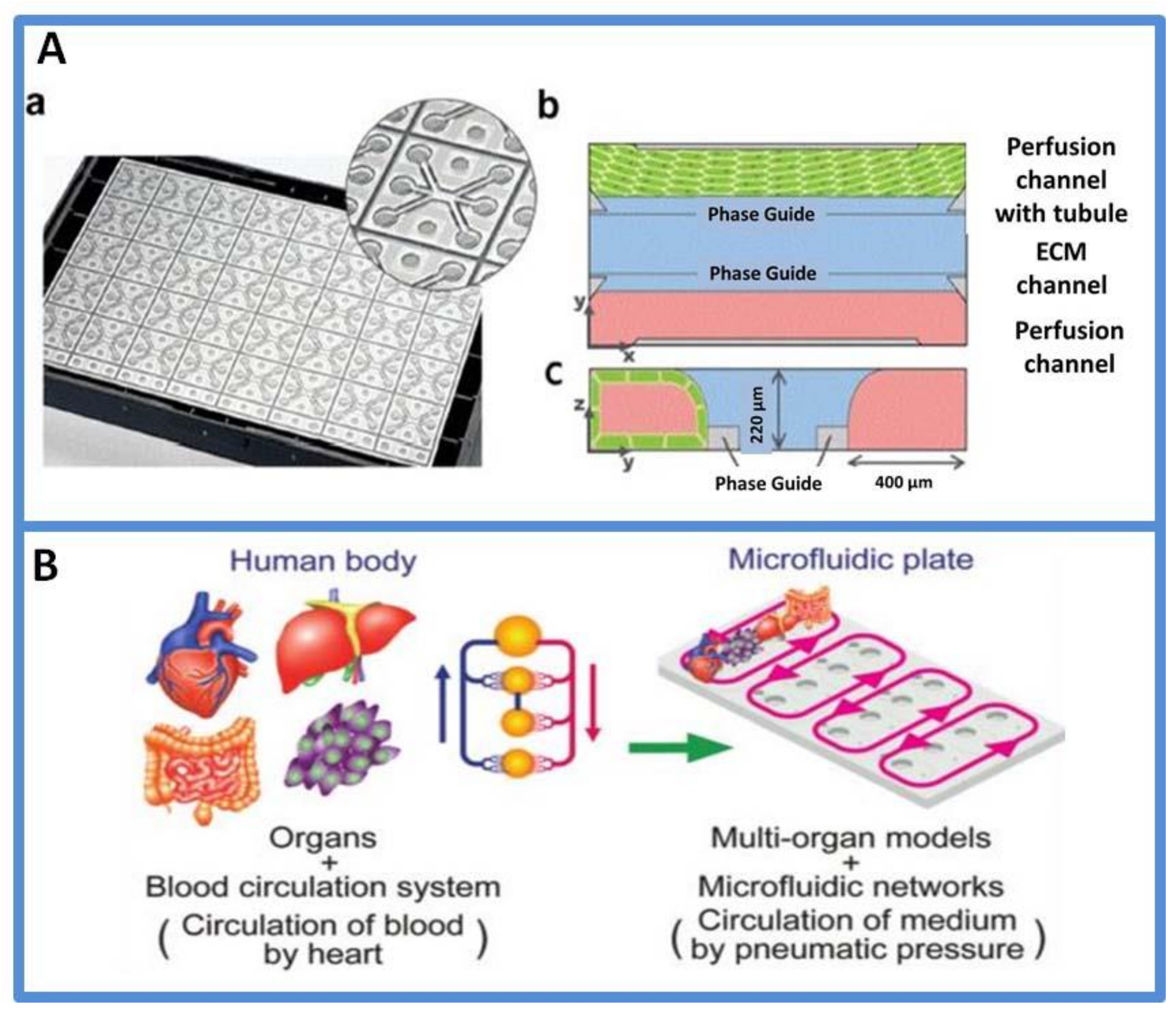
3.3. Organs-on-Chips
3.4. Outlook and Challenges
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Skommer, J.; Wlodkowic, D. Successes and future outlook for microfluidics-based cardiovascular drug discovery. Expert Opin. Drug Discov. 2015, 10, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Mailankody, S. Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval. JAMA Intern. Med. 2017, 177, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L.; Zhang, H.; Shang, L.; Zhao, Y. Microfluidics for Drug Development: From Synthesis to Evaluation. Chem. Rev. 2021, 121, 7468–7529. [Google Scholar] [CrossRef] [PubMed]
- Vladisavljevic, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliv. Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; She, Z.G.; Wang, S.; Sharma, G.; Smith, J.W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 2012, 28, 4459–4463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Pansare, V.J.; Prud’homme, R.K.; Priestley, R.D. Flash nanoprecipitation of polystyrenenanoparticles. Soft Matter 2012, 8, 86–93. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Thiele, J.; Windbergs, M.; Abate, A.R.; Trebbin, M.; Shum, H.C.; Forster, S.; Weitz, D.A. Early development drug formulation on a chip: Fabrication of nanoparticles using a microfluidic spray dryer. Lab Chip 2011, 11, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Allen, T.M.; Cullis, P.R. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv. Transl. Res. 2014, 4, 74–83. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. One-Step Production Using a Microfluidic Device of Highly Biocompatible Size-Controlled Noncationic Exosome-like Nanoparticles for RNA Delivery. ACS Appl. Biol. Mater. 2021, 4, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Attia, M.F.; Wallyn, J.; Taddei, C.; Serra, C.A.; Anton, N.; Kassem, M.; Schmutz, M.; Er-Rafik, M.; Messaddeq, N.; et al. Microfluidic-Assisted Production of Size-Controlled Superparamagnetic Iron Oxide Nanoparticles-Loaded Poly(methyl methacrylate) Nanohybrids. Langmuir 2018, 34, 1981–1991. [Google Scholar] [CrossRef]
- Brzeziński, M.; Socka, M.; Kost, B. Microfluidics for producing polylactide nanoparticles and microparticles and their drug delivery application. Polym. Int. 2019, 68, 997–1014. [Google Scholar] [CrossRef]
- Vyawahare, S.; Zhang, Q.; Lau, A.; Austin, R.H. In vitro microbial culture models and their application in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 217–224. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, H.; Sun, M.; Xu, J.; Zhao, S.; Liu, Z.; Gooch, K.J.; Zhao, Y.; Lu, X.; He, X. Microfluidics Enabled Bottom-Up Engineering of 3D Vascularized Tumor for Drug Discovery. ACS Nano 2017, 11, 6691–6702. [Google Scholar] [CrossRef]
- Cui, P.; Wang, S. Application of microfluidic chip technology in pharmaceutical analysis: A review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef]
- Seeto, W.J.; Tian, Y.; Pradhan, S.; Kerscher, P.; Lipke, E.A. Rapid Production of Cell-Laden Microspheres Using a Flexible Microfluidic Encapsulation Platform. Small 2019, 15, 1902058–1902071. [Google Scholar] [CrossRef]
