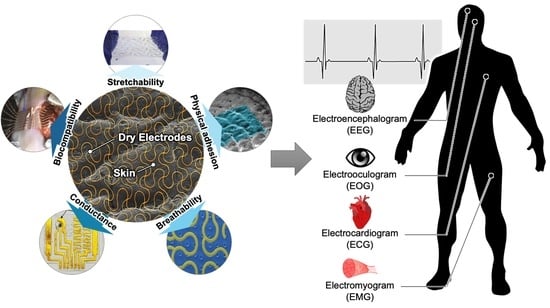
Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices
Abstract
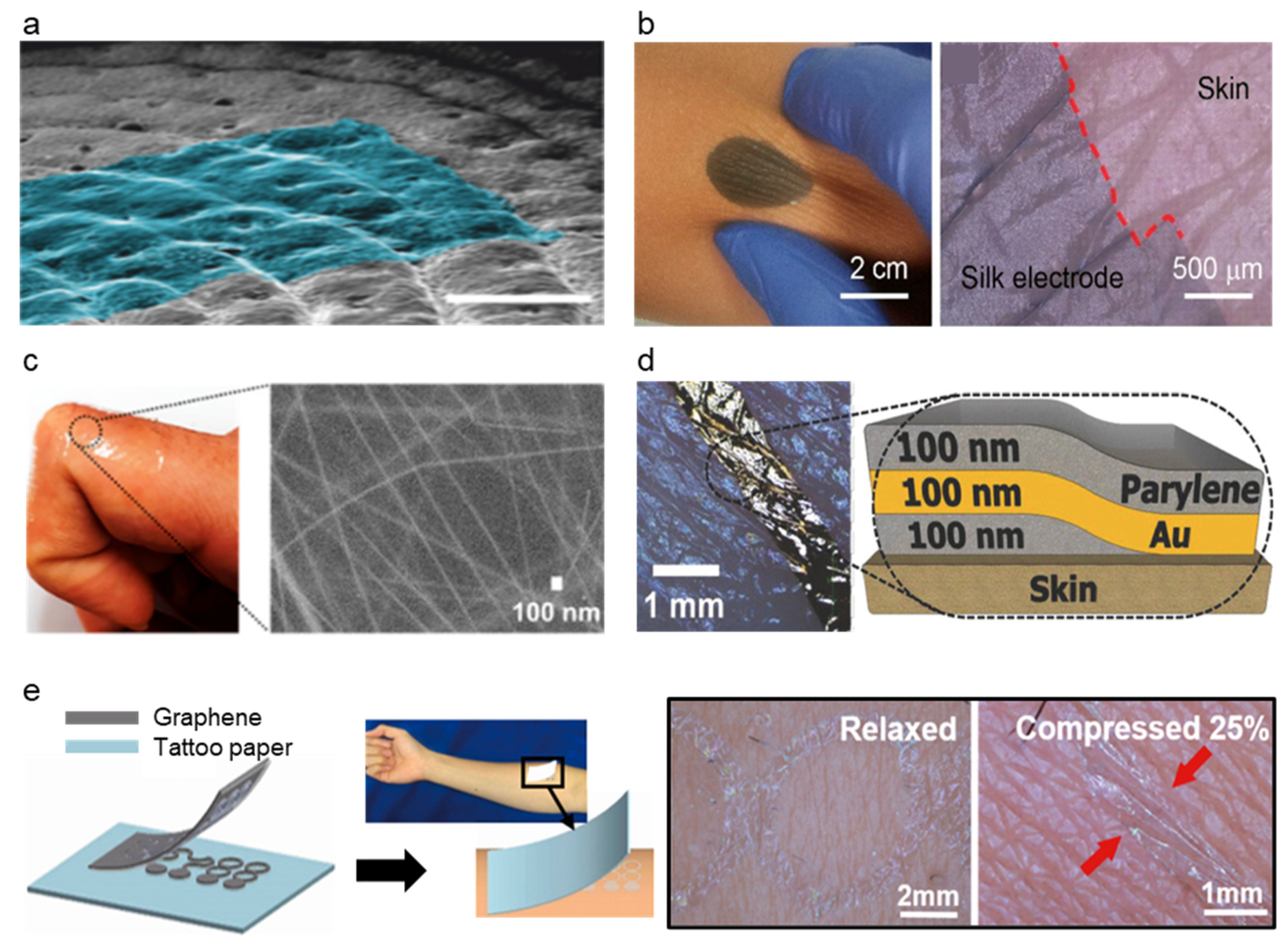
:1. Introduction
2. Dry Electrodes with Various Materials
2.1. Significance of Material Selection in Dry Electrode Fabrication
2.2. Metal-Based Electrodes
| Electrode Material | Manufacturing Method | Conductivity/ Resistivity | SNR/Contact Impedance | Stretchability | Thickness | Ref. |
|---|---|---|---|---|---|---|
| Ag flake, Au | 3D Direct ink write, Steam etching, Metallization, Electroplating | 1.0 kΩ (@ 40, 150, 1000 Hz) | 2.1, 1.5, 1.0 kΩ (@ 40, 150, 1000 Hz) | >100% | ≥50 μm | [41] |
| Ag-PTFE | Magnetron sputtering system | 3.09–17.23 Ω/sq | N/A | 40% | 20 nm | [42] |
| Au, PDMS | Thermal release tape transfer, Laser patterning, MPTMS treatment, Spin coating, Stencil masking | 10–150 Ω | N/A | <50% | 250 μm | [43] |
| Au nanoparticle | Electrodeposition | 270 mΩ/sq | ECG 51% EMG 63% | N/A | N/A | [44] |
| Ti thin film | Magnetron sputtering with glancing/oblique angle deposition (GLAD/OAD) | 1–15 × 10−6 Ωm | SS: 10 kΩ TPU: 200–250 kΩ | N/A | 1–1.5 mm | [45] |
| Pt thin film with Cu co-deposition | Electrodeposition | N/A | <5 kΩ @ 20 Hz | N/A | 5–20 μm | [46] |

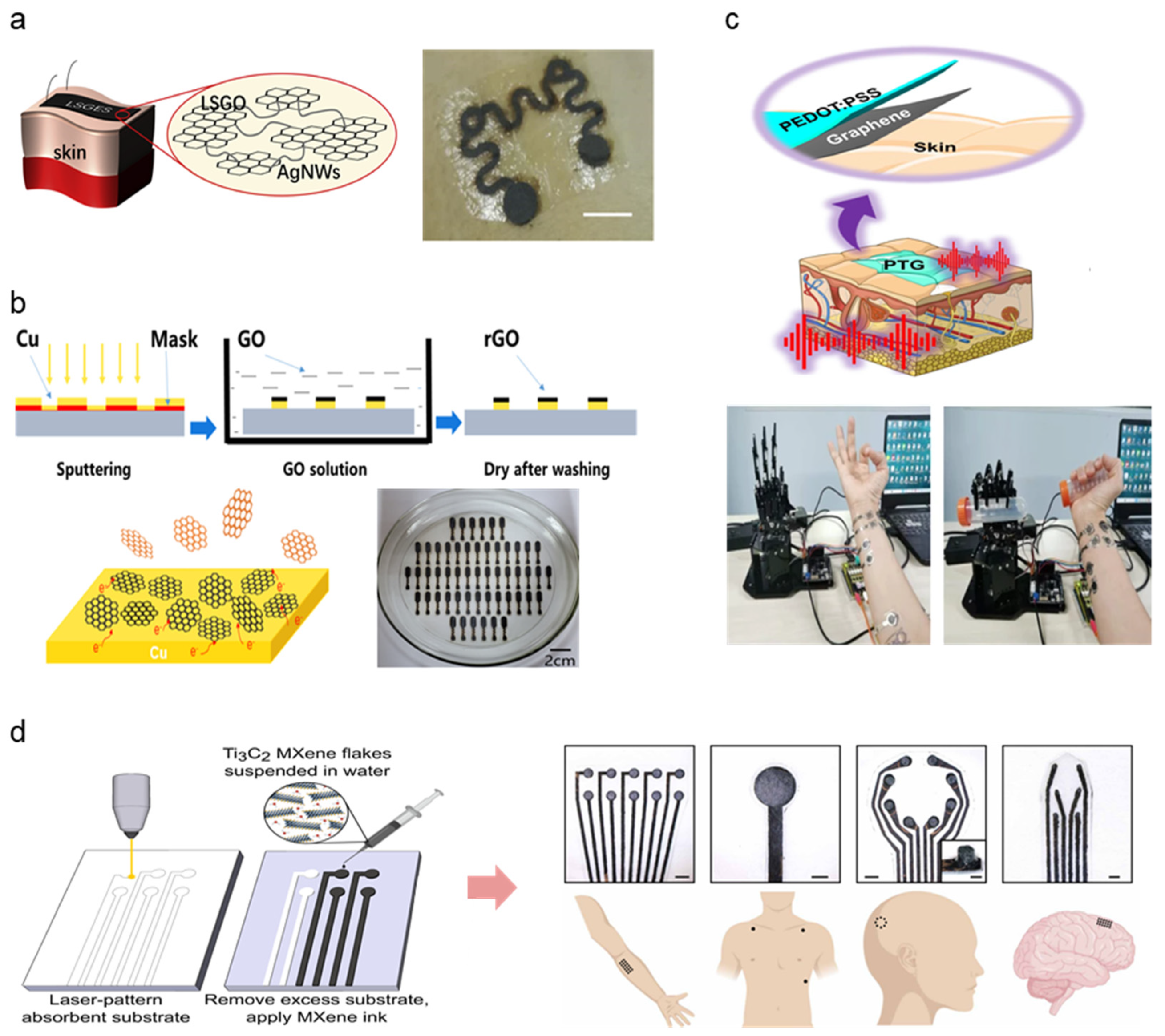
2.3. Carbon-Based Electrodes
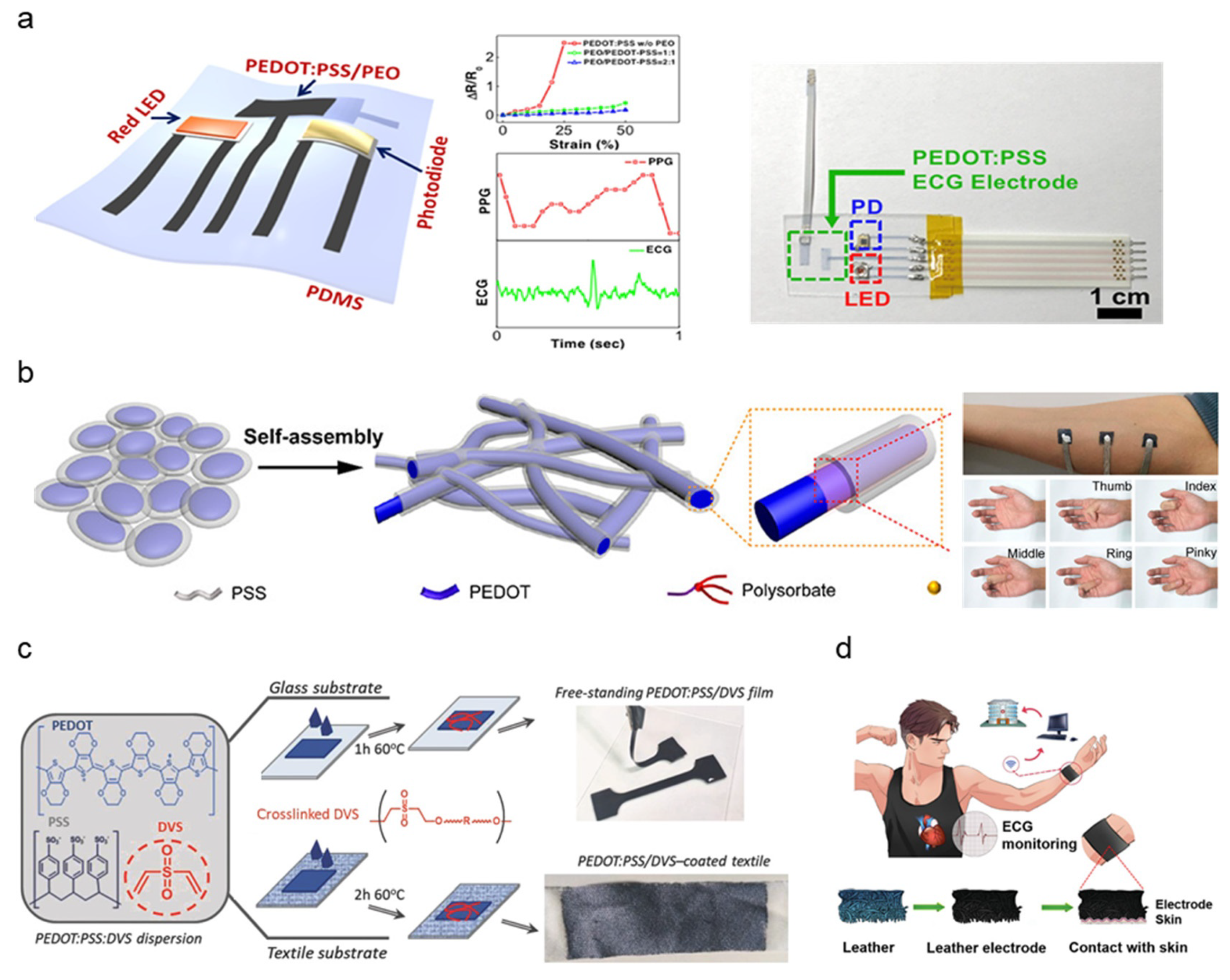
2.4. Conductive Polymer-Based Electrodes
2.5. Other/Hybrid Electrodes
| Electrode Material | Manufacturing Method | Conductivity/ Resistivity | SNR/Contact Impedance | Stretchability | Thickness | Ref. |
|---|---|---|---|---|---|---|
| AgNW, Laser scribed graphene oxide (LSGO) | Mixture volatilization on transfer paper to form a thin film | 700 Ω | 20–100 kΩ (@ 10–1000 Hz) | 1–4% | N/A | [77] |
| Cu/rGO | Magnetron sputtering | N/A | 100 k–900 kΩ (@ 10–1000 Hz) | N/A | 0.4–4.9 μm | [79] |
| Graphene, PEDOT:PSS | Spin-coating | ~24 Ω/sq (4142 S/cm) | 4 × 102–7 × 104 Ω (@ 10–105 Hz) | 40% | ~100 nm | [80] |
| MXene | Spray, Spin, Dip Coating, Direct writing, IJP | 15,000–20,000 S/cm | 241.4 ± 14.7–1343.3 ± 81.6 Ω (0.05–3 mm) | N/A | N/A | [81] |
| Nanofiber membrane (PVDF/PEDOT:PSS), CB/rGO | Electrospinning | 2.5 × 10 Ω/sq | 2 × 102–7 × 105 Ω | 10–50% | 100 μm | [82] |
| CNTs/AgNw/PDMS | Sonication | N/A | 105 Z (@ 10 Hz) | 30% | 200 μm | [78] |
| PEDOT:PSS covering laser-induced graphene (LIG) | Spray coating, Laser cutting, mask peel-off | 13.1–33.5 Ω/sq | 17.4 U/sq 385 kU (@ 10 Hz) | N/A | N/A | [83] |
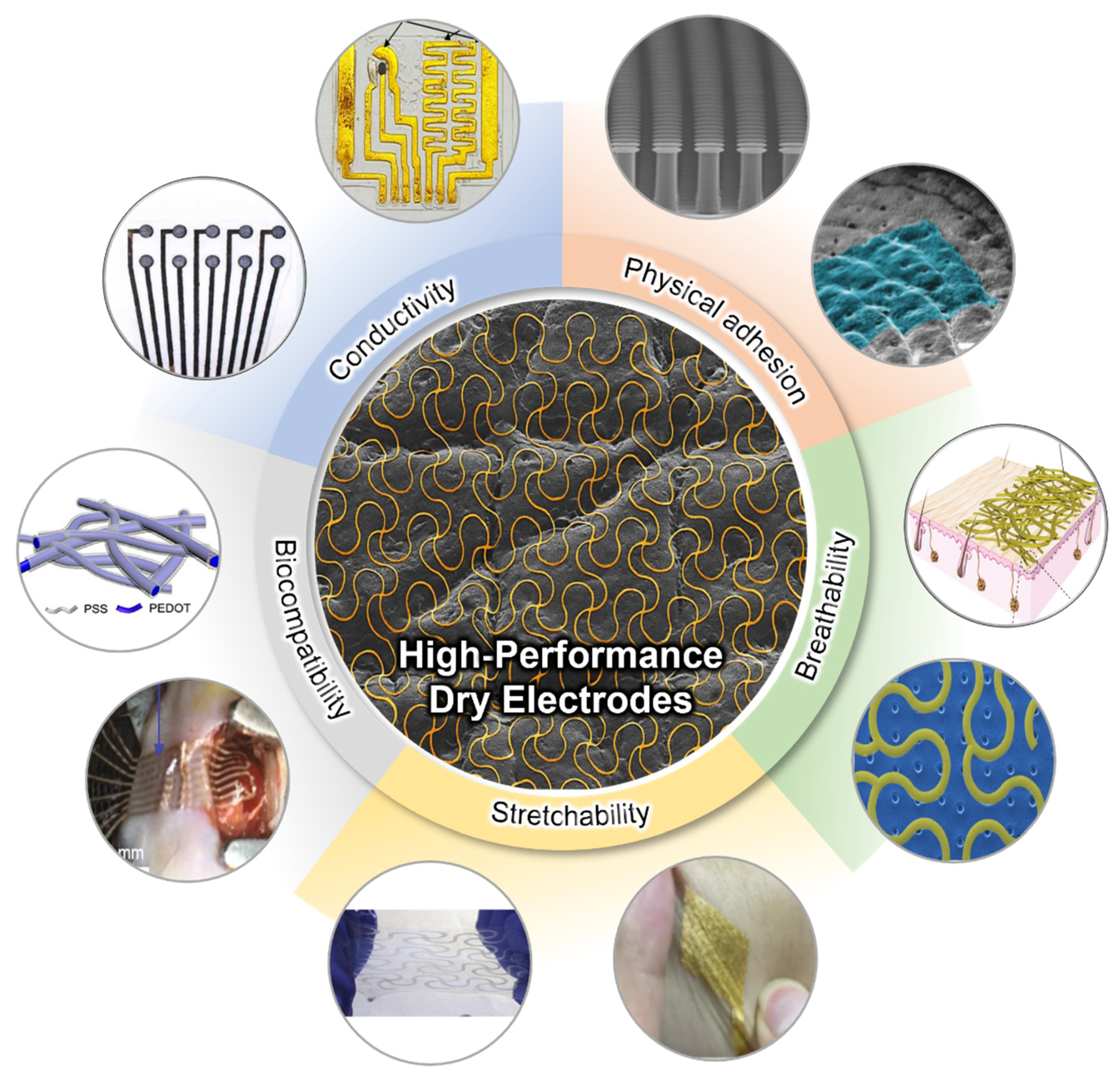
3. Physical Adhesion
3.1. Significance of Adhesion in Soft–Dry Electrode Performance
3.2. Adhesive
3.2.1. Dry Adhesive

3.2.2. Pressure-Sensitive Adhesive
3.3. Conformal Contact
3.3.1. Material Modification
3.3.2. Structural Modification
4. Breathability
5. Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machemer, H. Electrophysiology. In Paramecium, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Kumar, A.; Komaragiri, R.; Kumar, M. From Pacemaker to Wearable: Techniques for ECG Detection Systems. J. Med. Syst. 2018, 42, 34. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Chen, L.; Marinov, O.; Chen, C.H.; Mondal, T.; Deen, M.J. Noncontact Wearable Wireless ECG Systems for Long-Term Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mitra, S.; Van Hoof, C.; Yazicioglu, R.F.; Makinwa, K.A.A. Active Electrodes for Wearable EEG Acquisition: Review and Electronics Design Methodology. IEEE Rev. Biomed. Eng. 2017, 10, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Sethi, A.; Ting, J.; Allen, M.; Clark, W.; Weber, D. Advances in motion and electromyography based wearable technology for upper extremity function rehabilitation: A review. J. Hand Ther. 2020, 33, 180–187. [Google Scholar] [CrossRef]
- Mishra, S.; Kim, Y.S.; Intarasirisawat, J.; Kwon, Y.T.; Lee, Y.; Mahmood, M.; Lim, H.R.; Herbert, R.; Yu, K.J.; Ang, C.S.; et al. Soft, wireless periocular wearable electronics for real-time detection of eye vergence in a virtual reality toward mobile eye therapies. Sci. Adv. 2020, 6, eaay1729. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Mahmood, M.; Lee, Y.; Kim, N.K.; Kwon, S.; Herbert, R.; Kim, D.; Cho, H.C.; Yeo, W.H. All-in-One, Wireless, Stretchable Hybrid Electronics for Smart, Connected, and Ambulatory Physiological Monitoring. Adv. Sci. 2019, 6, 1900939. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Acharya, U.R.; Vinitha Sree, S.; Swapna, G.; Martis, R.J.; Suri, J.S. Automated EEG analysis of epilepsy: A review. Knowl.-Based Syst. 2013, 45, 147–165. [Google Scholar] [CrossRef]
- Bi, L.; Feleke, A.G.; Guan, C. A review on EMG-based motor intention prediction of continuous human upper limb motion for human-robot collaboration. Biomed. Signal Process. Control 2019, 51, 113–127. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Hossain, M.F.; Heo, J.S.; Nelson, J.; Kim, I. Paper-Based Flexible Electrode Using Chemically-Modified Graphene and Functionalized Multiwalled Carbon Nanotube Composites for Electrophysiological Signal Sensing. Information 2019, 10, 325. [Google Scholar] [CrossRef]
- Rajaraman, S.; Bragg, J.A.; Ross, J.D.; Allen, M.G. Micromachined three-dimensional electrode arrays for transcutaneous nerve tracking. J. Micromech. Microeng. 2011, 21, 085014. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef] [PubMed]
- Malghan, P.G.; Hota, M.K. A review on ECG filtering techniques for rhythm analysis. Res. Biomed. Eng. 2020, 36, 171–186. [Google Scholar] [CrossRef]
- Huigen, E.; Peper, A.; Grimbergen, C.A. Investigation into the origin of the noise of surface electrodes. Med. Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Ying, B.; Wu, Q.; Li, J.; Liu, X. An ambient-stable and stretchable ionic skin with multimodal sensation. Mater. Horiz. 2020, 7, 477–488. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Unal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, e1706910. [Google Scholar] [CrossRef]
