Langasite Bonding via High Temperature for Fabricating Sealed Microcavity of Pressure Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
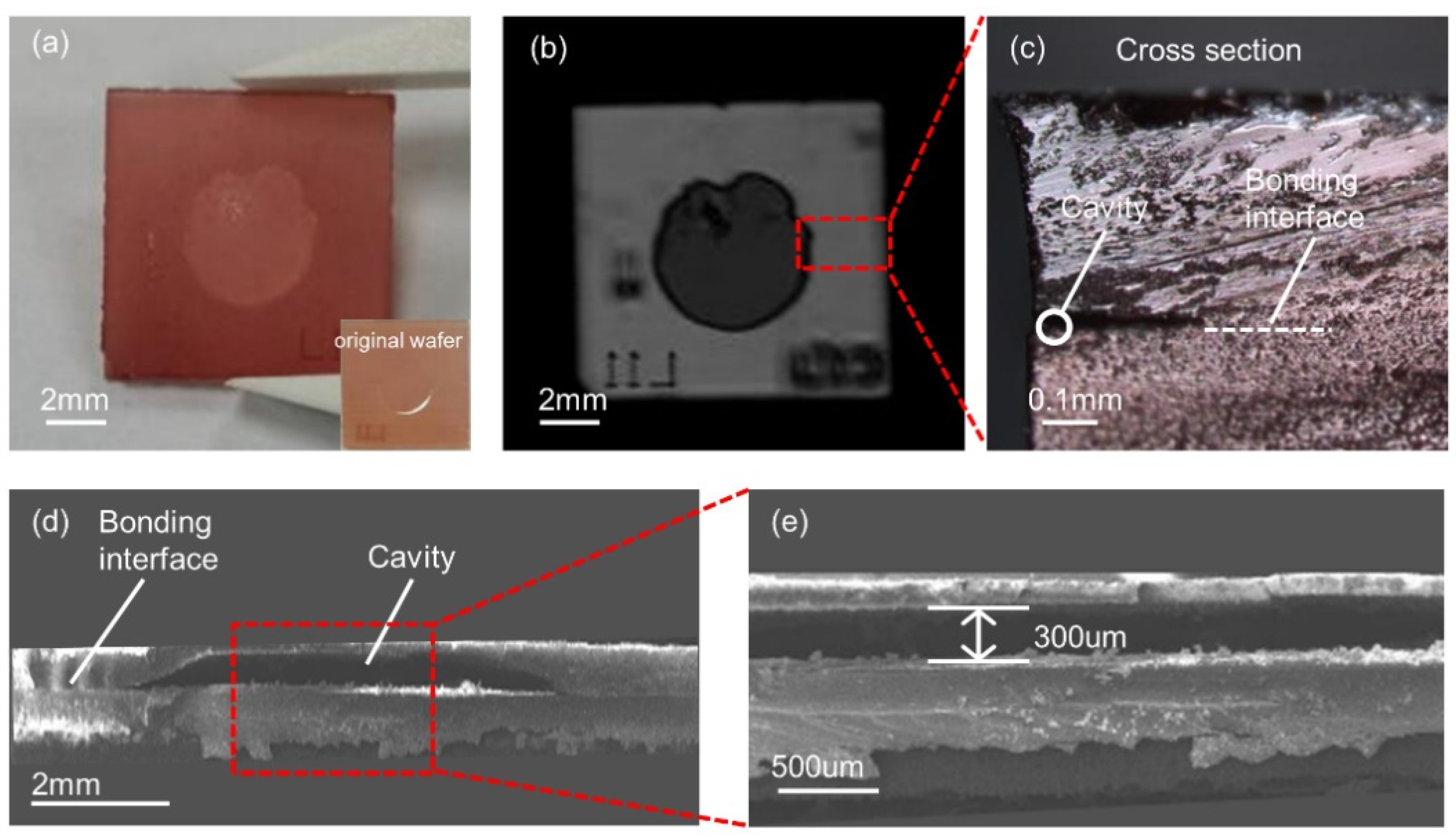
3.1. Bonding Interface Observation
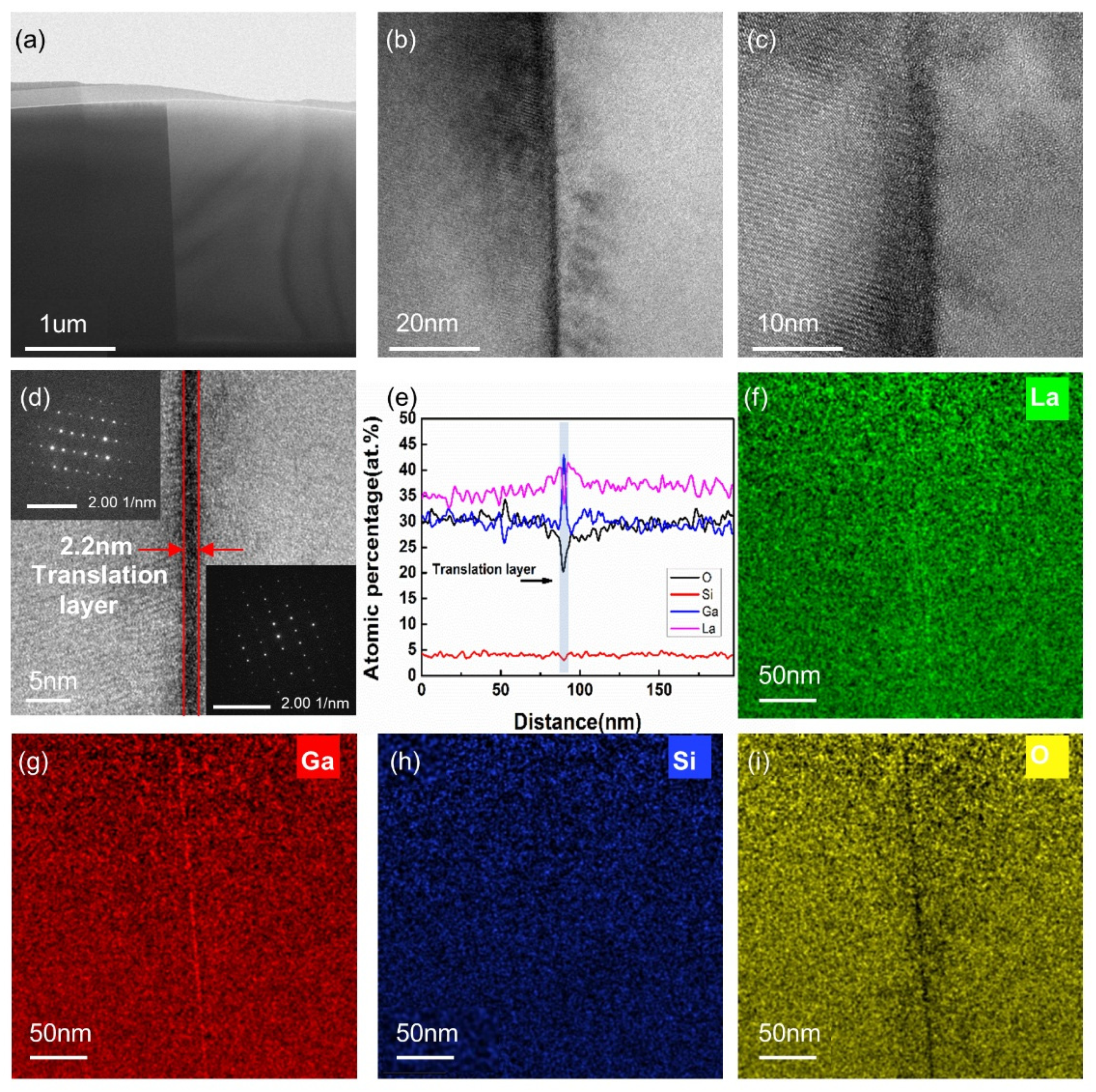
3.2. TEM Analysis of Bonding Interface
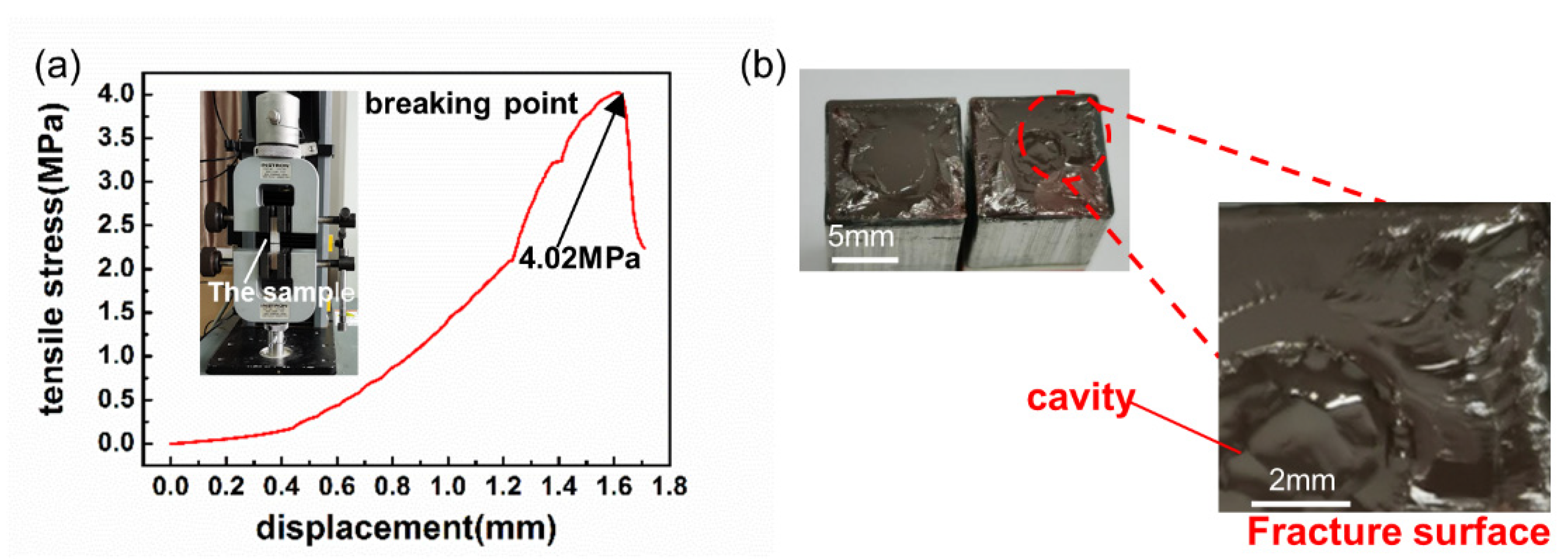
3.3. Bonding Strength
3.4. Seal Performance
3.5. Piezoelectric Property
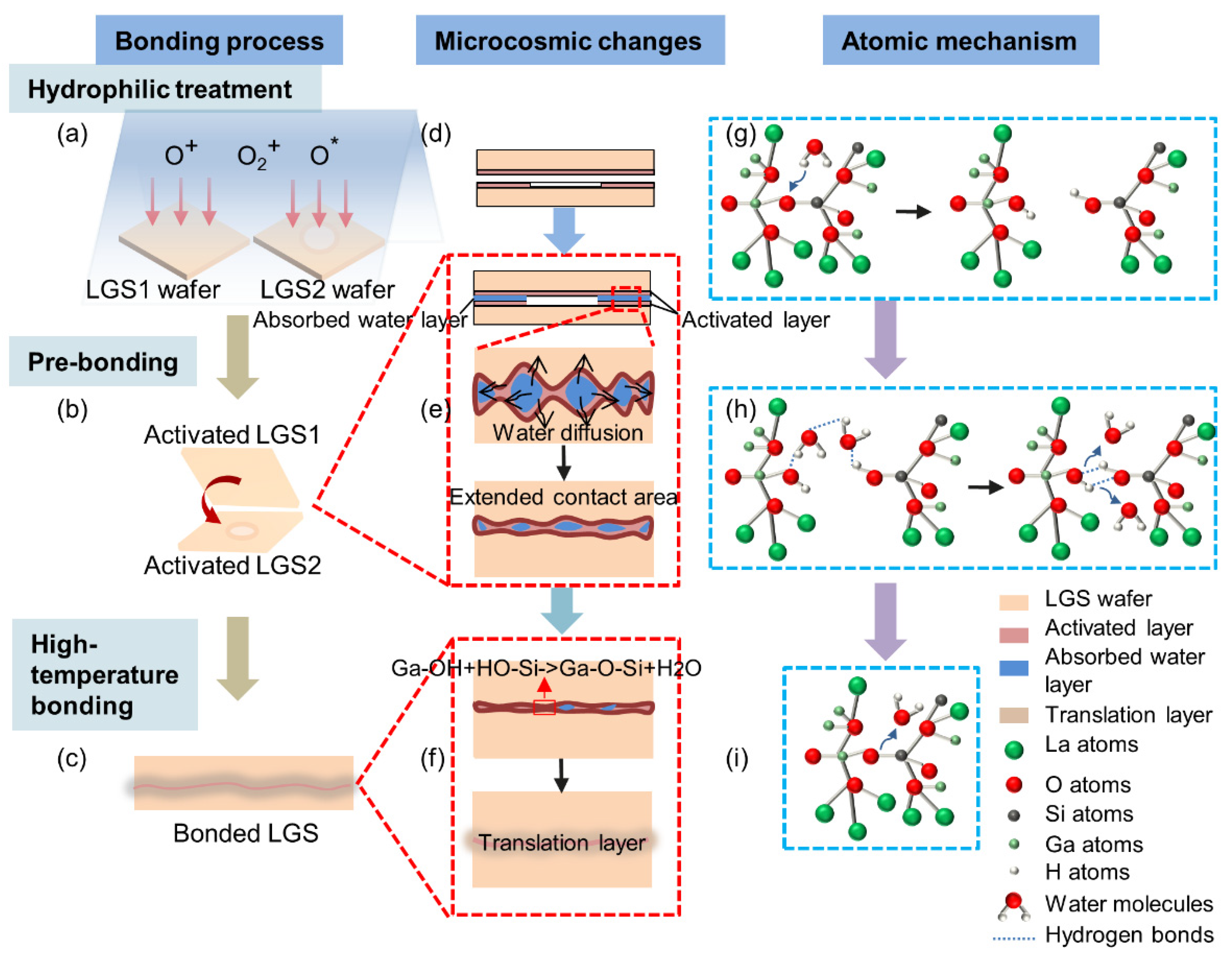
3.6. Bonding Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tortissier, G.; Blanc, L.; Tetelin, A.; Lachaud, J.L.; Benoit, M.; Conédéra, V.; Dejous, C.; Rebière, D. Langasite based surface acoustic wave sensors for high temperature chemical detection in harsh environment: Design of the transducers and packaging. Sens. Actuators B Chem. 2011, 156, 510–516. [Google Scholar] [CrossRef]
- Da Cunha, M.P.; Maskay, A.; Lad, R.J.; Coyle, T.; Harkay, G. Langasite 2. 45 GHz ISM band SAW resonator for harsh environment wireless applications. In Proceedings of the IEEE International Ultrasonics Symposium, Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Ansorge, E.; Schmidt, B.; Sauerwald, J.; Fritze, H. Langasite for microelectromechanical systems. Phys. Status Solidi Appl. Mater. Sci. 2011, 208, 377–389. [Google Scholar] [CrossRef]
- Chvets, V.B.; Ivanov, P.G.; Makarov, V.M.; Orlov, V.S. Design of SAW filters on langasite. In Proceedings of the IEEE Ultrasonics Symposium, Tahoe, NV, USA, 17–20 October 1999; Volume 1, pp. 295–299. [Google Scholar]
- Hornsteiner, J.; Born, E.; Fischerauer, G.; Riha, E. Surface acoustic wave sensors for high-temperature applications. In Proceedings of the Annual IEEE International Frequency Control Symposium, Pasadena, CA, USA, 27–29 May 1998; pp. 615–620. [Google Scholar]
- Zhang, S.; Yu, F. Piezoelectric materials for high temperature sensors. J. Am. Ceram. Soc. 2011, 94, 3153–3170. [Google Scholar] [CrossRef]
