Microscopic Imaging Methods for Organ-on-a-Chip Platforms
Abstract
1. Introduction
2. Transillumination Imaging
2.1. Brightfield Imaging with Microfluidic Devices
2.2. Brightfield Imaging with OOC Platforms
2.3. Phase-Contrast Imaging with Microfluidic Devices
2.4. Phase-Contrast Imaging with OOC Platforms
2.5. Holographic Optofluidic Microscopy with Microfluidic Devices
2.6. Holographic Optofluidic Microscopy with OOC Platforms
3. Fluorescence Imaging
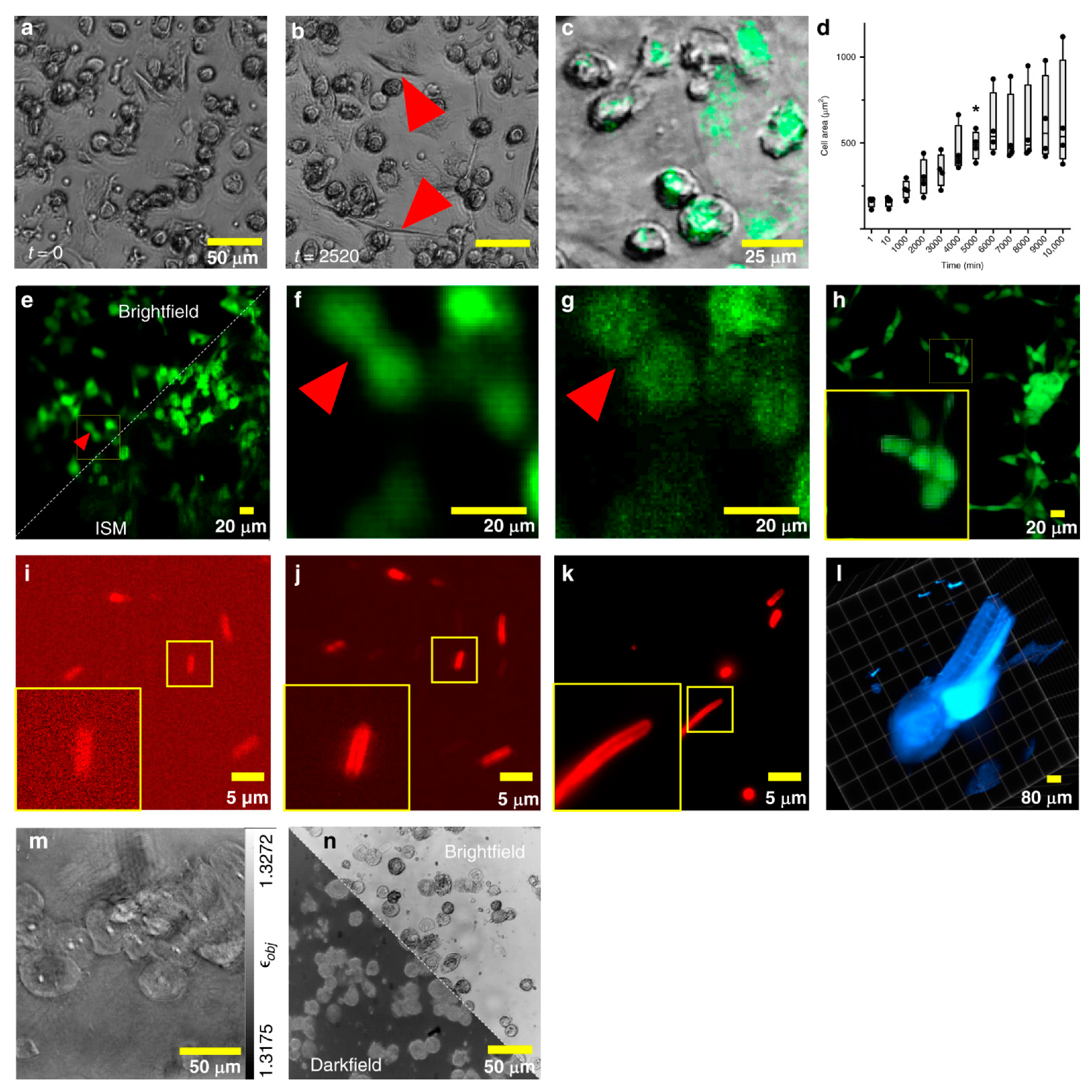
3.1. Confocal Fluorescence Microscopy with Microfluidic Devices
3.2. Confocal Fluorescence Microscopy with OOC Platforms
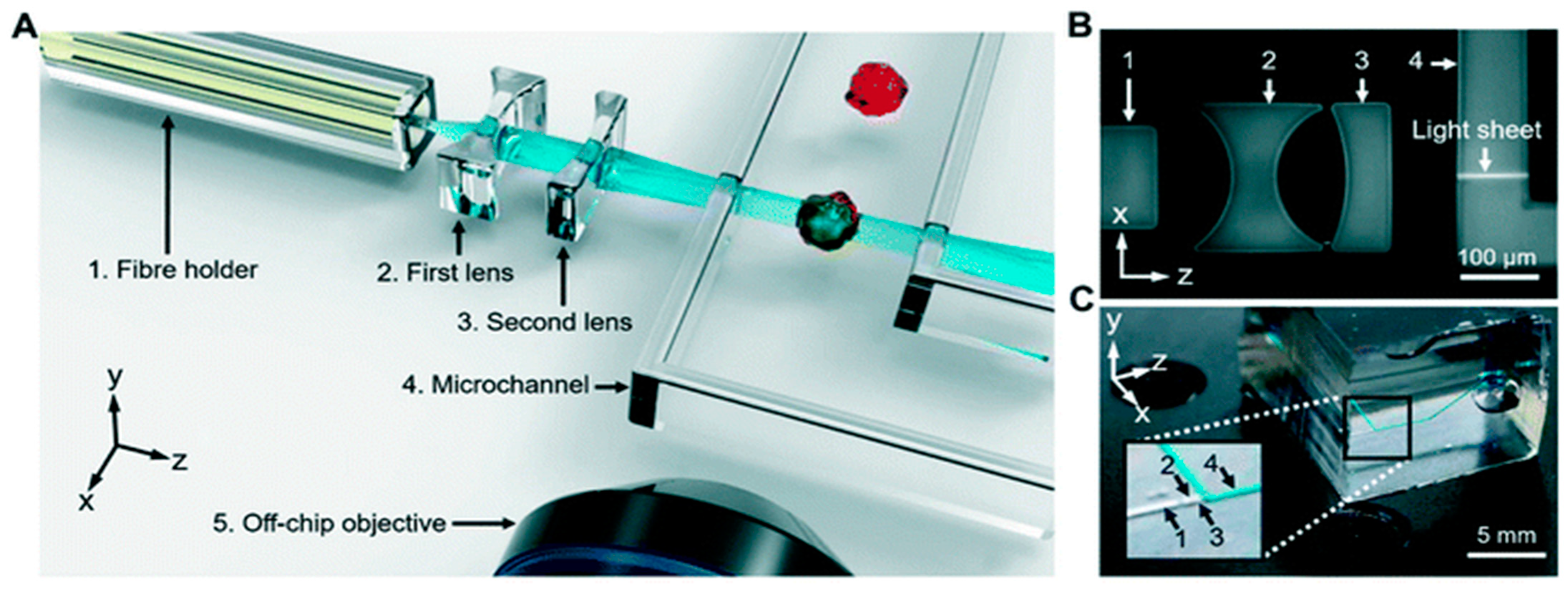
3.3. Light-Sheet Fluorescence Microscopy (LSFM) with Microfluidic Devices
3.4. Light-Sheet Fluorescence Microscopy (LSFM) with OOC Platforms
4. Smartphone-Based Imaging
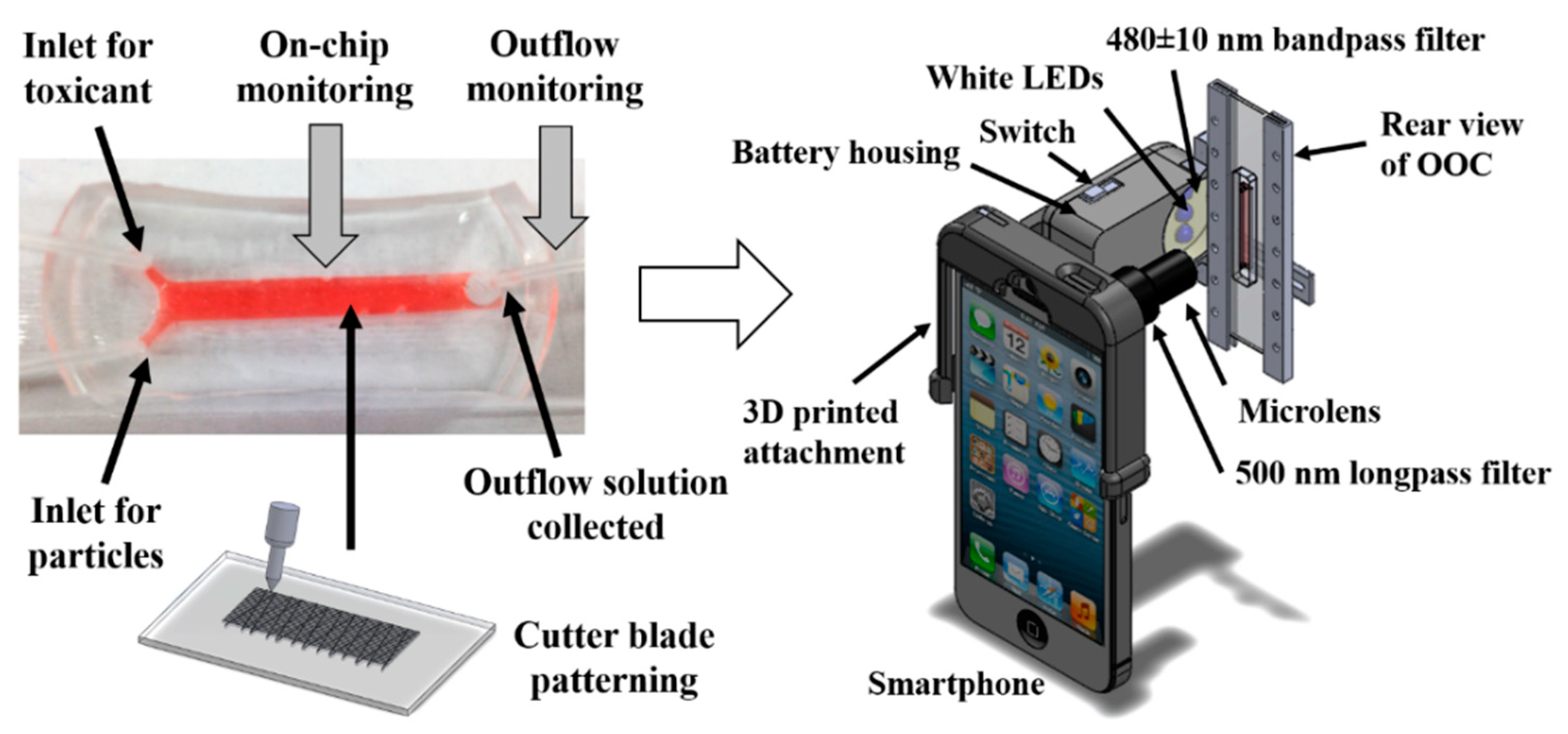
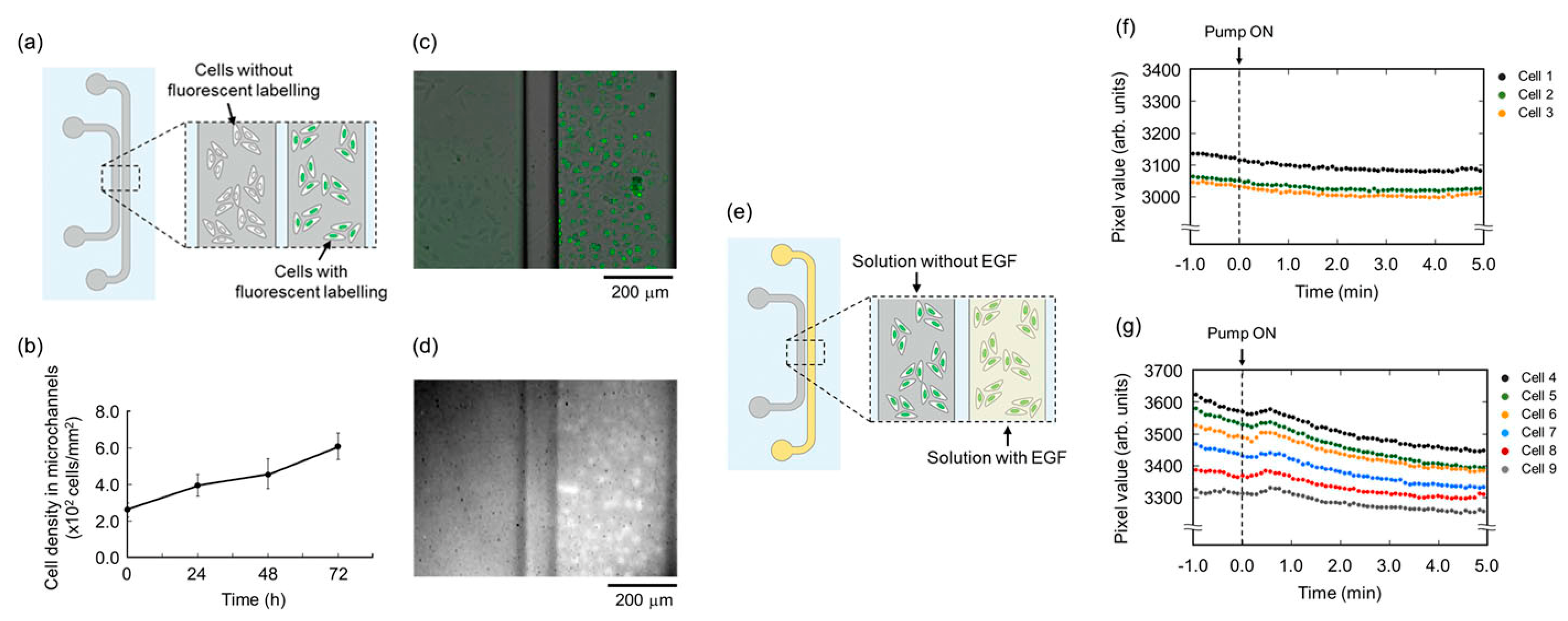
4.1. Microscope Attachment-Based Smartphone Microscopy with Microfluidic Devices
4.2. Microscope Attachment-Based Smartphone Microscopy with OOC Platforms
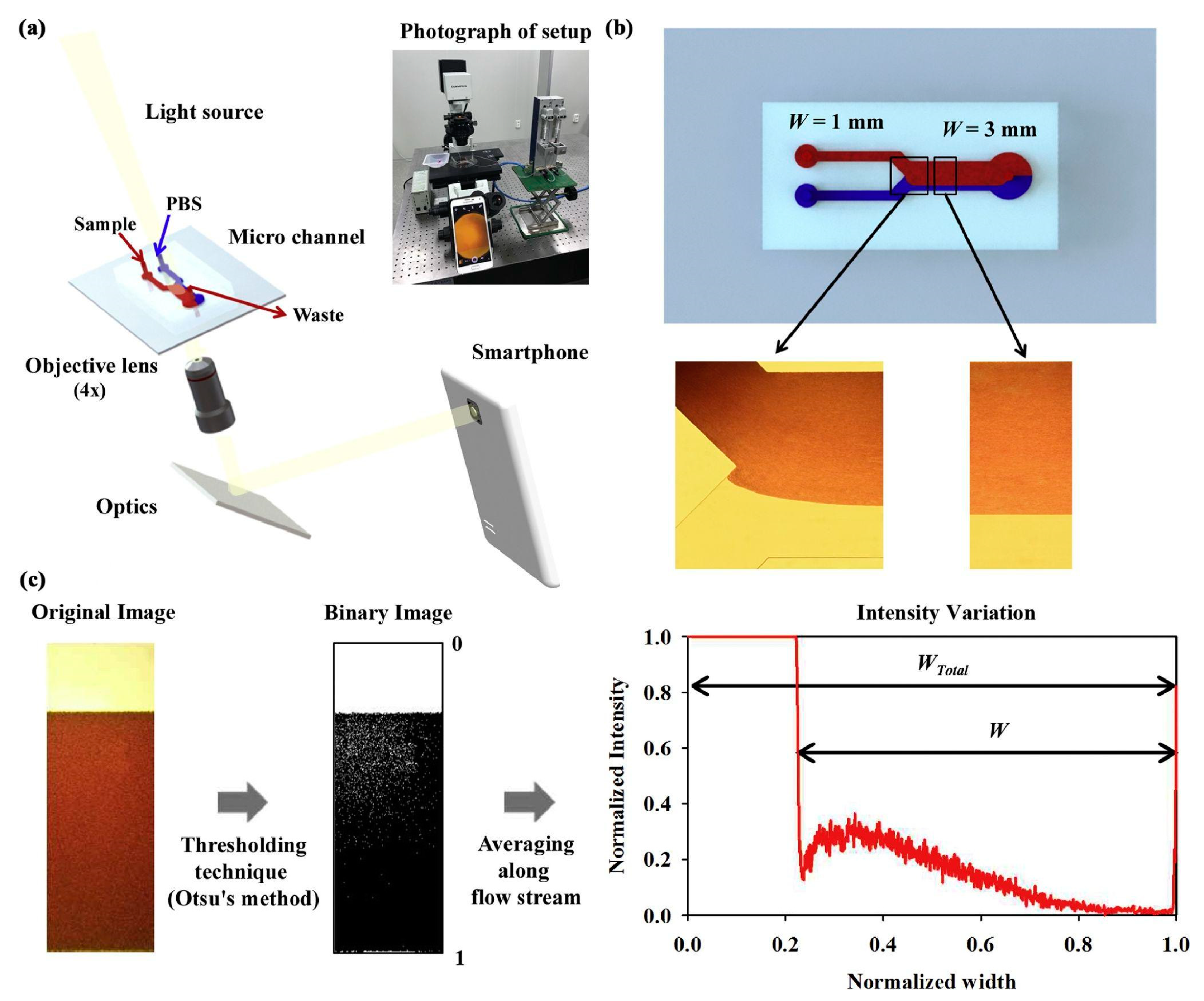
4.3. Quantitative Phase Smartphone Microscopy with Microfluidic Devices
4.4. Quantitative Phase Smartphone Microscopy with OOC Platforms
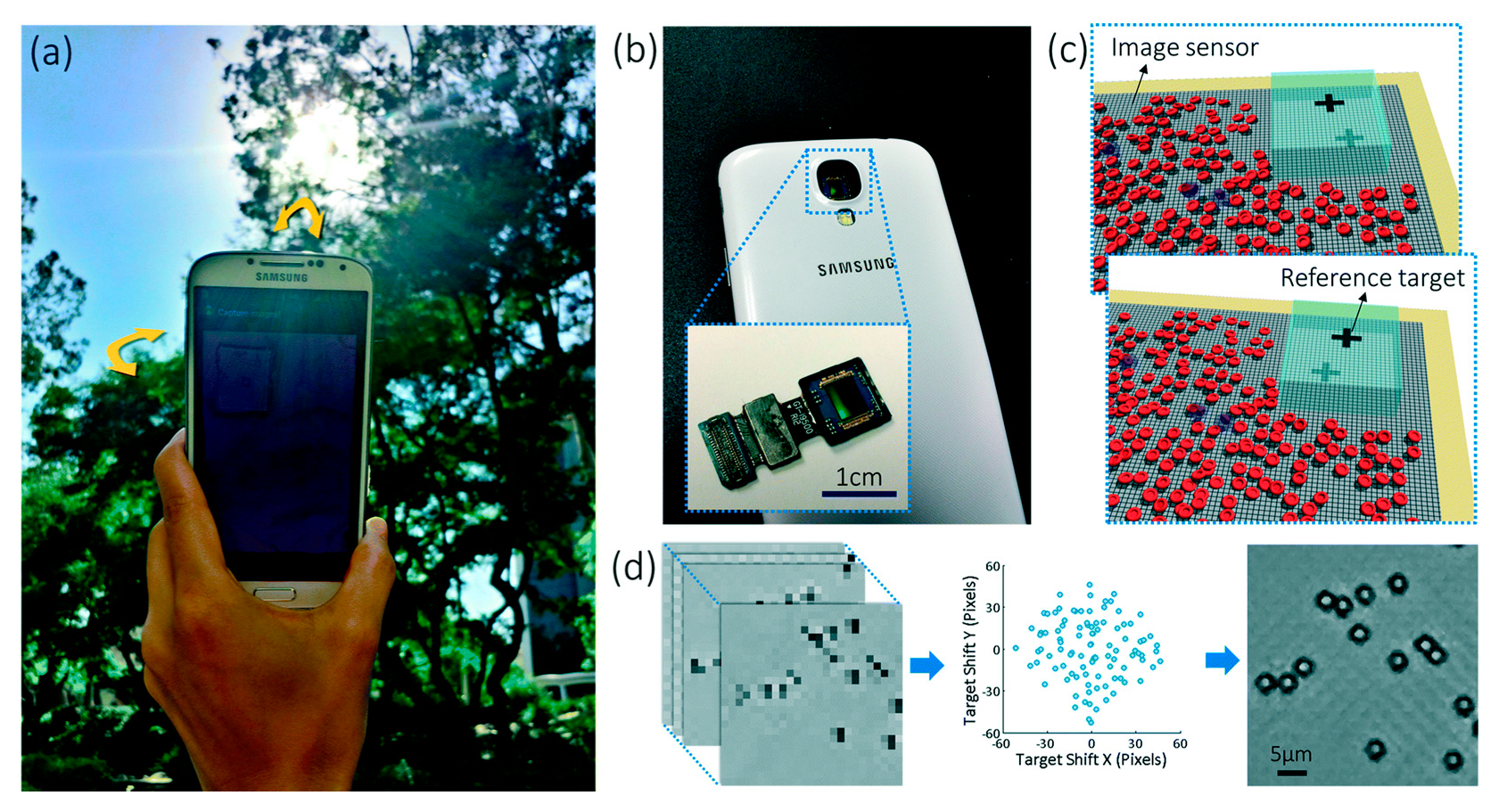
4.5. Lens-Free Smartphone Microscopy with Microfluidic Devices
4.6. Lens-Free Smartphone Microscopy with OOC Platforms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Gao, D.; Wang, Y.; Lin, S.; Jiang, Y. A novel 3D breast-cancer-on-chip platform for therapeutic evaluation of Drug Delivery Systems. Anal. Chim. Acta 2018, 1036, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, G.; Qin, J.; Lin, B. Characterization of drug metabolites and cytotoxicity assay simultaneously using an integrated microfluidic device. Lab Chip 2009, 9, 232–238. [Google Scholar] [CrossRef]
- Cho, S.; Islas-Robles, A.; Nicolini, A.M.; Monks, T.J.; Yoon, J.-Y. In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens. Bioelectron. 2016, 86, 697–705. [Google Scholar] [CrossRef][Green Version]
- Kaarj, K.; Ngo, J.; Loera, C.; Akarapipad, P.; Cho, S.; Yoon, J.-Y. Simple paper-based liver cell model for drug screening. BioChip J. 2020, 14, 218–229. [Google Scholar] [CrossRef]
- Akarapipad, P.; Kaarj, K.; Liang, Y.; Yoon, J.-Y. Environmental toxicology assays using organ-on-chip. Annu. Rev. Anal. Chem. 2021, 14, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoon, J.-Y. Organ-on-a-chip for assessing environmental toxicants. Curr. Opin. Biotechnol. 2017, 45, 34–42. [Google Scholar] [CrossRef]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef][Green Version]
- Kaarj, K.; Madias, M.; Akarapipad, P.; Cho, S.; Yoon, J.-Y. Paper-based in vitro tissue chip for delivering programmed mechanical stimuli of local compression and shear flow. J. Biol. Eng. 2020, 14, 20. [Google Scholar] [CrossRef]
- Yoon, J.-Y. Cell Metabolism. In Tissue Engineering: A Primer with Laboratory Demonstrations; Springer: Cham, Switzerland, 2021; pp. 33–47. [Google Scholar] [CrossRef]
- Liang, Y.; Yoon, J.-Y. In situ sensors for blood-brain barrier (BBB) on a chip. Sens. Actuat. Rep. 2021, 3, 100031. [Google Scholar] [CrossRef]
- Yoon, J.-Y. Introduction to Biosensors: From Electric Circuits to Immunosensors, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.-C.; Ning, R.-Z.; Ma, Y.; Lin, J.-M. Recent developments in microfluidic chip for in vitro cell-based research. Chin. J. Anal. Chem. 2016, 44, 522–532. [Google Scholar] [CrossRef]
