Effects of Miniaturization on the Quality of Metallized Plastic Parts
Abstract
:1. Introduction
2. Electroless Metallization of Plastics and State-of-the-Arts
3. Experimental Investigation
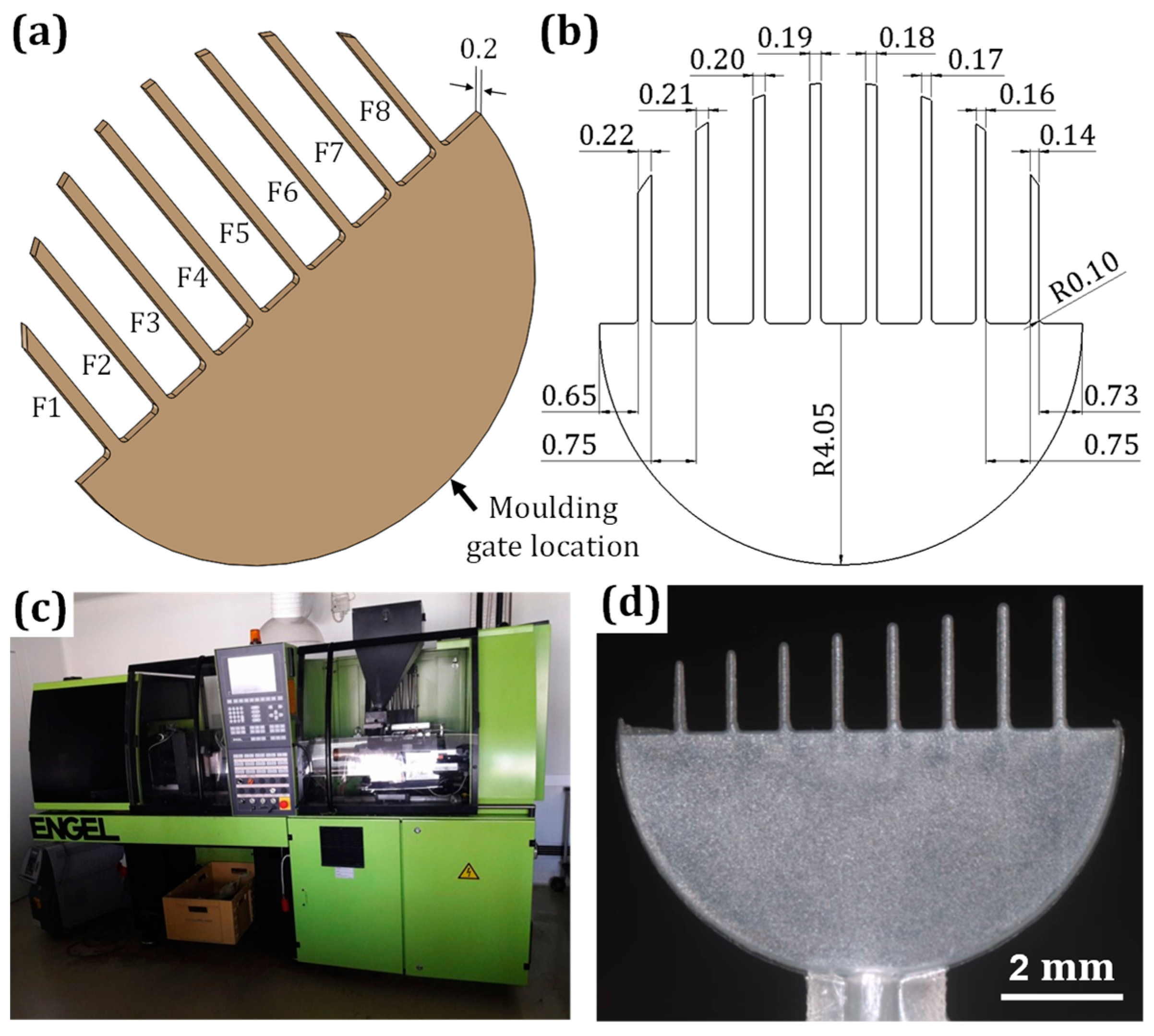
3.1. Materials and Methods
3.2. Characterizations
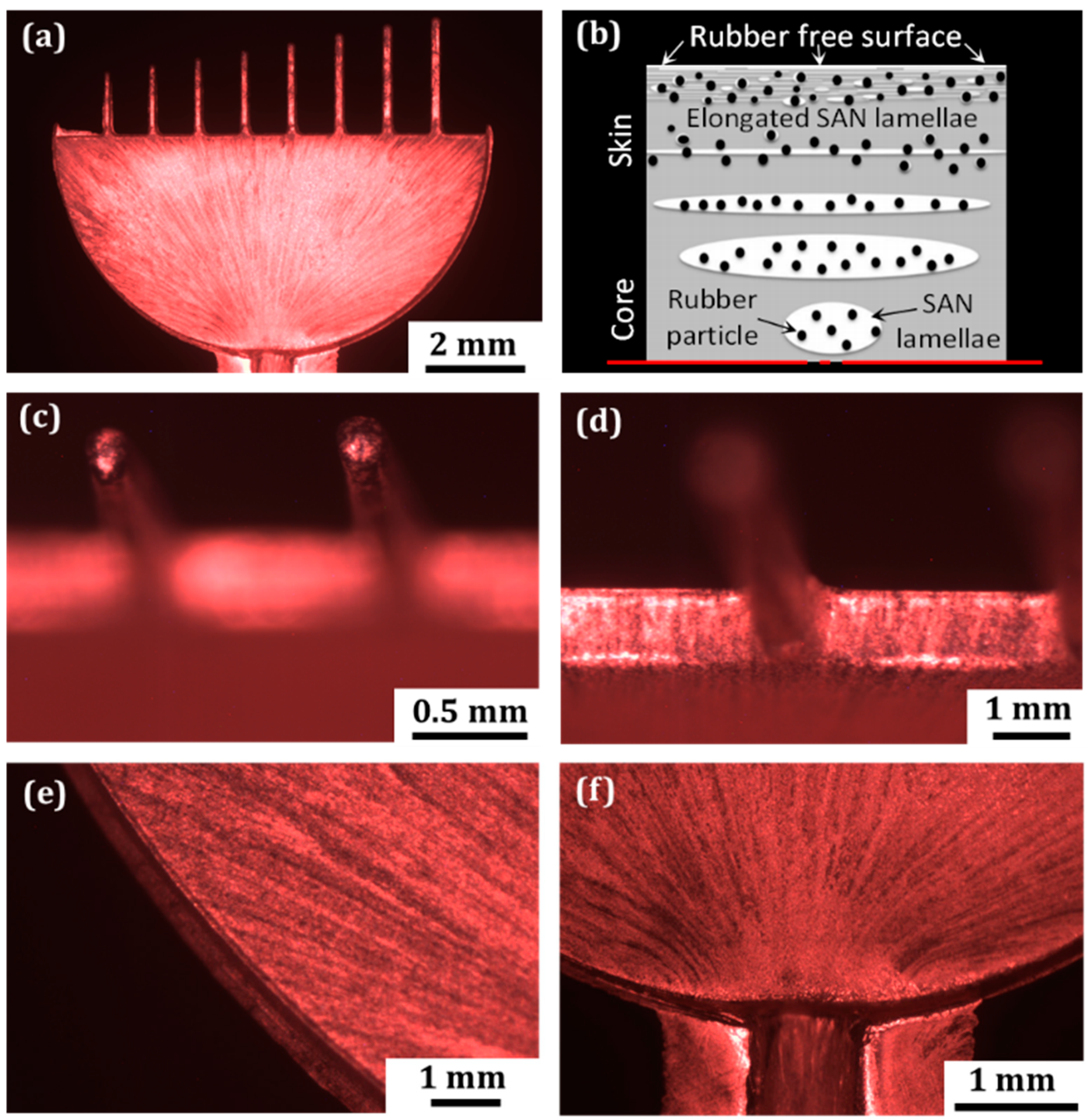
3.3. Results and Analysis
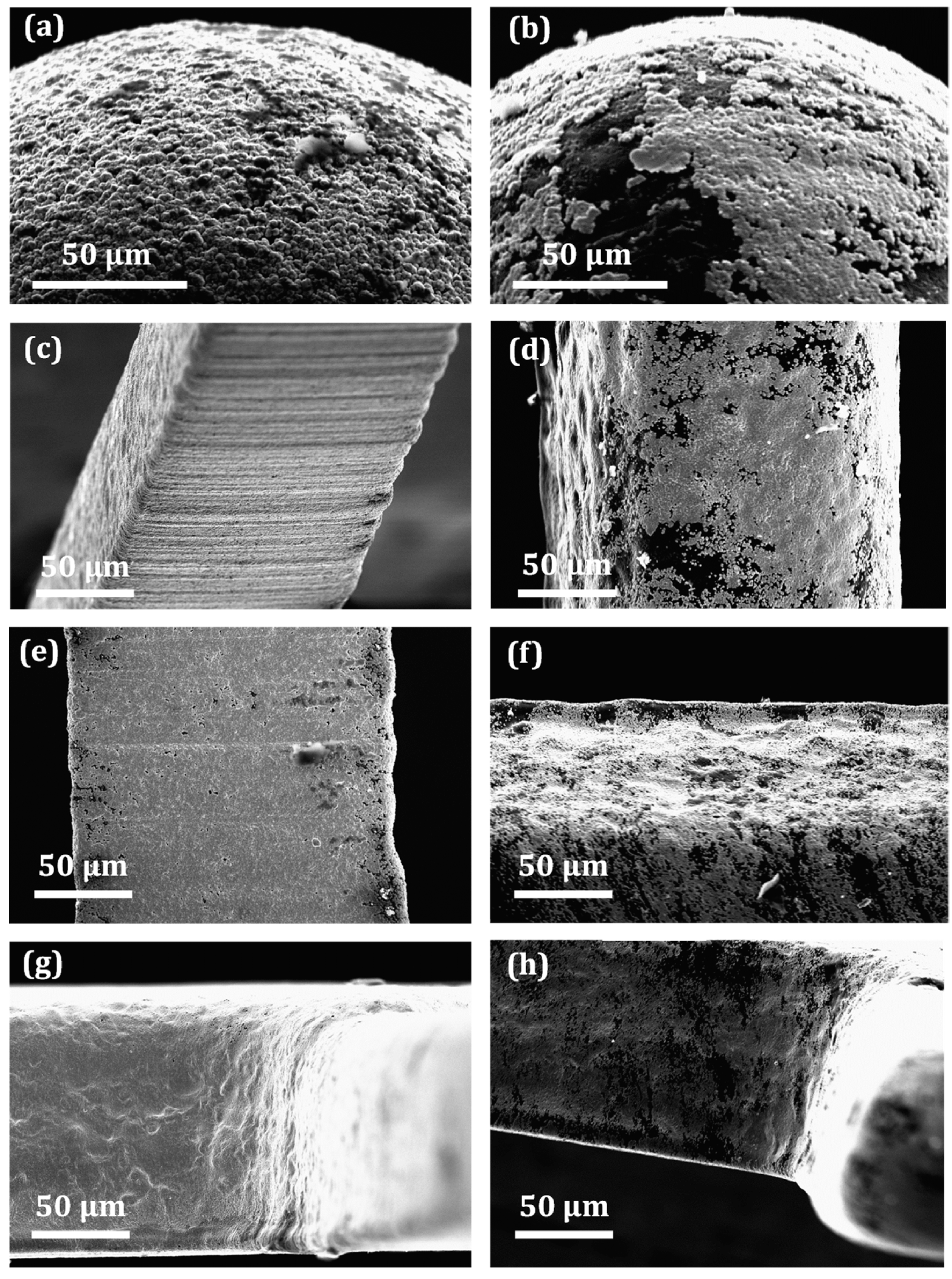
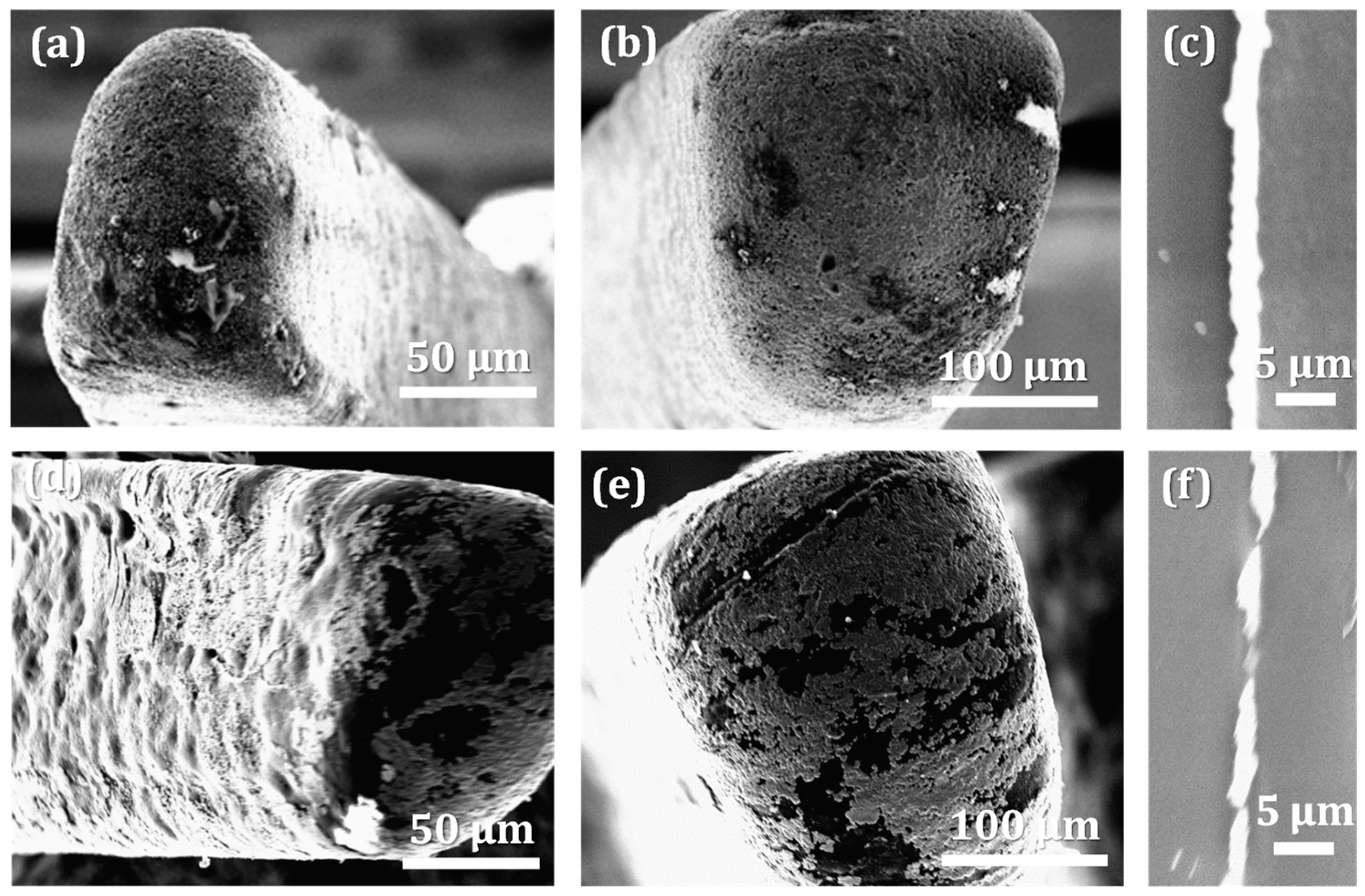
3.3.1. Metallization Quality on Different Microfeatures
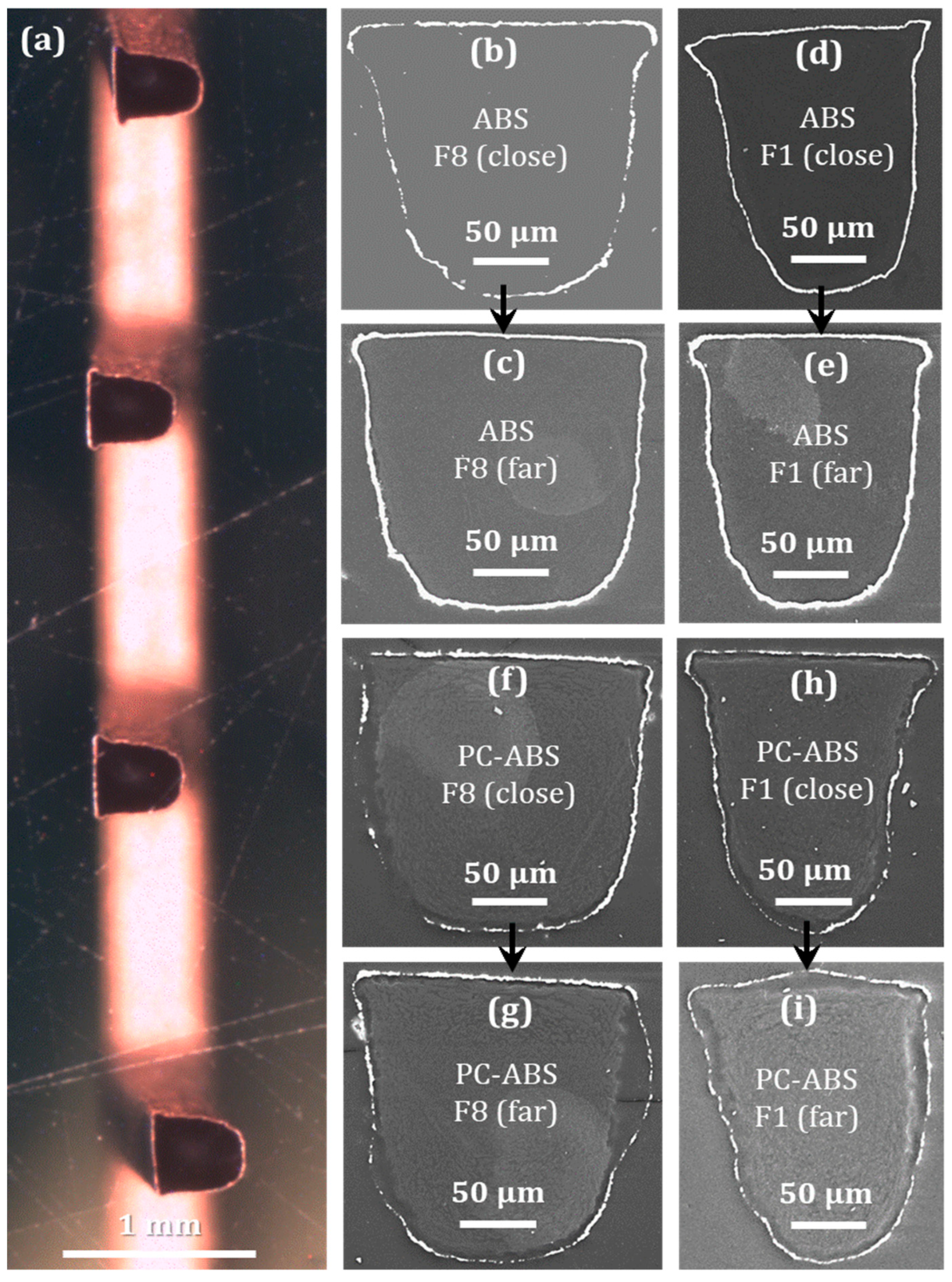
3.3.2. Metallization Quality on Different Fingers
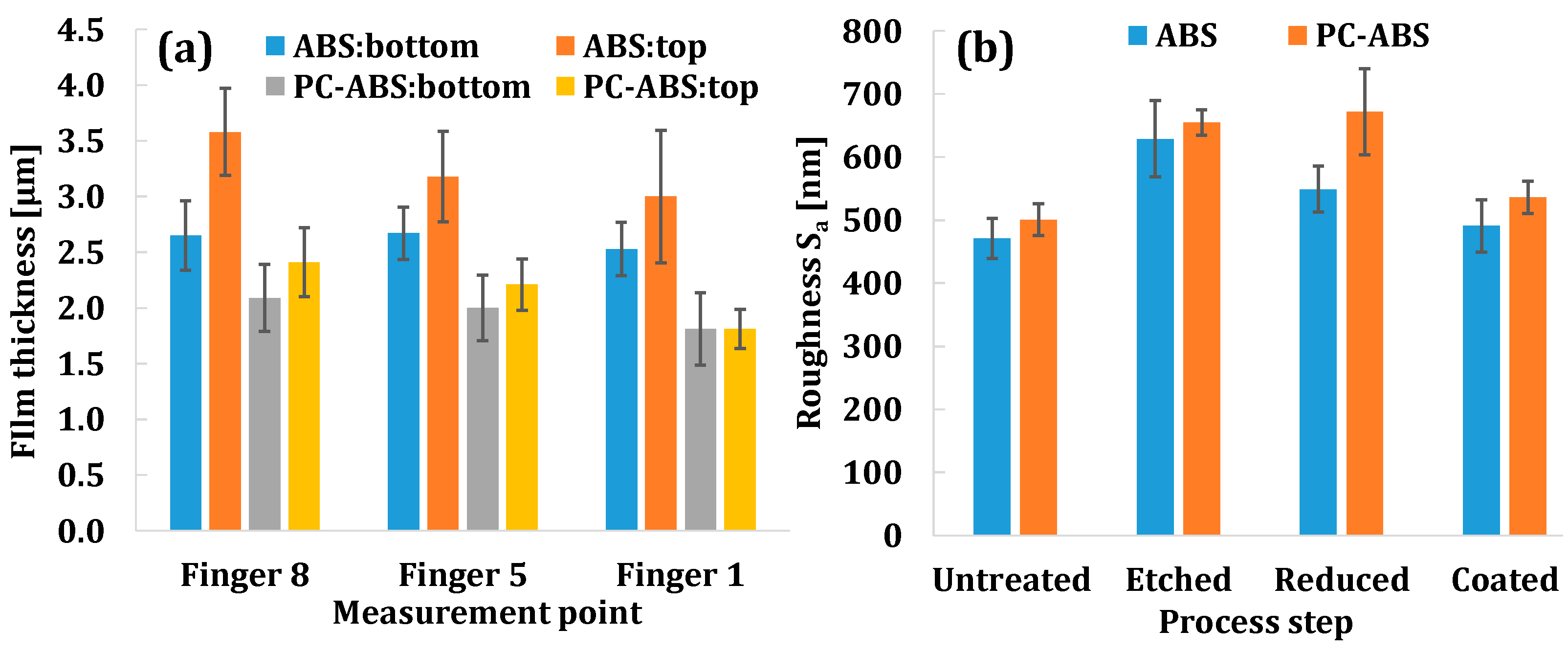
3.3.3. Film Thickness on Different Fingers