- Damjanovic, D. Materials for high temperature piezoelectric transducers. Curr. Opin. Solid State Mater. Sci. 1998, 3, 469–473. [Google Scholar] [CrossRef]
- Hornsteiner, J.; Born, E.; Riha, E. Langasite for high temperature surface acoustic wave applications. Phys. Status Solidi Appl. Res. 1997, 163, R3–R4. [Google Scholar] [CrossRef]
- Da Cunha, M.P.; Lad, R.J.; Moonlight, T.; Bernhardt, G.; Frankel, D.J. High temperature stability of langasite surface acoustic wave devices. In Proceedings of the IEEE Ultrasonics Symposium, Beijing, China, 2–5 November 2008; pp. 205–208. [Google Scholar]
- Chen, J.B.; Fang, Q.H.; Wang, C.C.; Du, J.K.; Liu, F. Theoretical study on brittle–ductile transition behavior in elliptical ultrasonic assisted grinding of hard brittle materials. Precis. Eng. 2016, 46, 104–117. [Google Scholar] [CrossRef]
- Ansorge, E.; Schimpf, S.; Hirsch, S.; Schmidt, B.; Sauerwald, J.; Fritze, H. Microelectromechanical structures in langasite (La3Ga 5SiO14) by wet chemical etching. In Proceedings of the Digest of Technical Papers-International Conference on Solid State Sensors and Actuators and Microsystems, TRANSDUCERS ’05, Seoul, Korea, 5–9 June 2005; Volume 1, pp. 908–911. [Google Scholar]
- Ansorge, E.; Schimpf, S.; Hirsch, S.; Schmidt, B.; Sauerwald, J.; Fritze, H. Gas detecting langasite membranes by wet chemical etching. In Proceedings of the IEEE Sensors, Irvine, CA, USA, 30 October–3 November 2005; Volume 2005, pp. 235–238. [Google Scholar]
- Cho, H.; Pearton, S.J. Removal of chemical–mechanical polishing-induced damage layer in single crystal La3Ga5SiO14by inductively coupled plasma etching. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2005, 23, 2236. [Google Scholar] [CrossRef]
- Lee, D.M.; Hwang, S.; Lee, B.W.; Shim, K.B.; Pearton, S.J.; Cho, H. Post-CMP dry etching for the removal of the nanoscale subsurface damage layer from a single crystal La3Ga5SiO14 for a high quality wide band SAW filter device. J. Ceram. Process. Res. 2007, 8, 98–102. [Google Scholar]