- Takehara, H.; Sakaguchi, K.; Sekine, H.; Okano, T.; Shimizu, T. Microfluidic vascular-bed devices for vascularized 3D tissue engineering: Tissue engineering on a chip. Biomed. Microdevices 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, D.T.T.; Wang, X.; Craver, B.M.; Sobrino, A.; Zhao, D.; Chen, J.C.; Lee, L.Y.N.; George, S.C.; Lee, A.P.; Hughes, C.C.W. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 2017, 17, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, W.J.; Arriaga, L.R.; Ung, W.L.; Kopechek, J.A.; Porter, T.M.; Weitz, D.A. Microfluidic Fabrication of Perfluorohexane-Shelled Double Emulsions for Controlled Loading and Acoustic-Triggered Release of Hydrophilic Agents. Langmuir 2014, 30, 13765–13770. [Google Scholar] [CrossRef]
- Kong, T.; Wu, J.; Yeung, K.W.K.; To, M.K.T.; Shum, H.C.; Wang, L. Microfluidic fabrication of polymeric core-shell microspheres for controlled release applications. Biomicrofluidics 2013, 7, 044128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, M.; Chu, L. Functional Polymeric Microparticles Engineered from Controllable Microfluidic Emulsions. Accounts Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef]
- Utada, A.; Lorenceau, E.; Link, D.; Kaplan, P.; Stone, H.; Weitz, D. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of monodisperse particles by using microfluidics: Control over size, shape, and composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Li, Y.; Wang, X.; Chen, C.; Liu, G. Microfluidic Technology for the Production of Well-Ordered Porous Polymer Scaffolds. Polymers 2020, 12, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics a review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, X.; Wang, J.; Tian, H.; Zhao, P.; Tian, Y.; Gu, Y.; Wang, L.; Wang, C. Droplet Microfluidics for the Production of Microparticles and Nanoparticles. Micromachines 2017, 8, 22. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.A.; Zhang, H.; Zhang, Y.; Cui, W. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021, 1, 210036. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Duncanson, W.J.; Shum, H.C.; Weitz, D.A. Emulsion Templating of Poly(lactic acid) Particles: Droplet Formation Behavior. Langmuir 2012, 28, 12948–12954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.; Stokke, B.T. Fabrication of monodisperse alginate microgel beads by microfluidic picoinjection: A chelate free approach. Lab Chip 2021, 21, 2232–2243. [Google Scholar] [CrossRef]
- Kumar, A.A.; Lim, C.; Moreno, Y.; Mace, C.R.; Syed, A.; Tyne, D.V.; Wirth, D.F.; Duraisingh, M.T.; Whitesides, G.M. Enrichment of reticulocytes from whole blood using aqueous multiphase systems of polymers. Am. J. Hematol. 2015, 90, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Chan, Y.K.; Ma, Q.; Liu, Z.; Shum, H.C. All-Aqueous Electrosprayed Emulsion for Templated Fabrication of Cytocompatible Microcapsules. ACS Appl. Mater. Interfaces 2015, 7, 13925–13933. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, Z.Y.; Tang, S.Y.; Li, W.; Inglis, D.W.; Hosokawa, Y.; Yalikun, Y.; Li, M. Focusing of sub-micrometer particles in microfluidic devices. Lab Chip 2020, 20, 35–53. [Google Scholar] [CrossRef]