- Rudmann, D.G. The emergence of microphysiological systems (organs-on-chips) as paradigm-changing tools for toxicologic pathology. Toxicol. Pathol. 2018, 47, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Gerver, R.E.; Gómez-Sjöberg, R.; Baxter, B.C.; Thorn, K.S.; Fordyce, P.M.; Diaz-Botia, C.A.; Helms, B.A.; DeRisi, J.L. Programmable microfluidic synthesis of spectrally encoded microspheres. Lab Chip 2012, 12, 4716–4723. [Google Scholar] [CrossRef][Green Version]
- Kazama, Y.; Carlen, E.T.; van den Berg, A.; Hibara, A. Top-and-side dual-view microfluidic device with embedded prism. Sens. Actuat. B Chem. 2017, 248, 753–760. [Google Scholar] [CrossRef]
- Guan, W.; Chen, L.; Rane, T.D.; Wang, T.-H. Droplet digital enzyme-linked oligonucleotide hybridization assay for absolute RNA quantification. Sci. Rep. 2015, 5, 13795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jégouic, S.M.; Jones, I.M.; Edwards, A.D. Affordable mobile microfluidic diagnostics: Minimum requirements for smartphones and digital imaging for colorimetric and fluorometric anti-dengue and anti-SARS-cov-2 antibody detection. Wellcome Open Res. 2021, 6, 57. [Google Scholar] [CrossRef]
- Nagy, M.; Heemskerk, J.W.; Swieringa, F. Use of microfluidics to assess the platelet-based control of coagulation. Platelets 2017, 28, 441–448. [Google Scholar] [CrossRef]
- Uranga, J.; Rodríguez-Pena, A.; Gahigiro, D.; Ortiz de Solórzano, C. Miniaturized microscope for high throughput screening of tumor spheroids in microfluidic devices. Proc. SPIE 2018, 10497, 104970A. [Google Scholar] [CrossRef]
- Paiè, P.; Martínez Vázquez, R.; Osellame, R.; Bragheri, F.; Bassi, A. Microfluidic based optical microscopes on chip. Cytometry A 2018, 93, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Sapuppo, F.; Llobera, A.; Schembri, F.; Intaglietta, M.; Cadarso, V.J.; Bucolo, M. A polymeric micro-optical interface for flow monitoring in biomicrofluidics. Biomicrofluidics 2010, 4, 024108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jagannadh, V.K.; Mackenzie, M.D.; Pal, P.; Kar, A.K.; Gorthi, S.S. Imaging flow cytometry with Femtosecond Laser-micromachined glass microfluidic channels. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 370–375. [Google Scholar] [CrossRef]
- Lange, D.; Storment, C.W.; Conley, C.A.; Kovacs, G.T.A. A microfluidic shadow imaging system for the study of the nematode caenorhabditis elegans in space. Sens. Actuat. B Chem. 2005, 107, 904–914. [Google Scholar] [CrossRef]
- Asghar, W.; Yuksekkaya, M.; Shafiee, H.; Zhang, M.; Ozen, M.O.; Inci, F.; Kocakulak, M.; Demirci, U. Engineering long shelf life multi-layer biologically active surfaces on microfluidic devices for point of care applications. Sci. Rep. 2016, 6, 21163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ceffa, N.G.; Bouzin, M.; D’Alfonso, L.; Sironi, L.; Marquezin, C.A.; Auricchio, F.; Marconi, S.; Chirico, G.; Collini, M. Spatiotemporal image correlation analysis for 3D flow field mapping in microfluidic devices. Anal. Chem. 2018, 90, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Travagliati, M.; Girardo, S.; Pisignano, D.; Beltram, F.; Cecchini, M. Easy monitoring of velocity fields in microfluidic devices using spatiotemporal image correlation spectroscopy. Anal. Chem. 2013, 85, 8080–8084. [Google Scholar] [CrossRef]
- Dee, F.R. Virtual microscopy in pathology education. Human Pathol. 2009, 40, 1112–1121. [Google Scholar] [CrossRef]
- Koch, L.H.; Lampros, J.N.; Delong, L.K.; Chen, S.C.; Woosley, J.T.; Hood, A.F. Randomized comparison of virtual microscopy and traditional glass microscopy in diagnostic accuracy among dermatology and pathology residents. Hum. Pathol. 2009, 40, 662–667. [Google Scholar] [CrossRef]