- Wu, N.; Wan, S.; Su, S.; Huang, H.; Dou, G.; Sun, L. Electrode materials for brain–machine interface: A review. InfoMat 2021, 3, 1174–1194. [Google Scholar] [CrossRef]
- Plenk, H., Jr. The role of materials biocompatibility for functional electrical stimulation applications. Artif. Organs 2011, 35, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh Tonekabony Shad, E.; Molinas, M.; Ytterdal, T. Impedance and Noise of Passive and Active Dry EEG Electrodes: A Review. IEEE Sens. J. 2020, 20, 14565–14577. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Temko, A.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Popovici, E. Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors 2019, 19, 2637. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Hossain, M.M. Nanomaterials-patterned flexible electrodes for wearable health monitoring: A review. J. Mater. Sci. 2021, 56, 14900–14942. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Bellu, E.; Medici, S.; Coradduzza, D.; Cruciani, S.; Amler, E.; Maioli, M. Nanomaterials in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 7095. [Google Scholar] [CrossRef]
- Drees, C.; Makic, M.B.; Case, K.; Mancuso, M.P.; Hill, A.; Walczak, P.; Limon, S.; Biesecker, K.; Frey, L. Skin Irritation during Video-EEG Monitoring. Neurodiagn. J. 2016, 56, 139–150. [Google Scholar] [CrossRef]
- Cánovas, R.; Padrell Sánchez, S.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Cytotoxicity Study of Ionophore-Based Membranes: Toward On-Body and In Vivo Ion Sensing. ACS Sens. 2019, 4, 2524–2535. [Google Scholar] [CrossRef] [Green Version]
- Beard, R.B.; Hung, B.N.; Schmukler, R. Biocompatibility considerations at stimulating electrode interfaces. Ann. Biomed. Eng. 1992, 20, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Albulbul, A. Evaluating Major Electrode Types for Idle Biological Signal Measurements for Modern Medical Technology. Bioengineering 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Massè, A.; Tobin, E.; Cannas, M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef]
- Paladini, F.; Sannino, A.; Pollini, M. In vivo testing of silver treated fibers for the evaluation of skin irritation effect and hypoallergenicity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1031–1037. [Google Scholar] [CrossRef]
- Toropov, N.; Vartanyan, T. Noble Metal Nanoparticles: Synthesis and Optical Properties. In Comprehensive Nanoscience and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–88. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Soepriatna, A.H.; Park, W.; Moon, H.; Cox, A.; Zhao, J.; Gupta, N.S.; Park, C.H.; Kim, K.; Jeon, Y.; et al. Rapid custom prototyping of soft poroelastic biosensor for simultaneous epicardial recording and imaging. Nat. Commun. 2021, 12, 3710. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, Y.J.; Lee, Y.R.; Lee, N.-E.; Won, Y.; Gandla, S.; Kim, S.; Kim, H.-K. Highly stretchable metal-polymer hybrid conductors for wearable and self-cleaning sensors. NPG Asia Mater. 2021, 13, 4. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, D.H.; Youn, J.; Lee, H.; Jeong, J. Simple and cost-effective microfabrication of flexible and stretchable electronics for wearable multi-functional electrophysiological monitoring. Sci. Rep. 2021, 11, 14823. [Google Scholar] [CrossRef] [PubMed]