- Laffey, S.; Hendrickson, M.; Vig, J.R. Polishing and etching langasite and quartz crystals. In Proceedings of the IEEE 48th Annual Symposium on Frequency Control, Boston, MA, USA, 1–3 June 2002; pp. 245–250. [Google Scholar]
- Takeda, H.; Okamura, S.; Shiosaki, T. Chemical etching of lanthanum gallium silicate La3Ga5SiO14 single crystals. J. Mater. Sci. Lett. 2002, 21, 1117–1119. [Google Scholar] [CrossRef]
- Shu, L.; Peng, B.; Cui, Y.; Gong, D.; Yang, Z.; Liu, X.; Zhang, W. Effects of ALN coating layer on high temperature characteristics of langasite SAW sensors. Sensors 2016, 16, 1436. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Xue, T.; Liang, X.; Tan, Q. High-temperature direct bonding of langasite using oxygen plasma activation. Scr. Mater. 2021, 194, 113681. [Google Scholar] [CrossRef]
- Schmidt, M.A. Wafer-to-wafer bonding for microstructure formation. Proc. IEEE 1998, 86, 1575–1585. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Ejeckam, F.E.; Qian, Y.; Zhang, J.; Zhang, Z.; Christenson, G.L.; Lo, Y.H. Wafer bonding technology and its applications in optoelectronic devices and materials. IEEE J. Sel. Top. Quantum Electron. 1997, 3, 927–936. [Google Scholar] [CrossRef]
- Fan, J.; Lim, D.F.; Peng, L.; Li, K.H.; Tan, C.S. Low temperature Cu-to-Cu bonding for wafer-level hermetic encapsulation of 3D microsystems. Electrochem. Solid State Lett. 2011, 14, H470–H474. [Google Scholar] [CrossRef]
- Kühne, S.; Hierold, C. Wafer-level packaging and direct interconnection technology based on hybrid bonding and through silicon vias. J. Micromech. Microeng. 2011, 21, 85032. [Google Scholar] [CrossRef]
- Esch, J. Wafer direct bonding: From advance substrate engineering to future applications in micro/nanoelectronics. Proc. IEEE 2006, 94, 2058–2059. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Behanan, R.; Lad, R.J.; Da Cunha, M.P. Langasite SAW pressure sensor for harsh environments. In Proceedings of the IEEE International Ultrasonics Symposium, IUS, Dresden, Germany, 7–10 October 2012; pp. 1224–1227. [Google Scholar]