- van der Meer, A.D.; Orlova, V.V.; ten Dijke, P.; van den Berg, A.; Mummery, C.L. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 2013, 13, 3562–3568. [Google Scholar] [CrossRef]
- Peel, S.; Corrigan, A.M.; Ehrhardt, B.; Jang, K.-J.; Caetano-Pinto, P.; Boeckeler, M.; Rubins, J.E.; Kodella, K.; Petropolis, D.B.; Ronxhi, J.; et al. Introducing an automated high content confocal imaging approach for organs-on-chips. Lab Chip 2019, 19, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.N.; Kim, K.T.; Kim, C.; Teitell, M.A.; Zangle, T.A. Soft lithography fabrication of index-matched microfluidic devices for reducing artifacts in fluorescence and quantitative phase imaging. Microfluid. Nanofluid. 2017, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Ochs, C.J.; Kasuya, J.; Pavesi, A.; Kamm, R.D. Oxygen levels in thermoplastic microfluidic devices during cell culture. Lab Chip 2014, 14, 459–462. [Google Scholar] [CrossRef][Green Version]
- Weinhausen, B.; Köster, S. Microfluidic devices for X-ray studies on hydrated cells. Lab Chip 2013, 13, 212–215. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Zheng, G.; Lee, L.M. Optical imaging techniques in microfluidics and their applications. Lab Chip 2012, 12, 3566–3575. [Google Scholar] [CrossRef][Green Version]
- Jang, J.; Bae, C.Y.; Park, J.-K.; Ye, J.C. Self-reference quantitative phase microscopy for microfluidic devices. Opt. Lett. 2010, 35, 514. [Google Scholar] [CrossRef][Green Version]
- Paguirigan, A.; Beebe, D.J. Gelatin based microfluidic devices for cell culture. Lab Chip 2006, 6, 407–413. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef][Green Version]
- Bianco, V.; Paturzo, M.; Marchesano, V.; Gallotta, I.; Di Schiavi, E.; Ferraro, P. Optofluidic holographic microscopy with custom field of view (FOV) using a linear array detector. Lab Chip 2015, 15, 2117–2124. [Google Scholar] [CrossRef]
- Bishara, W.; Zhu, H.; Ozcan, A. Holographic opto-fluidic microscopy. Opt. Express 2010, 18, 27499. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Mandracchia, B.; Marchesano, V.; Pagliarulo, V.; Olivieri, F.; Coppola, S.; Paturzo, M.; Ferraro, P. Endowing a plain fluidic chip with micro-optics: A holographic microscope slide. Light Sci. Appl. 2017, 6, e17055. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Li, H.; Bashir, R. Real-time virus trapping and fluorescent imaging in microfluidic devices. Nano Lett. 2003, 4, 257–259. [Google Scholar] [CrossRef][Green Version]
- Barker, S.L.; Ross, D.; Tarlov, M.J.; Gaitan, M.; Locascio, L.E. Control of flow direction in microfluidic devices with polyelectrolyte multilayers. Anal. Chem. 2000, 72, 5925–5929. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.D.; Matthews, S.M.; Swartling, J.; Yunus, K.; Frank, J.H.; Brennan, C.M.; Fisher, A.C.; Kaminski, C.F. The application of frequency-domain fluorescence lifetime imaging microscopy as a quantitative analytical tool for microfluidic devices. Opt. Express 2006, 14, 5456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eteshola, E.; Balberg, M. Microfluidic ELISA: On-chip fluorescence imaging. Biomed. Microdev. 2004, 6, 7–9. [Google Scholar] [CrossRef]
- Gutierrez, E.; Petrich, B.G.; Shattil, S.J.; Ginsberg, M.H.; Groisman, A.; Kasirer-Friede, A. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 2008, 8, 1486–1495. [Google Scholar] [CrossRef][Green Version]
- Beckwitt, C.H.; Clark, A.M.; Wheeler, S.; Taylor, D.L.; Stolz, D.B.; Griffith, L.; Wells, A. Liver ‘organ on a chip’. Exp. Cell Res. 2018, 363, 15–25. [Google Scholar] [CrossRef][Green Version]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2018, 18, 115–125. [Google Scholar] [CrossRef]