- Yun, I.; Jeung, J.; Lim, H.; Kang, J.; Lee, S.; Park, S.; Seong, S.; Park, S.; Cho, K.; Chung, Y. Stable Bioelectric Signal Acquisition Using an Enlarged Surface-Area Flexible Skin Electrode. ACS Appl. Electron. Mater. 2021, 3, 1842–1851. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Fiedler, P.; Küchler, N.; Domingues, R.P.; Lopes, C.; Borges, J.; Haueisen, J.; Vaz, F. Dry Electrodes for Surface Electromyography Based on Architectured Titanium Thin Films. Materials 2020, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Eickenscheidt, M.; Schäfer, P.; Baslan, Y.; Schwarz, C.; Stieglitz, T. Highly Porous Platinum Electrodes for Dry Ear-EEG Measurements. Sensors 2020, 20, 3176. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Chung, K.H.; Liu, G.T.; Duh, J.G.; Wang, J.H. Biocompatibility of a titanium–aluminum nitride film coating on a dental alloy. Surf. Coat. Technol. 2004, 188–189, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Sidambe, A. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Luo, G.; Xing, M. Two-Dimensional Graphene Family Material: Assembly, Biocompatibility and Sensors Applications. Sensors 2019, 19, 2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Li, H.; Dou, J.; Wang, Q.; He, W.; Wang, C.; Li, D.; Lin, J.-M.; Zhang, Y. Stable and Biocompatible Carbon Nanotube Ink Mediated by Silk Protein for Printed Electronics. Adv. Mater. 2020, 32, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Lim, H.-R.; Park, S.-W.; Kang, S.-O.; Choi, J.J.; Herbert, R.; Jang, Y.C.; et al. All-printed nanomembrane wireless bioelectronics using a biocompatible solderable graphene for multimodal human-machine interfaces. Nat. Commun. 2020, 11, 3450. [Google Scholar] [CrossRef]
- Li, B.M.; Yildiz, O.; Mills, A.C.; Flewwellin, T.J.; Bradford, P.D.; Jur, J.S. Iron-on carbon nanotube (CNT) thin films for biosensing E-Textile applications. Carbon 2020, 168, 673–683. [Google Scholar] [CrossRef]
- Sun, B.; Mccay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-Permeable, Multifunctional On-Skin Electronics Based on Laser-Induced Porous Graphene and Sugar-Templated Elastomer Sponges. Adv. Mater. 2018, 30, 1804327. [Google Scholar] [CrossRef]
- Cheng, X.; Bao, C.; Wang, X.; Zhang, F.; Dong, W. Soft surface electrode based on PDMS-CB conductive polymer for electrocardiogram recordings. Appl. Phys. A 2019, 125, 876. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.J.; Holzinger, M.; Cosnier, S. Buckypaper bioelectrodes: Emerging materials for implantable and wearable biofuel cells. Energy Environ. Sci. 2018, 11, 1670–1687. [Google Scholar] [CrossRef]