- Wu, J.; Kong, T.; Yeung, K.W.; Shum, H.C.; Cheung, K.M.; Wang, L.; To, M.K. Fabrication and characterization of monodisperse PLGA-alginate core-shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013, 9, 7410–7419. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Yang, X.; Ma, B.; Liu, Y.; Xie, R.; Ju, X.; Liu, Z.; Chu, L. Uniform Microparticles with Controllable Highly Interconnected Hierarchical Porous Structures. ACS Appl. Mater. Interfaces 2015, 7, 13758–13767. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.E.; Nabavi, S.A.; Vladisavljevic, G.T.; Gu, S. Structured Biodegradable Polymeric Microparticles for Drug Delivery Produced Using Flow Focusing Glass Microfluidic Devices. ACS Appl. Mater. Interfaces 2015, 7, 23132–23143. [Google Scholar] [CrossRef] [Green Version]
- De Rutte, J.M.; Koh, J.; Di Carlo, D. Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater. 2019, 29, 1900071. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, K.; Liu, S.; Yang, Y.; Liang, B.; Zheng, C.; Yang, X.; Xu, H.; Shen, A.Q. X-ray Visible and Uniform Alginate Microspheres Loaded with in Situ Synthesized BaSO4 Nanoparticles for in Vivo Transcatheter Arterial Embolization. Biomacromolecules 2015, 16, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Ji, X.; Wang, C.; Shen, R.; Zhang, L. Preparation of solid, hollow, hole-shell and asymmetric silica microspheres by microfluidic-assisted solvent extraction process. Chem. Eng. J. 2014, 250, 112–118. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Yang, F.; Gan, L.; Yang, X.; Yang, Y. Magnetic alginate microspheres detected by MRI fabricated using microfluidic technique and release behavior of encapsulated dual drugs. Int. J. Nanomed. 2017, 12, 4335–4347. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, S.; Machado, A.; Lecommandoux, S.; Sandre, O.; Gu, F.; Colin, A. Controllable Microfluidic Production of Drug-Loaded PLGA Nanoparticles Using Partially Water-Miscible Mixed Solvent Microdroplets as a Precursor. Sci Rep. 2017, 7, 4794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, R.J.; Huang, X.; Li, Y.; Lv, B.; Wang, T.; Qi, Y.; Hao, F.; Lu, J.; Meng, Q.; et al. Single-step microfluidic synthesis of transferrin-conjugated lipid nanoparticles for siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; Shao, C.; Omiatek, D.M.; Vreeland, W.N.; DeVoe, D.L. Microfluidic synthesis of PEG- and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers. Pharm. Res. 2013, 30, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youan, B.B.C. Impact of nanoscience and nanotechnology on controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2008, 3, 401–406. [Google Scholar] [CrossRef]
- Anton, N.; Bally, F.; Serra, C.A.; Ali, A.; Arntz, Y.; Mely, Y.; Zhao, M.; Marchioni, E.; Jakhmolaa, A.; Vandamme, T.F. A new microfluidic setup for precise control of the polymer nanoprecipitation process and lipophilic drug encapsulation. Soft Matter 2012, 8, 10628–10635. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Vandamme, T.F.; Serra, C.A. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: An overview. Expert Opin. Drug Deliv. 2016, 13, 1447–1460. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T.F. Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin. Drug Deliv. 2014, 12, 1–16. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Biol. Mater. 2019, 3, 107–120. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef] [Green Version]