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.-T.; Ertl, P. Multi-layered, membrane-integrated microfluidics based on replica molding of a thiol–ene epoxy thermoset for organ-on-a-chip applications. Lab Chip 2015, 15, 4542–4554. [Google Scholar] [CrossRef]
- Kobayashi, H.; Lei, C.; Wu, Y.; Mao, A.; Jiang, Y.; Guo, B.; Ozeki, Y.; Goda, K. Label-free detection of cellular drug responses by high-throughput bright-field imaging and machine learning. Sci. Rep. 2017, 7, 12454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin-Fernandez, M.; Clarke, D. Single Molecule fluorescence detection and tracking in mammalian cells: The state-of-the-art and future perspectives. Int. J. Mol. Sci. 2012, 13, 14742–14765. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Nagano, T. Fluorescent probes for sensing and imaging. Nat. Meth. 2011, 8, 642–645. [Google Scholar] [CrossRef]
- Cullander, C. Fluorescent probes for confocal microscopy. In Confocal Microscopy: Methods and Protocols; Paddock, S.W., Ed.; Springer: New York, NY, USA, 1999; pp. 59–73. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Gnanasammandhan, M.K.; Zhang, Y. Small upconverting fluorescent nanoparticles for biomedical applications. Small 2010, 6, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Scharff-Poulsen, A.M.; Gu, H.; Almdal, K. Synthesis and characterization of ratiometric, pH sensing nanoparticles with covalently attached fluorescent dyes. Chem. Mater. 2006, 18, 3381–3384. [Google Scholar] [CrossRef]
- Vujanovic, S.; Goh, Y.K.; Vujanovic, V. Natural fruit extracts as non-toxic fluorescent dyes for staining fungal chlamydospores. World J. Microbiol. Biotechnol. 2011, 28, 387–390. [Google Scholar] [CrossRef]
- Ichiyanagi, M.; Sakai, K.; Kidani, S.; Kakinuma, Y.; Sato, Y.; Hishida, K. Evaluation methodology of gas permeable characterization in a polymer-based microfluidic device by confocal fluorescence imaging. J. Micromech. Microeng. 2012, 22, 065023. [Google Scholar] [CrossRef]
- Kuricheti, K.K.; Buschmann, V.; Brister, P.; Weston, K.D. Velocity imaging in microfluidic devices using fluorescence correlation spectroscopy. Proc. SPIE 2004, 5345, 194–205. [Google Scholar] [CrossRef]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef]
- Sagar, D.M.; Aoudjane, S.; Gaudet, M.; Aeppli, G.; Dalby, P.A. Optically induced thermal gradients for protein characterization in nanolitre-scale samples in microfluidic devices. Sci. Rep. 2013, 3, 2130. [Google Scholar] [CrossRef][Green Version]
- Simonnet, C.; Groisman, A. High-throughput and high-resolution flow cytometry in molded microfluidic devices. Anal. Chem. 2006, 78, 5653–5663. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Fukuyama, H.; Ishihara, Y.; Tanaka, H.; Takamatsu, T. In situ fluorescence imaging of organs through compact scanning head for confocal laser microscopy. J. Biomed. Opt. 2005, 10, 024010. [Google Scholar] [CrossRef] [PubMed]
- Diaspro, A.; Chirico, G.; Usai, C.; Ramoino, P.; Dobrucki, J. Photobleaching. In Handbook of Biological Confocal Microscopy; Pawley, J., Ed.; Springer: Boston, MA, USA, 2006; pp. 690–702. [Google Scholar] [CrossRef]
- Kuang, C.; Luo, D.; Liu, X.; Wang, G. Study on factors enhancing photobleaching effect of fluorescent dye. Measurement 2013, 46, 1393–1398. [Google Scholar] [CrossRef]
- Mosier, B.; Molho, J.; Santiago, J. Photobleached-fluorescence imaging of microflows. Exp. Fluids 2002, 33, 545–554. [Google Scholar] [CrossRef]
