Effect of Wetting Characteristics of Polishing Fluid on the Quality of Water-Dissolution Polishing of KDP Crystals
Abstract
:1. Introduction
2. Correlation between Surface Roughness and Water Content during the Water-Dissolution Polishing Process of KDP Crystals
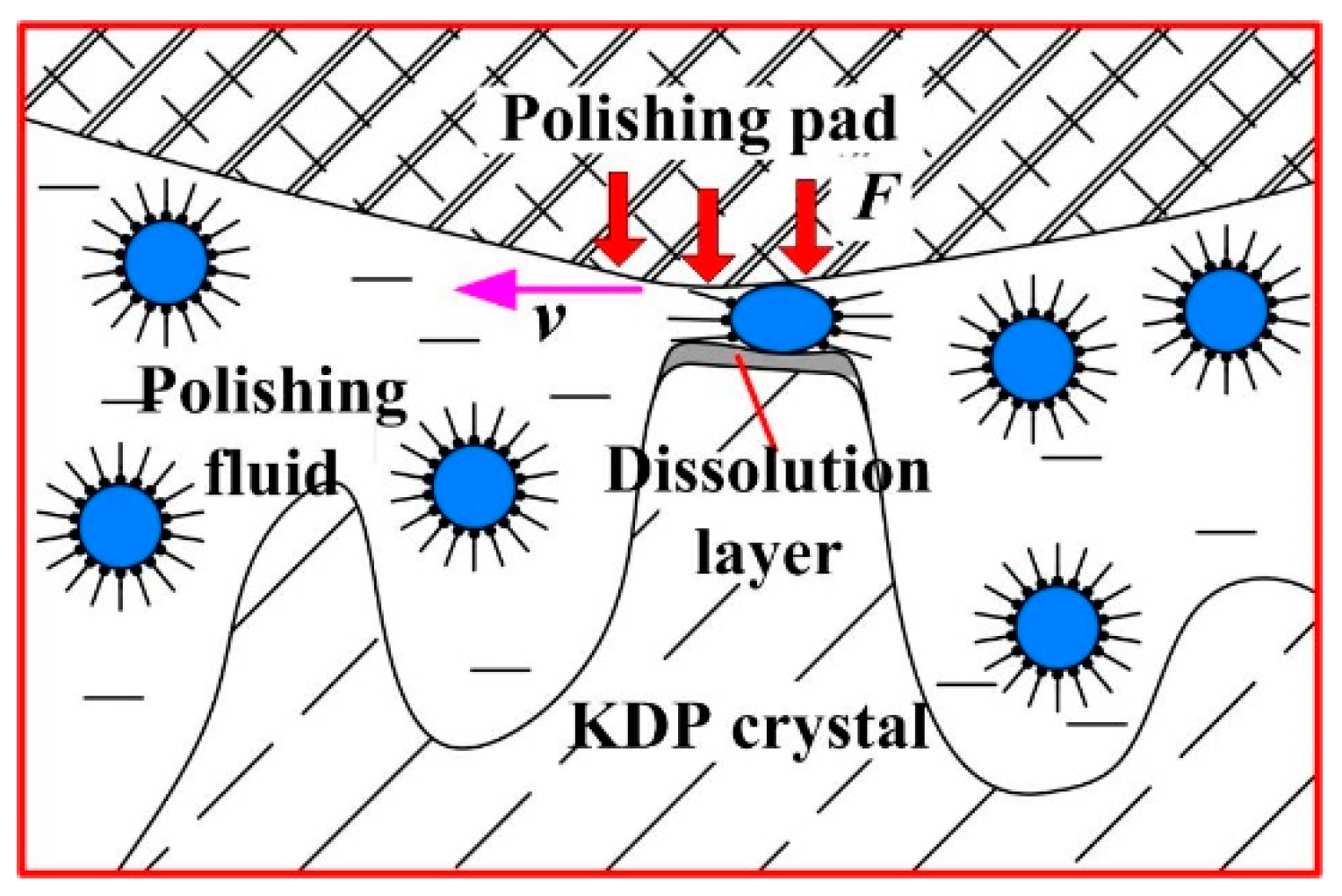
2.1. Selective Removal Mechanism of Water-Dissolution Polishing
2.2. Analysis of the Effect of Water Content of Water-Dissolution Polishing Fluid on the Polished Surface Roughness
3. Analysis of the Effect of Polishing Fluid Viscosity on the Surface Quality of Water-Dissolution Polishing
4. Analysis of the Effect of the Wetting Characteristics of Polishing Fluid on the Surface Quality of Water-Dissolution Polishing
5. Conclusions
- The radius distribution of micro water droplets in water-dissolution polishing fluid was within 10 nm, and it increased significantly with the increase in water content. The mean value of the micro water droplet radius was approximately 0.6 nm when the polishing fluid contained 5 wt.% of water, it was approximately 1.2 nm with a water content of 7.5 wt.%, and it was approximately 3.8 nm when the water content was 10 wt.%.
- The viscosity of the polishing fluid increased with the increase of water content; theoretically, a lower viscosity should be more conducive to polishing the surface quality. Thus, the polishing fluid viscosity was not considered as a key factor affecting the quality of water-dissolution polishing of KDP crystals.