3.3.4. Roughness Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harper, C.A.; Petrie, E.M. Plastic Materials and Processes: A Concise Encyclopedia; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sharma, T.; Brüning, R.; Nguyen, T.C.L.; Bernhard, T.; Brüning, F. Properties of electroless Cu films optimized for horizontal plating as a function of deposit thickness. Microelectron. Eng. 2015, 140, 38–46. [Google Scholar] [CrossRef]
- Islam, A.; Hansen, H.N.; Giannekas, N. Quality investigation of miniaturized Moulded Interconnect Devices (MIDs) for hearing aid applications. CIRP Ann. Manuf. Technol. 2015, 64, 539–544. [Google Scholar] [CrossRef]
- Ijeri, V.; Shah, K.; Bane, S. Chromium-free etching and palladium-free plating of plastics. NASF Surf. Tech. White Pepers 2014, 78, 1–8. [Google Scholar]
- Bernasconi, R.; Credi, C.; Tironi, M.; Levi, M.; Magagnin, L. Electroless Metallization of Stereolithographic Photocurable Resins for 3D Printing of Functional Microdevices. J. Electrochem. Soc. 2017, 164, B3059–B3066. [Google Scholar] [CrossRef]
- Feldmann, K.; Franke, J.; Schußler, F. Development of micro assembly processes for further miniaturization in electronics production. CIRP Ann. Manuf. Technol. 2010, 59, 1–4. [Google Scholar] [CrossRef]
- Equbal, A.; Dixit, N.K.; Sood, A.K. Electroless Plating on Plastic. Int. J. Sci. Eng. Res. 2013, 8, 12–18. [Google Scholar]