- Krzysztoń, R.; Salem, B.; Lee, D.J.; Schwake, G.; Wagner, E.; Rädler, J.O. Microfluidic self-assembly of folate-targeted monomolecular siRNA-lipid nanoparticles. Nanoscale 2017, 7, 7442–7453. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, N.M.; Jens, H.; Lin, P.J.; Chen, S.; Leung Alex, K.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Kolishetti, N.; Valencia, S.D.P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langerb, R.; Farokhzada, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Toth, M.J.; Kim, T.; Kim, Y. Robust manufacturing of lipid-polymer nanoparticles through feedback control of parallelized swirling microvortices. Lab Chip 2017, 17, 2805–2813. [Google Scholar] [CrossRef]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.M.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass Production and Size Control of Lipid–Polymer Hybrid Nanoparticles through Controlled Microvortices. Nano Letters 2012, 12, 3587–3591. [Google Scholar] [CrossRef] [Green Version]
- Hao, N.; Wang, Z.; Liu, P.; Becker, R.; Yang, S.; Yang, K.; Pei, Z.; Zhang, P.; Xia, J.; Shen, L.; et al. Acoustofluidic multimodal diagnostic system for Alzheimer’s disease. Biosens. Bioelectron. 2022, 196, 113730. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Zhao, S.; Bachman, H.; Nama, N.; Li, Z.; Chen, C.; Yang, S.; Wu, M.; Zhang, S.P.; Huang, T.J. Acoustofluidic Synthesis of Particulate Nanomaterials. Adv. Sci. 2019, 6, 1900913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, H.; Aoki, N.; Mae, K. Design of a New Micromixer for Instant Mixing Based on the Collision of Micro Segments. Chem. Eng. Technol. 2005, 28, 324–330. [Google Scholar] [CrossRef]
- Lim, J.-M.; Swami, A.; Gilson, L.M.; Chopra, S.; Choi, S.; Jun, W.; Langer, R.; Karnik, R.; Farokhzad, O.C. Ultra High Throughput Synthesis of Nanoparticles with Homogeneous Size Distribution Using a Coaxial Turbulent Jet Mixer. ACS Nano 2014, 8, 6056–6065. [Google Scholar] [CrossRef] [Green Version]
- Rhee, M.; Valencia, P.M.; Rodriguez, M.I.; Langer, R.; Farokhzad, O.C.; Karnik, R. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv. Mater. 2011, 23, H79–H83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazban-Shotorbani, S.; Dashtimoghadam, E.; Karkhaneh, A.; Hasani-Sadrabadi, M.M.; Jacob, K.I. Microfluidic Directed Synthesis of Alginate Nanogels with Tunable Pore Size for Efficient Protein Delivery. Langmuir 2016, 32, 4996. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Luo, C.; Wei, Q.; Xiong, C.; Chen, Q.; Chen, Y.; Ouyang, Q. Mass production of highly monodisperse polymeric nanoparticles by parallel flow focusing system. Microfluid. Nanofluid. 2013, 15, 337–345. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Wang, J.X.; Shao, L.; Zhang, H.X.; Zhang, Q.X.; Chen, J.F. Liquid antisolvent preparation of amorphous cefuroxime axetil nanoparticles in a tube-in-tube microchannel reactor. Int. J. Pharm. 2010, 395, 260. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-Up Design and Synthesis of Limit Size Lipid Nanoparticle Systems with Aqueous and Triglyceride Cores Using Millisecond Microfluidic Mixing. Langmuir ACS J. Surf. Colloids 2012, 28, 3633. [Google Scholar] [CrossRef] [PubMed]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Wang, H.; Yan, L.; Zhang, X.; Cheng, Y. Continuous preparation of itraconazole nanoparticles using droplet-based microreactor. Chem. Eng. J. 2020, 393, 124721. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawai, M.; Sato, Y.; Maeki, M.; Harashima, H. The Effect of Size and Charge of Lipid Nanoparticles Prepared by Microfluidic Mixing on Their Lymph Node Transitivity and Distribution. Mol. Pharm. 2020, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Q.; Liu, W.; He, Z.; Lin, J. Recent advances in microfluidic 3D cellular scaffolds for drug assays. TrAC Trend. Anal. Chem. 2017, 87, 19–31. [Google Scholar] [CrossRef]