- By measuring the contact angle and surface tension, it was concluded that the wetting characteristics of the polishing solution on the crystal surface were significantly affected by the water content. The higher the water content, the better the polishing fluid wet the crystal surface, and the easier to enter the micro-porosity between the polishing pad and the crystal surface, which is theoretically beneficial to obtain a high-quality surface.
- The surface roughness value of KDP was Ra 1.260 nm after being processed with a polishing fluid containing 7.5 wt.% water, while the surface roughness value increased to Ra 1.660 nm after being processed with a polishing fluid with a water content of 5 wt.%, and the surface became clearly scratched. Concerning the KDP crystal water-dissolution polishing method, a low water content of the polishing solution led to a decrease in the wettability, making it difficult to enter the gaps between the polishing pad and the crystal surface, and thus leading to a degradation in the quality of the polished surface. The polished surface quality of KDP crystals was jointly determined by the micro water droplet size and the wetting characteristics of the polishing fluid on the KDP surface. When the water content was 7.5 wt.%, the wetting characteristics of the polishing fluid and the effect of micro water droplet radius were balanced and the best polished surface quality was achieved.
- When using oil-based polishing fluids for ultra-precision processing, in addition to conventional factors such as viscosity, size of polishing particles (abrasive particles, microdroplets, etc.), and polishing speed, the wetting characteristics of the polishing fluid on the processed surface constituted one of the key factors that must be considered. In the future, we will further optimize the polishing fluid composition and incorporate appropriate additives to obtain a water-dissolution polishing fluid with good microemulsion radius and wetting characteristics to further improve the surface quality of KDP crystals after polishing. The present study also proved that the wetting characteristics of the polishing fluid should be improved during the optimization process of polishing fluid composition when using oil-based polishing fluids for ultra-precision polishing.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Wang, H.; Quan, X.; Pei, G.; Tian, M.; Liu, T.; Long, K.; Li, P.; Rong, Y. Optomechanical analysis and performance optimization of large-aperture KDP frequency converter. Opt. Laser Technol. 2019, 109, 633–642. [Google Scholar] [CrossRef]
- Zylstra, A.B.; Nora, R.; Patel, P.; Hurricane, O. Model validation for inferred Hot-Spot conditions in National Ignition Facility experiments. Phys. Plasmas 2021, 28, 122703. [Google Scholar] [CrossRef]
- Lindl, J.D.; Atherton, L.; Amednt, P.; Batha, S. Progress towards ignition on the National Ignition Facility. Nucl. Fusion 2011, 51, 0940249SI. [Google Scholar] [CrossRef]
- Hang, W.; Wei, L.; Debela, T.; Chen, H.; Zhou, L.; Yuan, J.; Ma, Y. Crystallographic orientation effect on the polishing behavior of LiTaO3 single crystal and its correlation with strain rate sensitivity. Ceram. Int. 2022, 48, 7766–7777. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Xu, D.; Luo, T.; Zhang, Z.; Wang, X. Solubility measurement and thermodynamics modelling for potassium dihydrogen phosphate in a water-ethanol system from 293.2 to 323.2 K. Fluid Phase Equil. 2020, 512, 112533. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Liu, J.; Zha, C.; Pan, R. Morphological analysis of KDP-crystal workpiece surfaces machined by ultra-precision fly cutting. Materials 2020, 13, 432. [Google Scholar] [CrossRef] [Green Version]
- An, C.; Feng, K.; Wang, W.; Xu, Q.; Lei, X.; Zhang, J.; Yao, X.; Li, H. Interaction mechanism of thermal and mechanical field in KDP fly-cutting process. Micromachines 2021, 12, 855. [Google Scholar] [CrossRef]
- Pang, Q.; Shu, Z.; Kuang, L.; Xu, Y. Effect of actual frequency features generated in machining process on the temperature and thermal stress of potassium dihydrogen phosphate crystal. Mater. Today Commun. 2021, 29, 102984. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Z.; Cheng, J.; Xu, D.; Chen, M. Mechanism of chip formation and surface-defects in orthogonal cutting of soft-brittle potassium dihydrogen phosphate crystals. Mater. Des. 2021, 198, 109327. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, N.; Zhang, L. Understanding the formation mechanism of subsurface damage in potassium dihydrogen phosphate crystals during ultra-precision fly cutting. Adv. Manuf. 2019, 7, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ji, Y. Experimental study on grinding damage control of optical materials. Diam. Abras. Eng. 2020, 40, 84–88. [Google Scholar]