- Mallory, G.O. Electroless Plating: Fundaments and Applications; Noyes Publications/William Andrew Publications: New York, NY, USA, 2002. [Google Scholar]
- Islam, A. Two Component Micro Moulding for MID Applications. Ph.D. Dissertation, Technical University of Denmark, Kgs. Lyngby, Denmark, 2008. [Google Scholar]
- Islam, A. Multimaterial Micro Injection Molding. In Micro Injection Molding; Tosello, G., Ed.; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2018. [Google Scholar]
- Islam, A.; Hansen, H.N.; Risager, F.; Tang, P.T. Experimental investigation on corrosion properties of LDS MID for Hearing Aid applications. In Proceedings of the ANTEC 2014 Conference Proceedings, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Diamand, S.Y.; Osaka, T.; Okinaka, Y.; Sugiyama, A.; Dubin, V. 30 years of electroless plating for semiconductor and polymer micro-systems. Microelectron. Eng. 2015, 132, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Kinge, S.; Calama, C.M.; Reinhoudt, D.N. Self-Assembling Nanoparticles at Surfaces and Interfaces. ChemPhysChem 2008, 9, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R.J. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar]
- Rodriguez, O.; Saxena, R.; Cho, W.; Plawsky, J.L.; Gill, W.N. Mechanism of Cu diffusion in porous low-κκ dielectrics. Ind. Eng. Chem. Res. 2005, 44, 1220–1225. [Google Scholar] [CrossRef]