- Zhai, J.; Yi, S.; Jia, Y.; Mak, P.-I.; Martins, R.P. Cell-based drug screening on microfluidics. TrAC Trend. Anal. Chem. 2019, 117, 231–241. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, Y.; Cao, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic technology and applications for anti-cancer drug screening. TrAC Trend. Anal. Chem. 2021, 134, 116118. [Google Scholar] [CrossRef]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Lin, R.; Lee, L.P. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol. Bioeng. 2005, 89, 1–8. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K.; Kim, J.H.; Shin, K.S.; Lee, K.-J.; Kim, T.S.; Kang, J.Y. A serial dilution microfluidic device using a ladder network generating logarithmic or linear concentrations. Lab Chip 2008, 8, 473–479. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Xu, Z.; Li, J.; Ding, X.; Wang, Q.; Chunyu, L. A multilayer microdevice for cell-based high-throughput drug screening. J. Micromech. Microeng. 2012, 22, 65008–65014. [Google Scholar] [CrossRef]
- Fu, F.; Shang, L.; Zheng, F.; Chen, Z.; Wang, H.; Wang, J.; Gu, Z.; Zhao, Y. Cells Cultured on Core-Shell Photonic Crystal Barcodes for Drug Screening. ACS Appl. Mater. Inter. 2016, 8, 13840–13848. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension-how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 12, 435–447. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Edgar, J.S.; Jeffries, G.D.; Lorenz, R.M.; Shelby, J.P.; Chiu, D.T. Selective encapsulation of single cells and subcellular organelles into picoliter-and femtoliter-volume droplets. Anal. Chem. 2005, 77, 1539–1544. [Google Scholar] [CrossRef]
- Clausell-Tormos, J.; Lieber, D.; Baret, J.-C.; El-Harrak, A.; Miller, O.J.; Frenz, L.; Blouwolff, J.; Humphry, K.J.; Köster, S.; Duan, H. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Köster, S.; Angile, F.E.; Duan, H.; Agresti, J.J.; Wintner, A.; Schmitz, C.; Rowat, A.C.; Merten, C.A.; Pisignano, D.; Griffiths, A.D. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 2008, 8, 1110–1115. [Google Scholar] [CrossRef]
- Niu, X.; Gulati, S.; Edel, J.B. Pillar-induced droplet merging in microfluidic circuits. Lab Chip 2008, 8, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Agresti, J.; Chong, H.; Marquez, M.; Weitz, D. Electrocoalescence of drops synchronized by size-dependent flow in microfluidic channels. Appl. Phys. Lett. 2006, 88, 264105. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Edit. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, F.; Prat, L.; Di Miceli, N.; Cristobal, G.; Link, D.; Weitz, D. Mixing characterization inside microdroplets engineered on a microcoalescer. Chem. Eng. Sci. 2007, 62, 1042–1048. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks inTime. Angew. Chem. Int. Edit. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.; Link, D.R.; Weitz, D. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef] [Green Version]