- Han, T.; Ji, X.; Shi, W. Langasite membranes for surface acoustic wave pressure sensors. Sens. Mater. 2006, 18, 173–181. [Google Scholar]
- Maskay, A.; Pereira da Cunha, M. High-temperature static strain langasite SAWR sensor: Temperature compensation and numerical calibration for direct strain reading. Sens. Actuators A Phys. 2017, 259, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Peng, B.; Zhu, J.; He, Z.; Yang, Y.; Zhang, W. Strain measurements with langasite SAW resonators at high temperature. IEEE Sens. J. 2021, 21, 4688–4695. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, C.; Niu, F.; Zhou, S.; Xu, J.; Tian, Y. Single-crystalline SiC integrated onto Si-based substrates via plasma-activated direct bonding. Ceram. Int. 2020, 46, 22718–22726. [Google Scholar] [CrossRef]
- Chung, T.R.; Yang, L.; Hosoda, N.; Takagi, H.; Suga, T. Wafer direct bonding of compound semiconductors and silicon at room temperature by the surface activated bonding method. Appl. Surf. Sci. 1997, 117–118, 808–812. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Li, D.; Cheng, J.; Wang, Y.; Hang, C.; Tian, Y. Fabrication of SiC/Si, SiC/SiO2, and SiC/glass heterostructures via VUV/O3 activated direct bonding at low temperature. Ceram. Int. 2019, 45, 4094–4098. [Google Scholar] [CrossRef]
- Xu, Y.; Mu, F.; Wang, Y.; Chen, D.; Ou, X.; Suga, T. Direct wafer bonding of Ga2O3–SiC at room temperature. Ceram. Int. 2019, 45, 6552–6555. [Google Scholar] [CrossRef]
- Chung, T.R.; Yang, L.; Hosoda, N.; Suga, T. Room temperature GaAs-Si and InP-Si wafer direct bonding by the surface activated bonding method. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1997, 121, 203–206. [Google Scholar] [CrossRef]