- Owens, C.E.; Headrick, R.J.; Williams, S.M.; Fike, A.J.; Pasquali, M.; McKinley, G.H.; Hart, A.J. Substrate-Versatile Direct-Write Printing of Carbon Nanotube-Based Flexible Conductors, Circuits, and Sensors. Adv. Funct. Mater. 2021, 31, 2100245. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Zhou, N.; Wang, X.; Deng, D.; Luo, L.; Chen, L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Zhang, H.; Li, Z. Characterization of PEDOT:PSS as a biocompatible conductive material. In Proceedings of the 10th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Xi’an, China, 7–11 April 2015. [Google Scholar]
- Liang, Y.; Offenhäusser, A.; Ingebrandt, S.; Mayer, D. PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals. Adv. Healthc. Mater. 2021, 10, 2100061. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Asano, D.K. Preparation and properties of polypyrrole. Synth. Met. 2003, 135–136, 43–44. [Google Scholar] [CrossRef]
- Lo, L.-W.; Zhao, J.; Wan, H.; Wang, Y.; Chakrabartty, S.; Wang, C. An Inkjet-Printed PEDOT:PSS-Based Stretchable Conductor for Wearable Health Monitoring Device Applications. ACS Appl. Mater. Interfaces 2021, 13, 21693–21702. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, Y.; Chen, S.; Yu, S.; Yang, Y.; Lin, J.; Yin, S.; Ma, Y.; Hu, B. Delamination-Resistant Imperceptible Bioelectrode for Robust Electrophysiological Signals Monitoring. ACS Mater. Lett. 2021, 3, 1385–1393. [Google Scholar] [CrossRef]
- Del Agua, I.; Mantione, D.; Ismailov, U.; Sanchez-Sanchez, A.; Aramburu, N.; Malliaras, G.G.; Mecerreyes, D.; Ismailova, E. DVS-Crosslinked PEDOT:PSS Free-Standing and Textile Electrodes toward Wearable Health Monitoring. Adv. Mater. Technol. 2018, 3, 1700322. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef]
- Tan, P.; Wang, H.; Xiao, F.; Lu, X.; Shang, W.; Deng, X.; Song, H.; Xu, Z.; Cao, J.; Gan, T.; et al. Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics. Nat. Commun. 2022, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, Q.; Hao, S.; Xu, F.; Yang, J. Self-adhesive, biodegradable silk-based dry electrodes for epidermal electrophysiological monitoring. Chem. Eng. J. 2022, 427, 131999. [Google Scholar] [CrossRef]
- Zhang, K.; Kang, N.; Zhang, B.; Xie, R.; Zhu, J.; Zou, B.; Liu, Y.; Chen, Y.; Shi, W.; Zhang, W.; et al. Skin Conformal and Antibacterial PPy-Leather Electrode for ECG Monitoring. Adv. Electron. Mater. 2020, 6, 2000259. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Y.; Jian, J.; Li, M.; Jiang, G.; Li, X.; Deng, G.; Ji, S.; Wei, Y.; Pang, Y.; et al. Multifunctional and high-performance electronic skin based on silver nanowires bridging graphene. Carbon 2020, 156, 253–260. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, J.-Y.; Zhu, J.; Hwang, H.R.; Lee, S.M.; Cheng, H.; Lee, S.-H.; Hwang, S.-W. Flexible Conductive Composite Integrated with Personal Earphone for Wireless, Real-Time Monitoring of Electrophysiological Signs. ACS Appl. Mater. Interfaces 2018, 10, 21184–21190. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.; Huang, Y.; Zhu, K.; Yi, H.; Wu, H. On-skin graphene electrodes for large area electrophysiological monitoring and human-machine interfaces. Carbon 2020, 164, 164–170. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Yu, T.; Zhang, Y.; Ye, G.; Cui, H.; He, C.; Jiang, W.; Zhai, Y.; Lu, C.; et al. Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low-motion artifact epidermal electrophysiology. Nat. Commun. 2021, 12, 4880. [Google Scholar] [CrossRef]
- Driscoll, N.; Erickson, B.; Murphy, B.B.; Richardson, A.G.; Robbins, G.; Apollo, N.V.; Mentzelopoulos, G.; Mathis, T.; Hantanasirisakul, K.; Bagga, P.; et al. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Transl. Med. 2021, 13, eabf8629. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiu, C.-W. Facile Fabrication of a Stretchable and Flexible Nanofiber Carbon Film-Sensing Electrode by Electrospinning and Its Application in Smart Clothing for ECG and EMG Monitoring. ACS Appl. Electron. Mater. 2021, 3, 676–686. [Google Scholar] [CrossRef]
- Zahed, M.A.; Das, P.S.; Maharjan, P.; Barman, S.C.; Sharifuzzaman, M.; Yoon, S.H.; Park, J.Y. Flexible and robust dry electrodes based on electroconductive polymer spray-coated 3D porous graphene for long-term electrocardiogram signal monitoring system. Carbon 2020, 165, 26–36. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Mahadevappa, M.; Mukhopadhyay, J. A 2D electrode-skin model for electrical & contact impedance characterization of Bio Impedance. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 2292–2295. [Google Scholar]
- Tong, D.A.; Bartels, K.A.; Honeyager, K.S. Adaptive reduction of motion artifact in the electrocardiogram. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; Volume 1402, pp. 1403–1404. [Google Scholar]
- Lu, F.; Wang, C.; Zhao, R.; Du, L.; Fang, Z.; Guo, X.; Zhao, Z. Review of Stratum Corneum Impedance Measurement in Non-Invasive Penetration Application. Biosensors 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, F.; Arpaia, P.; Manna, C. Characterization of human skin impedance after electrical treatment for transdermal drug delivery. Measurement 2013, 46, 3494–3501. [Google Scholar] [CrossRef]
- Ha, S.; Kim, C.; Wang, H.; Chi, Y.M.; Mercier, P.P.; Cauwenberghs, G. Chapter 6—Low-power integrated circuits for wearable electrophysiology. In Wearable Sensors, 2nd ed.; Sazonov, E., Ed.; Academic Press: Oxford, UK, 2021; pp. 163–199. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, M.; Li, Y.; Sun, Z.; Liu, Z.; He, L.; Liu, R. High-Adhesive Flexible Electrodes and Their Manufacture: A Review. Micromachines 2021, 12, 1505. [Google Scholar] [CrossRef]
- Ershad, F.; Thukral, A.; Yue, J.; Comeaux, P.; Lu, Y.; Shim, H.; Sim, K.; Kim, N.-I.; Rao, Z.; Guevara, R.; et al. Ultra-conformal drawn-on-skin electronics for multifunctional motion artifact-free sensing and point-of-care treatment. Nat. Commun. 2020, 11, 3823. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D-2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef]