- Benedict, D.J.; Parulekar, S.J.; Tsai, S.-P. Esterification of Lactic Acid and Ethanol with/without Pervaporation. Ind. Eng. Chem. Res. 2003, 42, 2282–2291. [Google Scholar] [CrossRef]
- Islam, A.; Hansen, H.N.; Tang, P.T.; Sun, J. Process chains for the manufacturing of molded interconnect devices. Int. J. Adv. Manuf. Technol. 2009, 42, 831–841. [Google Scholar] [CrossRef]
- Islam, A.; Hansen, H.; Tang, P.; Jørgensen, M.; Ørts, S. Two-component microinjection moulding for MID fabrication. Plast. Rubber Compos. 2010, 39, 300–307. [Google Scholar] [CrossRef]
- Alting, L.; Kimura, F.; Hansen, H.N.; Bissacco, G. Micro engineering. CIRP Ann. Manuf. Technol. 2003, 52, 635–657. [Google Scholar] [CrossRef]
- Struers. Selection Guide for Grinding and Polishing ID-09.2015/40500063; Struers: Ballerup, Denmark, 2015. [Google Scholar]
- ISO 25178-2, Geometrical Product Specifications (GPS)—Surface Texture: Areal-Part 2, ISO 25178-2:2012(EN). 2012. Available online: https://www.iso.org/standard/42785.html (accessed on 20 February 2022).
- Bärwinkel, S.; Seidel, A.; Hobeika, S.; Hufen, R.; Mörl, M.; Altstädt, V. Morphology formation in PC/ABS blends during thermal processing and the effect of the viscosity ratio of blend partners. Materials 2016, 9, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 2409, Paints and Varnishes-Cross-Cut Test (EN ISO 2409:2013). 2013. Available online: https://www.iso.org/obp/ui/#iso:std:iso:2409:ed-5:v1:en (accessed on 20 February 2022).