- Dean, K.M.; Lubbeck, J.L.; Davis, L.M.; Regmi, C.K.; Chapagain, P.P.; Gerstman, B.S.; Jimenez, R.; Palmer, A.E. Microfluidics-based selection of red-fluorescent proteins with decreased rates of photobleaching. Integr. Biol. 2015, 7, 263–273. [Google Scholar] [CrossRef][Green Version]
- Wu, H.-M.; Lee, T.-A.; Ko, P.-L.; Liao, W.-H.; Hsieh, T.-H.; Tung, Y.-C. Widefield frequency domain fluorescence lifetime imaging microscopy (FD-flim) for accurate measurement of oxygen gradients within microfluidic devices. Analyst 2019, 144, 3494–3504. [Google Scholar] [CrossRef]
- Lee, J.; Miyanaga, Y.; Ueda, M.; Hohng, S. Video-rate confocal microscopy for single-molecule imaging in live cells and superresolution fluorescence imaging. Biophys. J. 2012, 103, 1691–1697. [Google Scholar] [CrossRef][Green Version]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef][Green Version]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef][Green Version]
- Vidi, P.-A.; Maleki, T.; Ochoa, M.; Wang, L.; Clark, S.M.; Leary, J.F.; Lelièvre, S.A. Disease-on-a-chip: Mimicry of tumor growth in mammary ducts. Lab Chip 2014, 14, 172–177. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Albert-Smet, I.; Marcos-Vidal, A.; Vaquero, J.J.; Desco, M.; Muñoz-Barrutia, A.; Ripoll, J. Applications of light-sheet microscopy in microdevices. Front. Neuroanat. 2019, 13, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gualda, E.J.; Pereira, H.; Martins, G.G.; Gardner, R.; Moreno, N. Three-dimensional imaging flow cytometry through light-sheet fluorescence microscopy. Cytometry A 2017, 91, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhu, T.; Zhang, H.; Nie, J.; Guan, Z.; Ho, C.-M.; Liu, S.; Fei, P. Droplet-based light-sheet fluorescence microscopy for high-throughput sample preparation, 3-D imaging and quantitative analysis on a chip. Lab Chip 2017, 17, 2193–2197. [Google Scholar] [CrossRef]
- Memeo, R.; Paiè, P.; Sala, F.; Castriotta, M.; Guercio, C.; Vaccari, T.; Osellame, R.; Bassi, A.; Bragheri, F. Automatic imaging of Drosophila embryos with light sheet fluorescence microscopy on chip. J. Biophoton. 2020, 14, e20200396. [Google Scholar] [CrossRef]
- Power, R.M.; Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Meth. 2017, 14, 360–373. [Google Scholar] [CrossRef]
- Vargas-Ordaz, E.J.; Gorelick, S.; York, H.M.; Liu, B.; Halls, M.L.; Arumugam, S.; Neild, A.; de Marco, A.; Cadarso, V.J. Three-dimensional imaging on a chip using optofluidics light-sheet fluorescence microscopy. Lab Chip 2021, 21, 2945–2954. [Google Scholar] [CrossRef]
- von Wangenheim, D.; Hauschild, R.; Friml, J. Light sheet fluorescence microscopy of plant roots growing on the surface of a gel. J. Vis. Exp. 2017, 119, e55044. [Google Scholar] [CrossRef][Green Version]
- Escobet-Montalbán, A.; Gasparoli, F.M.; Nylk, J.; Liu, P.; Yang, Z.; Dholakia, K. Three-photon light-sheet fluorescence microscopy. Opt. Lett. 2018, 43, 5484. [Google Scholar] [CrossRef][Green Version]
- Icha, J.; Schmied, C.; Sidhaye, J.; Tomancak, P.; Preibisch, S.; Norden, C. Using light sheet fluorescence microscopy to image zebrafish eye development. J. Vis. Exp. 2016, 110, e53966. [Google Scholar] [CrossRef][Green Version]