- Drotlef, D.-M.; Amjadi, M.; Yunusa, M.; Sitti, M. Bioinspired Composite Microfibers for Skin Adhesion and Signal Amplification of Wearable Sensors. Adv. Mater. 2017, 29, 1701353. [Google Scholar] [CrossRef]
- Chun, S.; Kim, D.W.; Baik, S.; Lee, H.J.; Lee, J.H.; Bhang, S.H.; Pang, C. Conductive and Stretchable Adhesive Electronics with Miniaturized Octopus-Like Suckers against Dry/Wet Skin for Biosignal Monitoring. Adv. Funct. Mater. 2018, 28, 1805224. [Google Scholar] [CrossRef]
- Niu, X.; Wang, L.; Li, H.; Wang, T.; Liu, H.; He, Y. Fructus Xanthii-Inspired Low Dynamic Noise Dry Bioelectrodes for Surface Monitoring of ECG. ACS Appl. Mater. Interfaces 2022, 14, 6028–6038. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, D.; Takada, M.; Naito, H.; Arie, T.; Akita, S.; Takei, K. Efficient Skin Temperature Sensor and Stable Gel-Less Sticky ECG Sensor for a Wearable Flexible Healthcare Patch. Adv. Healthc. Mater. 2017, 6, 1700495. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Lu, L.; Du, R.; Ma, W.; Cai, Y.; An, X.; Wu, H.; Luo, Q.; Xu, Q.; et al. A Highly-Adhesive and Self-Healing Elastomer for Bio-Interfacial Electrode. Adv. Funct. Mater. 2021, 31, 2006432. [Google Scholar] [CrossRef]
- Kizilkan, E.; Gorb, S.N. Bioinspired Further Enhanced Dry Adhesive by the Combined Effect of the Microstructure and Surface Free-Energy Increase. ACS Appl. Mater. Interfaces 2018, 10, 26752–26758. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.G.; Kim, D.; Kwak, M.K.; Ha, L.; Kang, S.M.; Suh, K.Y. Enhanced Skin Adhesive Patch with Modulus-Tunable Composite Micropillars. Adv. Healthc. Mater. 2013, 2, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Jeong, H.-E.; Suh, K.Y. Rational Design and Enhanced Biocompatibility of a Dry Adhesive Medical Skin Patch. Adv. Mater. 2011, 23, 3949–3953. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bai, P.; Li, X.; Li, L.; Li, Y.; Lu, H.; Ma, L.; Meng, Y.; Tian, Y. Robust scalable reversible strong adhesion by gecko-inspired composite design. Friction 2021, 1–16. [Google Scholar] [CrossRef]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.D.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E.; et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Baik, S.; Min, H.; Chun, S.; Lee, H.J.; Kim, K.H.; Lee, J.Y.; Pang, C. Highly Permeable Skin Patch with Conductive Hierarchical Architectures Inspired by Amphibians and Octopi for Omnidirectionally Enhanced Wet Adhesion. Adv. Funct. Mater. 2019, 29, 1807614. [Google Scholar] [CrossRef]
- Chun, S.; Son, W.; Kim, D.W.; Lee, J.; Min, H.; Jung, H.; Kwon, D.; Kim, A.H.; Kim, Y.J.; Lim, S.K.; et al. Water-Resistant and Skin-Adhesive Wearable Electronics Using Graphene Fabric Sensor with Octopus-Inspired Microsuckers. ACS Appl. Mater. Interfaces 2019, 11, 16951–16957. [Google Scholar] [CrossRef]