- Boedicker, J.Q.; Li, L.; Kline, T.R.; Ismagilov, R.F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab Chip 2008, 8, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Brouzes, E.; Medkova, M.; Savenelli, N.; Marran, D.; Twardowski, M.; Hutchison, J.B.; Rothberg, J.M.; Link, D.R.; Perrimon, N.; Samuels, M.L. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA 2009, 106, 14195–14200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhamecha, D.; Le, D.; Movsas, R.; Gonsalves, A.; Menon, J.U. Porous Polymeric Microspheres With Controllable Pore Diameters for Tissue Engineered Lung Tumor Model Development. Front Bioeng. Biotechnol. 2020, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Cha, C. The Combined Effects of Co-Culture and Substrate Mechanics on 3D Tumor Spheroid Formation within Microgels Prepared via Flow-Focusing Microfluidic Fabrication. Pharmaceutics 2018, 10, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.; Sato, K.; Igawa, K.; Chung, U.I.; Kitamori, T. Development of an osteoblast-based 3D continuous-perfusion microfluidic system for drug screening. Anal. Bioanal. Chem. 2008, 390, 825–832. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Beebe, D.J.; Ingber, D.E.; Den, T.J. Organs on Chips 2013. Lab Chip 2013, 13, 3447–3448. [Google Scholar] [CrossRef]
- Selimović, S.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip for drug discovery. Curr. Opin. Pharmacol. 2013, 13, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Zhao, P.; Nandakumar, K.; Wang, L.; Song, Y. Microfluidics-Based Systems in Diagnosis of Alzheimer’s Disease and Biomimetic Modeling. Micromachines 2020, 2, 787. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. In Vitro Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hong, Y.H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; Mcalexander, M.A.; Ingber, D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 147. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber Membrane supported lung-on-a-chip Microdevice for Anti-cancer Drug Testing. Lab Chip 2018, 2, 465–478. [Google Scholar] [CrossRef]
- Toh, Y.C.; Lim, T.C.; Tai, D.; Xiao, G.; Noort, D.V.; Yu, H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 2009, 9, 2026–2035. [Google Scholar] [CrossRef]
- Kim, S.; Takayama, S. Organ-on-a-chip and the kidney. Kidney Res. Clin. Pract. 2015, 34, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Terrell-Hall, T.B.; Ammer, A.G.; Griffith, J.I.; Lockman, P.R. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, A.; Senutovitch, N.; Bale, S.S.; McCarty, W.J.; Hegde, M.; Jindal, R.; Golberg, I.; Usta, O.B.; Yarmush, M.L.; Vernetti, L.; et al. Towards a three-dimensional microfluidic liver platform for predicting drug efficacy and toxicity in humans. Stem Cell Res. Ther. 2013, 4, S16–S22. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotech. Bioeng. 2007, 97, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Mechanisms of Drug Toxicity and Relevance to Pharmaceutical Development. Drug Metab. Pharmacok. 2011, 26, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Vormann, M.K.; Vriend, J.; Lanz, H.L.; Gijzen, L.; van den Heuvel, A.; Hutter, S.; Joore, J.; Trietsch, S.J.; Stuut, C.; Nieskens, T.T.G.; et al. Implementation of a Human Renal Proximal Tubule on a Chip for Nephrotoxicity and Drug Interaction Studies. J. Pharm. Sci. 2021, 110, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Vormann, M.K.; Lanz, H.L.; Joore, J.; Trietsch, S.J.; Russel, F.G.M.; Jacobsen, B.; Roth, A.; Lu, S.; Polli, J.W.; et al. Nephroscreen: A robust and versatile renal tubule-on-a-chip platform for nephrotoxicity assessment. Curr. Opin. Toxicol. 2021, 25, 42–48. [Google Scholar] [CrossRef]