- Prahst, C.; Ashrafzadeh, P.; Mead, T.; Figueiredo, A.; Chang, K.; Richardson, D.; Venkaraman, L.; Richards, M.; Russo, A.M.; Harrington, K.; et al. Mouse retinal cell behaviour in space and time using light sheet fluorescence microscopy. eLife 2020, 9, e49779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ricci, P.; Gavryusev, V.; Müllenbroich, C.; Turrini, L.; de Vito, G.; Silvestri, L.; Sancataldo, G.; Pavone, F.S. Removing striping artifacts in light-sheet fluorescence microscopy: A review. Prog. Biophys. Mol. Biol. 2021, 168, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, E.H. Light-sheet fluorescence microscopy for quantitative biology. Nat. Meth. 2014, 12, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, E.H.; Strobl, F.; Chang, B.-J.; Preusser, F.; Preibisch, S.; McDole, K.; Fiolka, R. Light sheet fluorescence microscopy. Nat. Rev. Meth. Primers 2021, 1, 73. [Google Scholar] [CrossRef]
- Li, C.; Moatti, A.; Zhang, X.; Troy Ghashghaei, H.; Greenbaum, A. Deep learning-based autofocus method enhances image quality in light-sheet fluorescence microscopy. Biomed. Opt. Express 2021, 12, 5214. [Google Scholar] [CrossRef]
- Logan, S.L.; Dudley, C.; Baker, R.P.; Taormina, M.J.; Hay, E.A.; Parthasarathy, R. Automated high-throughput light-sheet fluorescence microscopy of larval zebrafish. PLoS ONE 2018, 13, e0198705. [Google Scholar] [CrossRef][Green Version]
- Sala, F.; Castriotta, M.; Paiè, P.; Farina, A.; D’Annunzio, S.; Zippo, A.; Osellame, R.; Bragheri, F.; Bassi, A. High-throughput 3D imaging of single cells with light-sheet fluorescence microscopy on chip. Biomed. Opt. Express 2020, 11, 4397–4407. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chen, K.-H.; Lin, C.-Y.; Yeh, Y.-C. Rapidly and simultaneously quantifying multiple biomarkers of L-tyrosine hydroxylase deficiency by using paper microfluidic devices and smartphone-based analysis system. Sens. Actuat. B Chem. 2021, 349, 130722. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Su, Y.; Chen, H. Recent progress in optical biosensors based on smartphone platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Kim, K.C.; Yeom, E. Microfluidic method for measuring viscosity using images from smartphone. Opt. Lasers Eng. 2018, 104, 237–243. [Google Scholar] [CrossRef]
- Needs, S.H.; Osborn, H.M.I.; Edwards, A.D. Counting bacteria in microfluidic devices: Smartphone compatible ‘dip-and-test’ viable cell quantitation using resazurin amplified detection in microliter capillary arrays. J. Microbiol. Meth. 2021, 187, 106199. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Peretz-Soroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kong, T.; You, J.B.; Zhang, B.; Nguyen, B.; Tarlan, F.; Jarvi, K.; Sinton, D. Accessory-free quantitative smartphone imaging of colorimetric paper-based assays. Lab Chip 2019, 19, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Li, A.; Li, B.; Liu, J.; Pan, L. A microfluidic colorimetric immunoassay for sensitive detection of altenariol monomethyl ether by UV spectroscopy and smart phone imaging. Anal. Chim. Acta 2019, 1092, 75–84. [Google Scholar] [CrossRef]
- McGonigle, A.; Wilkes, T.; Pering, T.; Willmott, J.; Cook, J.; Mims, F.; Parisi, A. Smartphone spectrometers. Sensors 2018, 18, 223. [Google Scholar] [CrossRef][Green Version]
- Stuart, M.B.; McGonigle, A.J.; Davies, M.; Hobbs, M.J.; Boone, N.A.; Stanger, L.R.; Zhu, C.; Pering, T.D.; Willmott, J.R. Low-cost hyperspectral imaging with a smartphone. J. Imaging 2021, 7, 136. [Google Scholar] [CrossRef]
- Wintergerst, M.W.; Petrak, M.; Li, J.Q.; Larsen, P.P.; Berger, M.; Holz, F.G.; Finger, R.P.; Krohne, T.U. Non-contact smartphone-based fundus imaging compared to conventional fundus imaging: A low-cost alternative for retinopathy of prematurity screening and documentation. Sci. Rep. 2019, 9, 19711. [Google Scholar] [CrossRef][Green Version]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.-L.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-based hand-held microplate reader for point-of-care testing of enzyme-linked immunosorbent assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef]