- Tomita, Y.; Sugimoto, M.; Eda, K. Direct bonding of LiNbO3 single crystals for optical waveguides. Appl. Phys. Lett. 1995, 66, 1484. [Google Scholar] [CrossRef]
- Takigawa, R.; Higurashi, E.; Asano, T. Room-temperature wafer bonding of LiNbO3 and SiO2 using a modified surface activated bonding method. Jpn. J. Appl. Phys. 2018, 57, 06HJ12. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Tian, Y.; Wu, B.; Wang, S.; Zhang, H. Glass-on-LiNbO3 heterostructure formed via a two-step plasma activated low-temperature direct bonding method. Appl. Surf. Sci. 2018, 459, 621–629. [Google Scholar] [CrossRef]
- Xu, F.; Tan, Q. Langasite micromachining technology applied to surface acoustic wave sensors in ultra-high temperatures. In Proceedings of the IEEE 34th International Conference on Micro Electro Mechanical Systems, Gainesville, FL, USA, 25–29 January 2021; pp. 674–677. [Google Scholar] [CrossRef]
- Jung, I.H.; Auh, K.H. Crystal growth and piezoelectric properties of langasite (La3Ga5SiO14) crystals. Mater. Lett. 1999, 41, 241–246. [Google Scholar] [CrossRef]
- Rayleigh, L. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 1885, s1–s17, 4–11. [Google Scholar] [CrossRef]
- Fritze, H. High-temperature piezoelectric crystals and devices. J. Electroceramics 2011, 26, 122–161. [Google Scholar] [CrossRef]
- Schulz, M.; Fritze, H.; Tuller, H.L.; Seh, H. Diffusion-related implications for langasite resonator operation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 1381–1387. [Google Scholar] [CrossRef]
- Ramm, P.; Lu, J.J.Q.; Taklo, M.M.V. Handbook of Wafer Bonding; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9783527326464. [Google Scholar]
- Bohm, J.; Chilla, E.; Flannery, C.; Fröhlich, H.J.; Hauke, T.; Heimann, R.B.; Hengst, M.; Straube, U. Czochralski growth and characterization of piezoelectric single crystals with langasite structure: La3Ga5SiO14 (LGS), La3Ga5.5Nb0.5O14 (LGN) and La3Ga5.5Ta0.5O14 (LGT). II. Piezoelectric and elastic properties. J. Cryst. Growth 2000, 216, 293–298. [Google Scholar] [CrossRef]