- Hovell, C.M.; Sei, Y.J.; Kim, Y. Microengineered vascular systems for drug development. J. Lab. Autom. 2015, 20, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, K.M.; Islam, M.M.; LeDuc, P.R.; Steward, R. 2D and 3D Mechanobiology in Human and Nonhuman Systems. ACS Appl. Mater. Inter. 2016, 8, 21869–21882. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; Li, R.; Uzel, S.G.M.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Moses, J.C.; Mandal, B.B. Surface Patterning and Innate Physicochemical Attributes of Silk Films Concomitantly Govern Vascular Cell Dynamics. ACS Biomater. Sci. Eng. 2018, 5, 933–949. [Google Scholar] [CrossRef]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakikic, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2017, 18, 115–125. [Google Scholar] [CrossRef]
- Wagner, I.; Materne, E.M.; Brincker, S.; Süssbier, U.; Frädrich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13, 3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materne, E.M.; Maschmeyer, I.; Lorenz, A.K.; Horland, R.; Schimek, K.M.S.; Busek, M.; Sonntag, F.; Lauster, R.; Marx, U. The Multi-organ Chip—A Microfluidic Platform for Long-term Multi-tissue Coculture. J. Vis. Exp. 2015, 2015, 52526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleaga, C.; Bernabini, C.; Smith, A.S.; Srinivasan, B.; Jackson, M.; Mclamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci. Rep. 2018, 8, 2841. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]

| Microfluidic Channels | MPs Synthesized | Diameter | Flow Rate (F)/Ratio | Reference |
|---|---|---|---|---|
| Flow focusing | Alginate microgels | ~35 μm | Faqueous phase = 20 μL/h Foil phase = 150 μL/h | [42] |
| T-junction | Silica microspheres | ~90–108 μm | Fcontinuous phase=5 mL/h, Fdispersed phase =0.5 mL/h | [43] |
| Cross-junction | Alginate microspheres | 150 μm | Faqueous phase = 30 μL/h Foil phase = 500 μL/h | [42] |
| T-junction | Alginate microspheres coencapsulated with superparamagnetic iron oxide NPs and dual drugs | ~500 μm | —— | [44] |
| Counter-current flow focusing | PLA and PLGA microparticles | 4–30 μm | Foil phase = 0.02–1.5 mL/h Faqueous phase = 0.25–8 mL/h | [40] |
| Parallelized step emulsification device | Microgels | 50 and 90 μm | Ftotal = 30 mL/h | [41] |
| Flow focusing | PLGA-alginate core–shell microspheres | 15–50 μm | Fpolymer phase = 0.8 mL/h Faqueous phase = 2–8 mL/h | [38] |
| Fabrication Method | NPs Synthesized | Diameter of NPs | Flow Rate (F)/Ratio | Reference |
|---|---|---|---|---|
| K-M impact jet mixer | Superparamagnetic iron oxide nanoparticles-loaded PMMA NPs | ~100 nm | Fpolymer = 0.2 mL/min Fwater = 2–4 mL/min | [12] |
| 3D hydrodynamic flow focusing | PLGA-PEG NPs | 30–230 nm | Forganic:Fwater = 1:10 | [67] |
| Flow focusing | Alginate nanogels | 68–138 nm | Forganic:Fwater = 0.02–0.2 | [68] |