- Chen, G.; Chai, H.H.; Yu, L.; Fang, C. Smartphone supported backlight illumination and image acquisition for microfluidic-based point-of-care testing. Biomed. Opt. Express 2018, 9, 4604. [Google Scholar] [CrossRef]
- Paterson, A.S.; Raja, B.; Mandadi, V.; Townsend, B.; Lee, M.; Buell, A.; Vu, B.; Brgoch, J.; Willson, R.C. A low-cost smartphone-based platform for highly sensitive point-of-care testing with persistent luminescent phosphors. Lab Chip 2017, 17, 1051–1059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Potluri, V.; Kathiresan, P.S.; Kandula, H.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Kota Sai Pavan, S.; Yarravarapu, D.; Soundararajan, A.; Baskar, K.; Gupta, R.; et al. An inexpensive smartphone-based device for point-of-care ovulation testing. Lab Chip 2019, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Stedtfeld, R.D.; Tourlousse, D.M.; Seyrig, G.; Stedtfeld, T.M.; Kronlein, M.; Price, S.; Ahmad, F.; Gulari, E.; Tiedje, J.M.; Hashsham, S.A. Gene-Z: A device for point of care genetic testing using a smartphone. Lab Chip 2012, 12, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.R.; Erikson, R.L.; Sheen, A.M.; Ozanich, R.M.; Kelly, R.T. Reagent-free and portable detection of bacillus anthracis spores using a microfluidic incubator and smartphone microscope. Analyst 2015, 140, 6269–6276. [Google Scholar] [CrossRef] [PubMed]
- Sami, M.A.; Tayyab, M.; Parikh, P.; Govindaraju, H.; Hassan, U. A modular microscopic smartphone attachment for imaging and quantification of multiple fluorescent probes using machine learning. Analyst 2021, 146, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Yafia, M.; Ahmadi, A.; Hoorfar, M.; Najjaran, H. Ultra-portable smartphone controlled integrated digital microfluidic system in a 3D-printed modular assembly. Micromachines 2015, 6, 1289–1305. [Google Scholar] [CrossRef]
- Zhu, H.; Ozcan, A. Wide-field fluorescent microscopy and fluorescent imaging flow cytometry on a cell-phone. J. Vis. Exp. 2013, 74, e50451. [Google Scholar] [CrossRef]
- Navruz, I.; Coskun, A.F.; Wong, J.; Mohammad, S.; Tseng, D.; Nagi, R.; Phillips, S.; Ozcan, A. Smart-phone based computational microscopy using multi-frame contact imaging on a fiber-optic array. Lab Chip 2013, 13, 4015–4023. [Google Scholar] [CrossRef]
- Meng, X.; Huang, H.; Yan, K.; Tian, X.; Yu, W.; Cui, H.; Kong, Y.; Xue, L.; Liu, C.; Wang, S. Smartphone based hand-held quantitative phase microscope using the transport of intensity equation method. Lab Chip 2017, 17, 104–109. [Google Scholar] [CrossRef]
- Yang, Z.; Zhan, Q. Single-shot smartphone-based quantitative phase imaging using a distorted grating. PLoS ONE 2016, 11, e0159596. [Google Scholar] [CrossRef] [PubMed]
- Diederich, B.; Lachmann, R.; Carlstedt, S.; Marsikova, B.; Wang, H.; Uwurukundo, X.; Mosig, A.S.; Heintzmann, R. A versatile and customizable low-cost 3D-printed open standard for microscopic imaging. Nat. Commun. 2020, 11, 5979. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.N.; Jian, H.; Lo, N.W.; Ip, M.; Chan, M.M.; Kam, K.M.; Wu, X. Low cost automated whole smear microscopy screening system for detection of acid fast bacilli. PLoS ONE 2018, 13, e0190988. [Google Scholar] [CrossRef]
- Lee, S.A.; Yang, C. A smartphone-based chip-scale microscope using ambient illumination. Lab Chip 2014, 14, 3056–3063. [Google Scholar] [CrossRef]
- Pirnstill, C.W.; Coté, G.L. Malaria diagnosis using a mobile phone polarized microscope. Sci. Rep. 2015, 5, 13368. [Google Scholar] [CrossRef][Green Version]