- Ohsato, H.; Iwataki, T.; Morikoshi, H. Mechanism of piezoelectricity for langasite based on the framework crystal structure. Trans. Electr. Electron. Mater. 2012, 13, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Araki, N.; Ohsato, H.; Kakimoto, K.; Kuribayashi, T.; Kudoh, Y.; Morikoshi, H. Origin of piezoelectricity for langasite A3Ga5SiO14 (A = La and Nd) under high pressure. J. Eur. Ceram. Soc. 2007, 27, 4099–4102. [Google Scholar] [CrossRef]
- Iwataki, T.; Ohsato, H.; Tanaka, K.; Morikoshi, H.; Sato, J.; Kawasaki, K. Mechanism of the piezoelectricity of langasite based on the crystal structures. J. Eur. Ceram. Soc. 2001, 21, 1409–1412. [Google Scholar] [CrossRef]
- Ohsato, H.; Iwataki, T.; Morikoshi, H. Crystal structure and piezoelectric properties of four component langasite A 3BGa3Si 2O14 (A = Ca or Sr, B = Ta or Nb). Trans. Electr. Electron. Mater. 2012, 13, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Sato, J.; Takeda, H.; Morikoshi, H.; Shimamura, K.; Rudolph, P.; Fukuda, T. Czochralski growth of RE3Ga5SiO14 (RE = La, Pr, Nd) single crystals for the analysis of the influence of rare earth substitution on piezoelectricity. J. Cryst. Growth 1998, 191, 746–753. [Google Scholar] [CrossRef]
- Maksimov, B.A.; Molchanov, V.N.; Mill, B.V.; Belokoneva, E.L.; Rabadanov, M.K.; Pugacheva, A.A.; Pisarevskiǐ, Y.V.; Simonov, V.I. Absolute structure of La3Ga5SiO14 langasite crystals. Crystallogr. Rep. 2005, 50, 751–758. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Tan, C.M.; Yu, W.; Wei, J. Comparison of medium-vacuum and plasma-activated low-temperature wafer bonding. Appl. Phys. Lett. 2006, 88, 114102. [Google Scholar] [CrossRef]
- Michalske, T.A.; Freiman, S.W. A molecular mechanism for stress corrosion in vitreous silica. J. Am. Ceram. Soc. 1983, 66, 284–288. [Google Scholar] [CrossRef]