- Eladl, A.; Mostafa, R.; Islam, A.; Loaldi Dario, S.; Hassan, H.; Tosello, G. Effect of process parameters on flow length and flash formation in injection moulding of high aspect ratio polymeric micro features. Micromachines 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
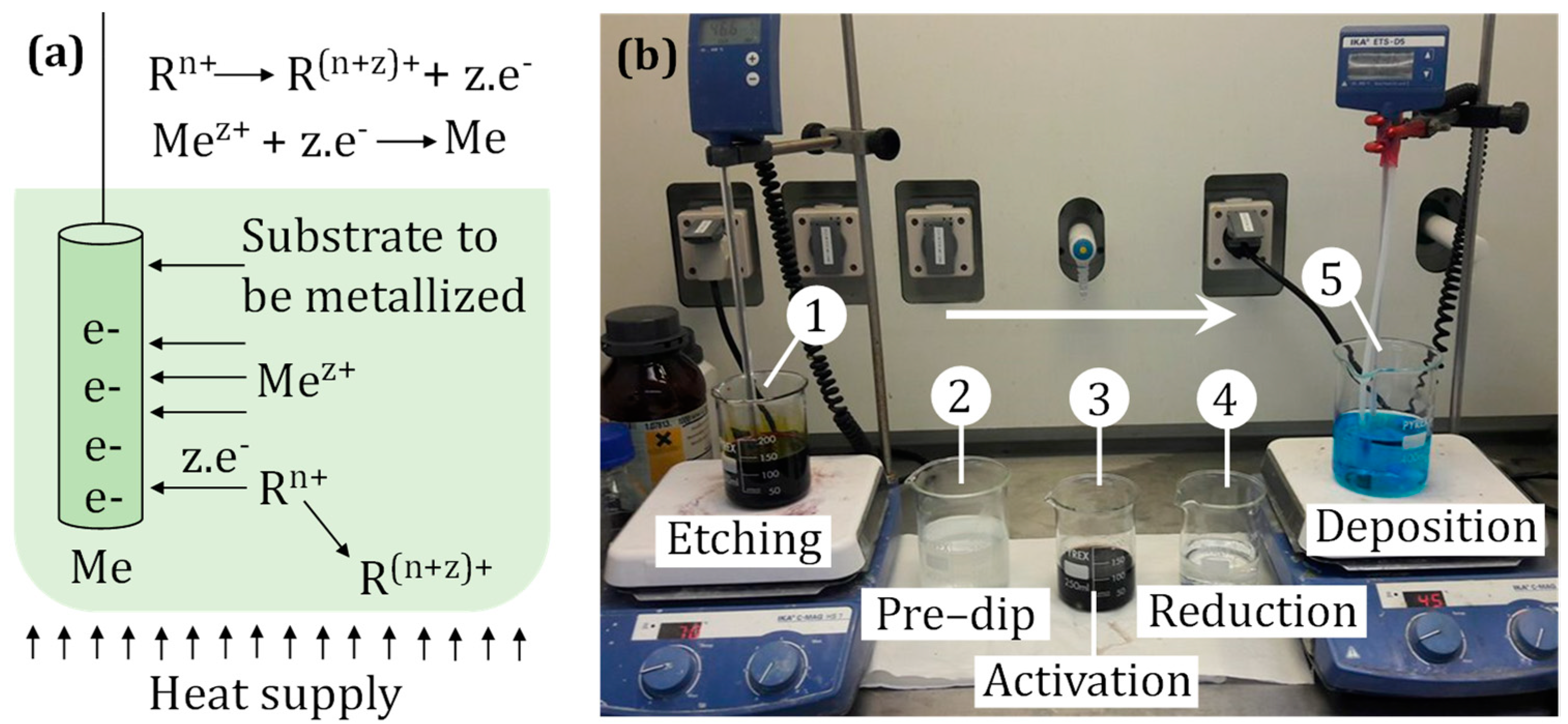


| Step | Composition | Temp. | Time |
|---|---|---|---|
| Etching | 375 g/L CrO3 + 400 g/L H2SO4 (conc.) | 63 °C | 10 min |
| Pre-dip | 2 g/L SnCl2 2 H2O + 180 g/L NaCl + 100 mL/L HCl (conc.) | Room temp. | 2 min |
| Activation | 180 g/L NaCl + 120 mL/L HCl (conc.) + 1 g/L SnCl2 ∙ 2 H2O + 30 mL/L Pd catalyst | Room temp. | 3 min |
| Reduction | 300 mL/L HCl (conc.) | Room temp. | 10 s |
| Deposition | Shipley Circuposit 3350 | 45 °C | 10 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, A.; Hansen, H.N. Effects of Miniaturization on the Quality of Metallized Plastic Parts. Micromachines 2022, 13, 515. https://doi.org/10.3390/mi13040515
Islam A, Hansen HN. Effects of Miniaturization on the Quality of Metallized Plastic Parts. Micromachines. 2022; 13(4):515. https://doi.org/10.3390/mi13040515
Chicago/Turabian StyleIslam, Aminul, and Hans Nørgaard Hansen. 2022. "Effects of Miniaturization on the Quality of Metallized Plastic Parts" Micromachines 13, no. 4: 515. https://doi.org/10.3390/mi13040515