- Tseng, D.; Mudanyali, O.; Oztoprak, C.; Isikman, S.O.; Sencan, I.; Yaglidere, O.; Ozcan, A. Lensfree microscopy on a cellphone. Lab Chip 2010, 10, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Tian, J.; Cao, R.; Li, M.; Cai, Z.; Shen, W. Barcode-like paper sensor for smartphone diagnostics: An application of blood typing. Anal. Chem. 2014, 86, 11362–11367. [Google Scholar] [CrossRef]
- Takehara, H.; Kazutaka, O.; Haruta, M.; Noda, T.; Sasagawa, K.; Tokuda, T.; Ohta, J. On-chip cell analysis platform: Implementation of contact fluorescence microscopy in microfluidic chips. AIP Adv. 2017, 7, 095213. [Google Scholar] [CrossRef][Green Version]
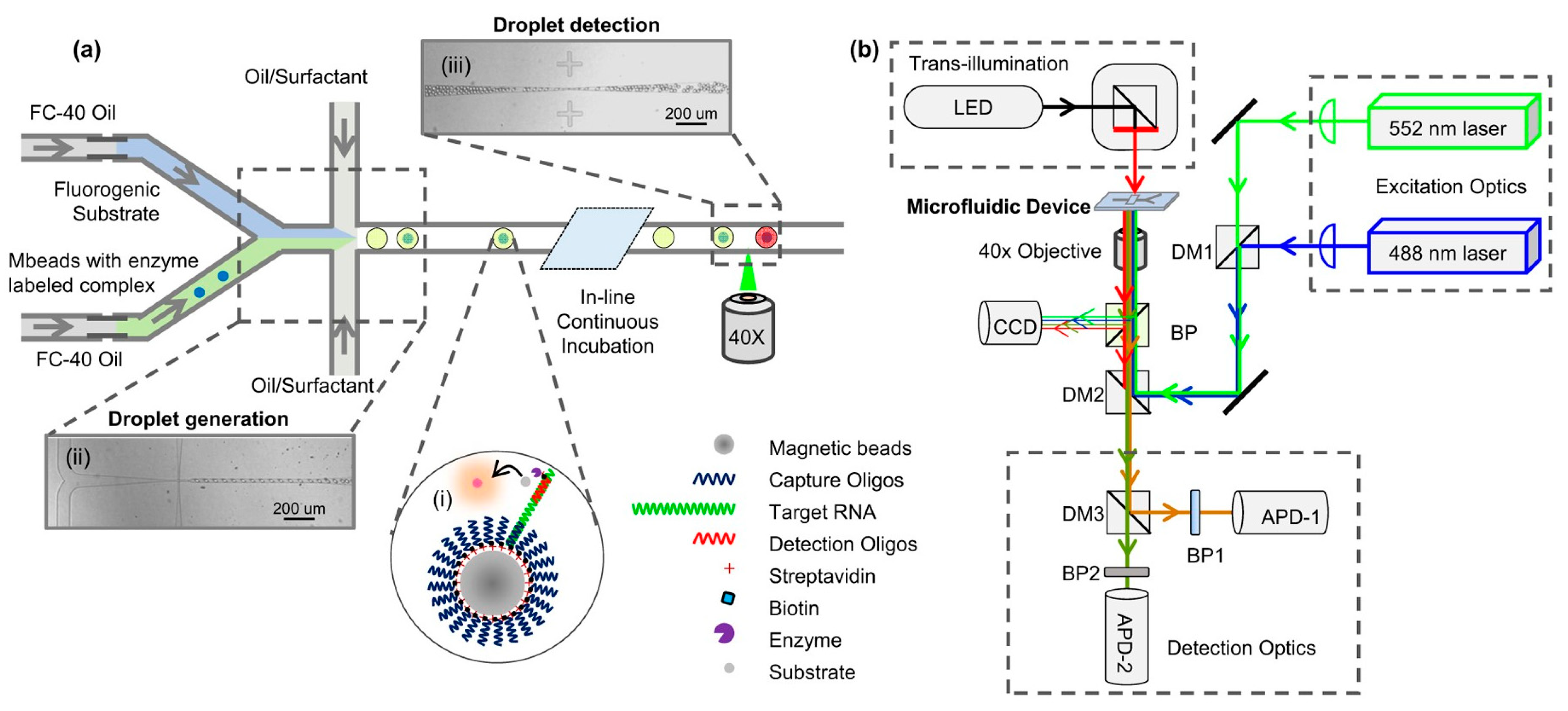
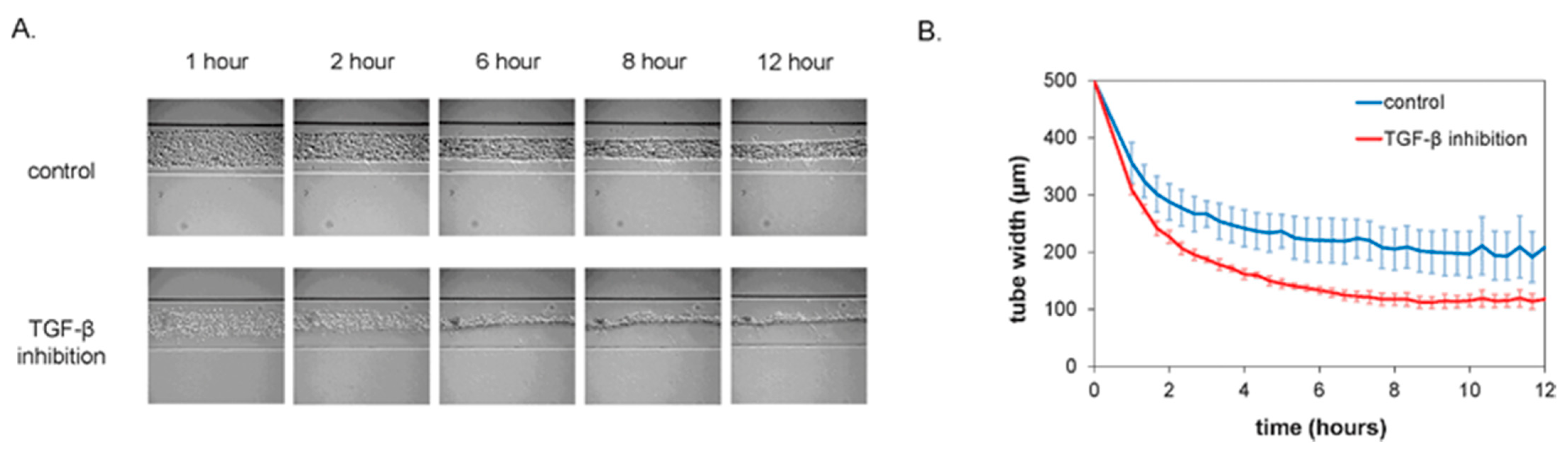


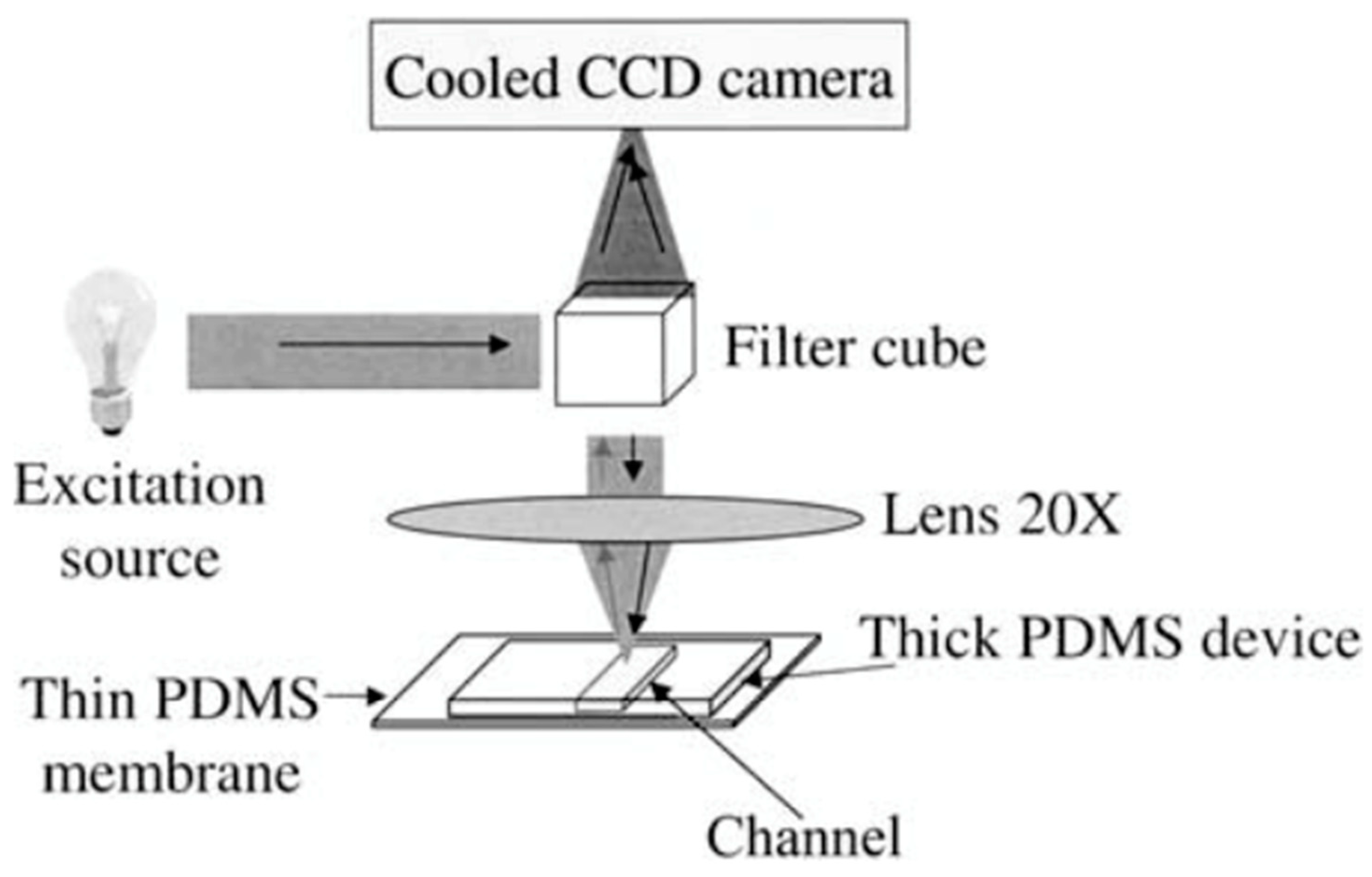
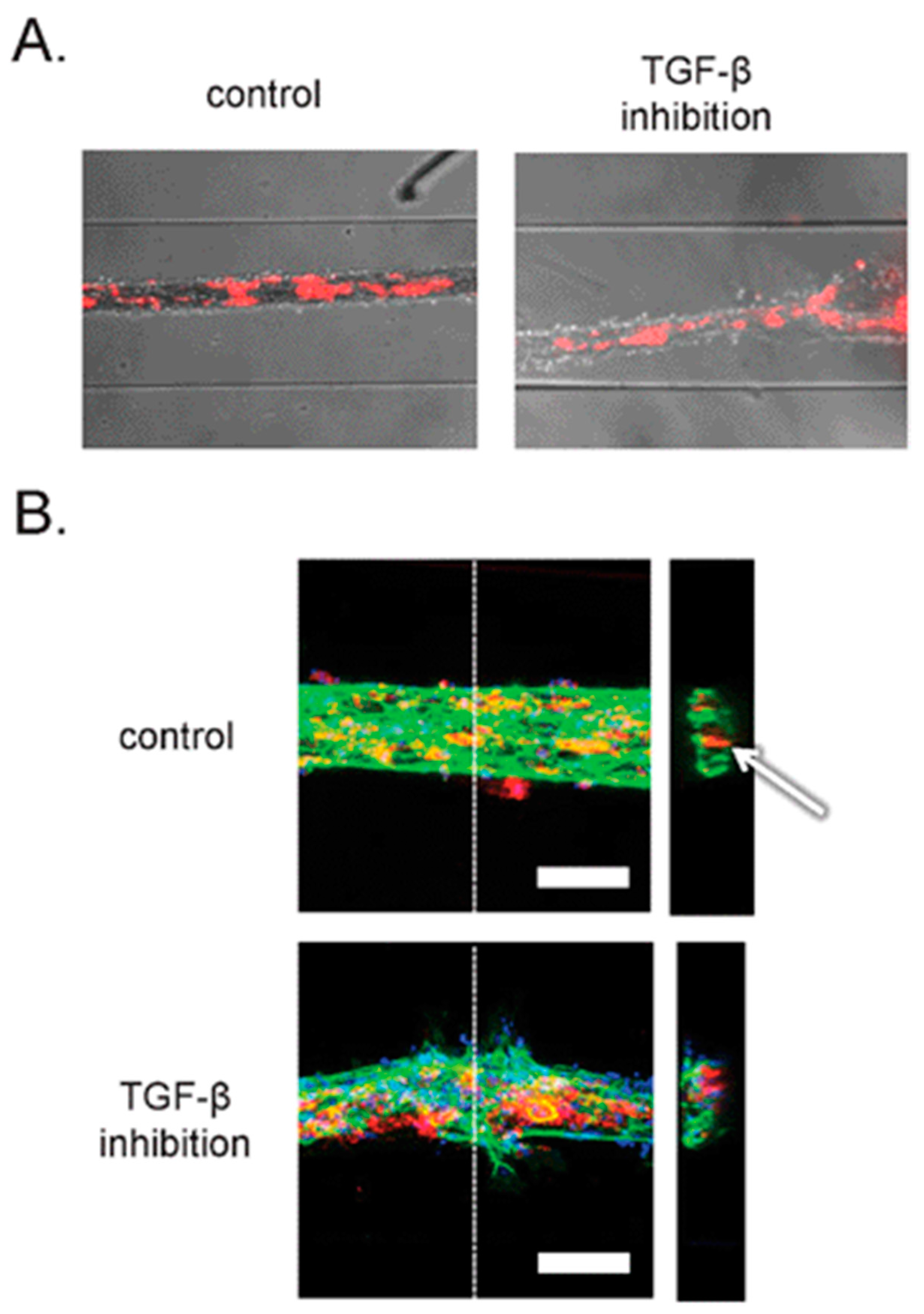


| Method | Sub-Method | Strengths for Microfluidic Devices | Weaknesses for Microfluidic Devices | Strengths for OOCs | Weaknesses for OOCs | Ref. |
|---|---|---|---|---|---|---|
| Transillumination | Brightfield | Ability to automatically count cells | Blur in images; need for traditional microscope; less cost-effective; not transportable | Preliminary method before higher resolution imaging | Low resolution | [26,27,30,31,32,33] |
| Phase-contrast | Extended field depth; Ability to measure phase changes | Low resolution | Ability to measure phase and morphological changes | Low resolution | [39,40] | |
| Holographic optofluidic | Phase images can be obtained; Allow portability to device | High signal-to-noise ratio; complicated; decent but not the best resolution | None at this time | Not currently demonstrated in OOC platforms | [42,43] | |
| Fluorescence | Confocal | High resolution | Photobleaching; phototoxicity; not label-free | High resolution; ability to measure cell–cell interactions | Photobleaching; phototoxicity; not label-free | [32,33,45,46,47,48,49,66] |
| LSFM | High acquisition rate; reduced phototoxicity | Some phototoxicity still exists | High acquisition rate; reduced phototoxicity | Some phototoxicity still exists | [23,81,83,84,85,86,87,88,91] | |
| Smartphone-based | Microscope attachment-based | Low-cost; portability | Compromised resolution compared to benchtop microscopy | Low cost; in situ monitoring | Compromised resolution compared to benchtop microscopy | [4,111] |
| Quantitative phase microscopy | Ability to measure phase and morphological changes; label-free; high resolution; reduced cost | Only suitable for imaging phase changes | Ability to measure phase and morphological changes; label-free | Focus drift can occur | [114,115] | |
| Lens-free microscopy | Reduced cost and size; high resolution | Slow processing speed | Low cost; can monitor cell activity in response to biomolecules | Lower resolution if image sensor is not close to cells | [118,120,122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchanan, B.C.; Yoon, J.-Y. Microscopic Imaging Methods for Organ-on-a-Chip Platforms. Micromachines 2022, 13, 328. https://doi.org/10.3390/mi13020328
Buchanan BC, Yoon J-Y. Microscopic Imaging Methods for Organ-on-a-Chip Platforms. Micromachines. 2022; 13(2):328. https://doi.org/10.3390/mi13020328
Chicago/Turabian StyleBuchanan, Bailey C., and Jeong-Yeol Yoon. 2022. "Microscopic Imaging Methods for Organ-on-a-Chip Platforms" Micromachines 13, no. 2: 328. https://doi.org/10.3390/mi13020328
APA StyleBuchanan, B. C., & Yoon, J.-Y. (2022). Microscopic Imaging Methods for Organ-on-a-Chip Platforms. Micromachines, 13(2), 328. https://doi.org/10.3390/mi13020328