- Chen, H.; Xu, Q.; Wang, J.; Li, P.; Yuan, J.; Lyu, B.; Wang, J.; Tokunaga, K.; Yao, G.; Luo, L.; et al. Effect of surface quality on hydrogen/helium irradiation behavior in tungsten. Nucl. Eng. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Qu, M.; Jin, T.; Xie, G.; Cai, R. Developing a novel binderless diamond grinding wheel with femtosecond laser ablation and evaluating its performance in grinding soft and brittle materials. J. Mater. Process. Technol. 2020, 275, 116359. [Google Scholar] [CrossRef]
- Qu, M.; Xie, G.; Jin, T.; Cai, R.; Lu, A. Realization of high efficiency and low damage machining of anisotropic KDP crystal by grinding. Precis. Eng. 2019, 55, 464–473. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Dai, Y.; Xiao, Q.; Tie, G. Novel magneto-rheological finishing process of KDP crystal by controlling fluid-crystal temperature difference to restrain deliquescence. CIRP Ann. Manuf. Technol. 2018, 67, 587–590. [Google Scholar] [CrossRef]
- Shi, F.; Qi, X.; Dong, W.; Zhu, Z. Improvement of surface laser damage resistance of KDP crystal under combined machining process. Opt. Eng. 2019, 57, 121911. [Google Scholar] [CrossRef]
- Gao, W.; Wei, Q.; Ji, J.; Sun, P.; Ji, F.; Wang, C.; Xu, M. Theoretical modeling and analysis of material removal characteristics for KDP crystal in abrasive-free jet processing. Opt. Express 2019, 27, 6268–6282. [Google Scholar] [CrossRef]
- Gao, H.; Wang, B.; Guo, D.; Li, Y. Experimental study on abrasive-free polishing for KDP crystal. J. Electrochem. Soc. 2010, 157, 853–856. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, H.; Liu, Z.; Guo, D. Investigation of the trajectory uniformity in water dissolution ultraprecision continuous polishing of large-sized KDP crystal. Int. J. Extrem. Manuf. 2020, 4, 45101. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H.; Yuan, J. Experimental investigation and analytical modelling of the tool influence function of the ultra-precision numerical control polishing method based on the water dissolution principle for KDP crystals. Precis. Eng. 2020, 65, 185–196. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H.; Chen, Y.; Guo, D. A water dissolution method for removing micro-waviness caused by SPDT process on KDP crystals. Int. J. Adv. Manuf. Technol. 2016, 85, 1347–1360. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Wang, X.; Guo, D.; Liu, Z. Laser induced damage of potassium dihydrogen phosphate (KDP) optical crystal machined by water dissolution ultra-precision polishing method. Materials 2018, 11, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, R.; Price, D.; Neyrick, J.; Saino, C.; Permana, D.; Muraka, S. CMP of copper-polymer interconnect structures. CMP-MIC Conf. 1998, 19, 257–266. [Google Scholar]
- Mullany, B.; Byrne, G. The effect of slurry viscosity on chemical-mechanical polishing of silicon wafers. J. Mater. Process. Technol. 2003, 132, 28–34. [Google Scholar] [CrossRef]








| Water Content | Measuring Results | Density (g/cm3) | Surface Tension (mN/m) |
|---|---|---|---|
| 0 wt.% |  | 0.820 | 24.78 |
| 5 wt.% |  | 0.842 | 24.90 |
| 7.5 wt.% |  | 0.849 | 25.02 |
| 10 wt.% |  | 0.856 | 25.29 |
| 15 wt.% |  | 0.867 | 25.58 |
| 20 wt.% |  | 0.887 | 25.73 |
| Water Content | Contact Angle (°) | Surface Tension (mN/m) | Wetting Work (mN/m) |
|---|---|---|---|
| 0 wt.% | 52.6 | 24.78 | 39.83 |
| 5 wt.% | 46.2 | 24.90 | 42.13 |
| 7.5 wt.% | 42.8 | 25.02 | 43.38 |
| 10 wt.% | 40.3 | 25.29 | 44.58 |
| 15 wt.% | 29.1 | 25.58 | 47.93 |
| 20 wt.% | 19.1 | 25.73 | 50.04 |
| 5 wt.% | 7.5 wt.% | |
|---|---|---|
| Point 1 |  |  |
| Point 2 |  |  |
| Point 3 |  |  |
| Point 4 |  |  |
| Point 5 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, H.; Deng, Q.; Wang, J.; Chen, H.; Yuan, J. Effect of Wetting Characteristics of Polishing Fluid on the Quality of Water-Dissolution Polishing of KDP Crystals. Micromachines 2022, 13, 535. https://doi.org/10.3390/mi13040535
Wang X, Gao H, Deng Q, Wang J, Chen H, Yuan J. Effect of Wetting Characteristics of Polishing Fluid on the Quality of Water-Dissolution Polishing of KDP Crystals. Micromachines. 2022; 13(4):535. https://doi.org/10.3390/mi13040535
Chicago/Turabian StyleWang, Xu, Hang Gao, Qianfa Deng, Jinhu Wang, Hongyu Chen, and Julong Yuan. 2022. "Effect of Wetting Characteristics of Polishing Fluid on the Quality of Water-Dissolution Polishing of KDP Crystals" Micromachines 13, no. 4: 535. https://doi.org/10.3390/mi13040535
APA StyleWang, X., Gao, H., Deng, Q., Wang, J., Chen, H., & Yuan, J. (2022). Effect of Wetting Characteristics of Polishing Fluid on the Quality of Water-Dissolution Polishing of KDP Crystals. Micromachines, 13(4), 535. https://doi.org/10.3390/mi13040535