- Michalske, T.A.; Bunker, B.C. Slow fracture model based on strained silicate structures. J. Appl. Phys. 1984, 56, 2686–2693. [Google Scholar] [CrossRef]
- Amirfeiz, P.; Bengtsson, S.; Bergh, M.; Zanghellini, E.; Börjesson, L. Formation of silicon structures by plasma-activated wafer bonding. J. Electrochem. Soc. 2000, 147, 2693. [Google Scholar] [CrossRef]
- Irene, E.A.; Tierney, E.; Angilello, J. A viscous flow model to explain the appearance of high density thermal SiO2 at low oxidation temperatures. J. Electrochem. Soc. 1982, 129, 2594–2597. [Google Scholar] [CrossRef]

| Bonding Conditions | Bonding Results | |
|---|---|---|
| Temperature (°C) | Time (h) | |
| 1100 | 2 | Failure |
| 1200 | 2 | Failure |
| 1300 | 2 | Failure |
| 1350 | 2 | Partially bonded |
| 1350 | 3 | Fully bonded |
| Wafer Type | Piezoelectric Constant (|d|/pC/N) | Decrease (%) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | ||
| Original wafer | 0.7 | 0.8 | 0.8 | 0.767 | - |
| Bonded wafer | 0.8 | 0.7 | 0.7 | 0.733 | 4.43% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tan, Q.; Zhang, L.; Zhao, N.; Liang, X. Langasite Bonding via High Temperature for Fabricating Sealed Microcavity of Pressure Sensors. Micromachines 2022, 13, 479. https://doi.org/10.3390/mi13030479
Zhang J, Tan Q, Zhang L, Zhao N, Liang X. Langasite Bonding via High Temperature for Fabricating Sealed Microcavity of Pressure Sensors. Micromachines. 2022; 13(3):479. https://doi.org/10.3390/mi13030479
Chicago/Turabian StyleZhang, Juan, Qiulin Tan, Lei Zhang, Nan Zhao, and Xiaorui Liang. 2022. "Langasite Bonding via High Temperature for Fabricating Sealed Microcavity of Pressure Sensors" Micromachines 13, no. 3: 479. https://doi.org/10.3390/mi13030479
APA StyleZhang, J., Tan, Q., Zhang, L., Zhao, N., & Liang, X. (2022). Langasite Bonding via High Temperature for Fabricating Sealed Microcavity of Pressure Sensors. Micromachines, 13(3), 479. https://doi.org/10.3390/mi13030479