| Parallel flow focusing | MPEG-PLGA NPs | 50–200 nm | Fwater = 5.0 mL/h Fpolymer = 0.5–2.0 mL/h | [69] |
| Microfluidic flow focusing | PLGA-NPs | 90–160 nm | Fdiserse/Fcontinuous = 50:5000–10,000 | [45] |
| Tube-in-tube microchannel reactor | Amorphous cefuroxime axetil NPs | ~440–760 nm | Ftotal = 1.5–6 L/min | [70] |
| Flash nanoprecipitation (FNP) mixing | Polystyrene NPs | Sub-150 nm | Fpolystyrene = 2mL/s Fwater = 2mL/s | [6] |
| Staggered herringbone mixer structures (SHM) | siRNA LNPs | ~70–80 nm | Fethanol = 0.5 mL/min Fwater = 0.5–4.5 mL/min | [71] |
| Staggered herringbone micromixer (SHM) | Liposome | ~50–450 nm | Ftotal = 0.5–6 mL/min | [72] |
| Fluidic nanoprecipitation system (FNPS) | Polymeric Janus NPs | Sub-μm | Fpolymer = 100 μL/h Fwater = 75 mL/min. | [5] |
| Coaxial turbulent jet mixer | PLGA-PEG NPs, lipid vesicles, iron oxide NPs, polystyrene NPs and siRNA/PEI polyplex NPs | ≤100 nm | Ftotal = 300–500 mL/min | [66] |
| Millisecond microfluidic mixing | LNP-siRNA | ≤100 nm | Fethanol = 0.5 mL/min Faqueous = 1.5 mL/min | [10] |
| Swirling microvortex reactors | LPNPs | ~50 nm | Ftotal = 15 μL/min | [61] |
| Gas/liquid Taylor flow micromixer | LNP | ~70 nm | Ftotal = 100–700 μL/min | [10] |
| Cross-junction T-junction | Itraconazole NPs | 130–340 nm | Fcontinuous = 10–250 μL/min Fdisperse = 50 μL/min | [73] |
| Microfluidic mixing | pH-sensitive LNPs | 30 nm 100 nm 200 nm | Flipid = 0.375 mL/min Facetate = 1.125 mL/min | [74] |
| Organ | Model | Chips | Drug Tested | Benefits | Reference |
|---|---|---|---|---|---|
| Brain | Blood–brain barrier | OrganoPlate | Organophosphate | High throughput and high-content imaging | [102,124] |
| Heart | Cardiac microphysiological systems | PDMS chips | Cardiac drugs | High throughput, simplify drug tests | [103] |
| Lung | Pulmonary edema-on-a-chip | PDMS chips | Angiopoietin-1 and transient receptor potential vanilloid 4 | Reproduce the intra-alveolar fluid accumulation, fibrin deposition and impaired gas exchange | [105] |
| Liver | Liver sinusoid with a microfluidic endothelial-like barrier | Chips including glass bottom, silicone middle and acrylic top | Diclofenac | Create a mass transport environment, improve hepatocyte viability | [111] |
| Kidney | Kidney proximal tubule-on-a-chip | PDMS chips | Cisplatin | Enhance epithelial cell polarization and primary cilia formation | [125] |
| Vascular vessel | Scaffold with a built-in branching microchannel network (AngioChip) | Poly(octamethylene maleate (anhydride) citrate) chips | Terfenadine | Tunable elasticity and permeability, enable extensive remodel | [115,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Wang, J.; Chen, C.; Wang, J.; Liu, G.; Nandakumar, K.; Li, Y.; Wang, L. Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening. Micromachines 2022, 13, 200. https://doi.org/10.3390/mi13020200
Zhao P, Wang J, Chen C, Wang J, Liu G, Nandakumar K, Li Y, Wang L. Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening. Micromachines. 2022; 13(2):200. https://doi.org/10.3390/mi13020200
Chicago/Turabian StyleZhao, Pei, Jianchun Wang, Chengmin Chen, Jianmei Wang, Guangxia Liu, Krishnaswamy Nandakumar, Yan Li, and Liqiu Wang. 2022. "Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening" Micromachines 13, no. 2: 200. https://doi.org/10.3390/mi13020200