- Baik, S.; Kim, J.; Lee, H.J.; Lee, T.H.; Pang, C. Highly Adaptable and Biocompatible Octopus-Like Adhesive Patches with Meniscus-Controlled Unfoldable 3D Microtips for Underwater Surface and Hairy Skin. Adv. Sci. 2018, 5, 1800100. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Tian, H.; Wang, C.; Wang, D.; Wu, Y.; Shao, J. Dytiscus lapponicus-Inspired Structure with High Adhesion in Dry and Underwater Environments. ACS Appl. Mater. Interfaces 2021, 13, 42287–42296. [Google Scholar] [CrossRef] [PubMed]
- Catron, N.D.; Lee, H.; Messersmith, P.B. Enhancement of poly (ethylene glycol) mucoadsorption by biomimetic end group functionalization. Biointerphases 2006, 1, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waiter, J.H. Reverse Engineering of Bioadhesion in Marine Mussels. Ann. N. Y. Acad. Sci. 1999, 875, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Wei, W.; Israelachvili, J.N.; Waite, J.H. Mussel adhesive protein provides cohesive matrix for collagen type-1α. Biomaterials 2015, 51, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, R.A.; Jin, H.; Lee, S.; Yokota, T.; Sekino, M.; Someya, T. Self-Adhesive and Ultra-Conformable, Sub-300 nm Dry Thin-Film Electrodes for Surface Monitoring of Biopotentials. Adv. Funct. Mater. 2018, 28, 1803279. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.; Cui, Y.; Ji, S.; Liu, Z.; Wan, C.; Liu, Y.; Lu, Y.; Wang, C.; Zhang, N.; et al. Highly Thermal-Wet Comfortable and Conformal Silk-Based Electrodes for On-Skin Sensors with Sweat Tolerance. ACS Nano 2021, 15, 9955–9966. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-R.; Kil, H.-J.; Kim, Y.-C.; Park, J.-W. Highly Conformable, Transparent Electrodes for Epidermal Electronics. Nano Lett. 2018, 18, 4531–4540. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Sudha, S.; Tarantino, S.; Esposti, R.; Bolzoni, F.; Cavallari, P.; Cipriani, C.; Mattoli, V.; Greco, F. Ultraconformable Temporary Tattoo Electrodes for Electrophysiology. Adv. Sci. 2018, 5, 1700771. [Google Scholar] [CrossRef] [Green Version]
- Kabiri Ameri, S.; Ho, R.; Jang, H.; Tao, L.; Wang, Y.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N. Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641. [Google Scholar] [CrossRef]
- Zhu, P.; Du, H.; Hou, X.; Lu, P.; Wang, L.; Huang, J.; Bai, N.; Wu, Z.; Fang, N.X.; Guo, C.F. Skin-electrode iontronic interface for mechanosensing. Nat. Commun. 2021, 12, 4731. [Google Scholar] [CrossRef]
- Lu, N.; Yang, S.; Wang, L. Stretchability, Conformability, and Low-Cost Manufacture of Epidermal Sensors. In Stretchable Bioelectronics for Medical Devices and Systems; Rogers, J.A., Ghaffari, R., Kim, D.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–51. [Google Scholar] [CrossRef]
- Mack, G.W.; Nadel, E.R. Body Fluid Balance During Heat Stress in Humans. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA; pp. 187–214. [CrossRef]
- Zhou, W.; Yao, S.; Wang, H.; Du, Q.; Ma, Y.; Zhu, Y. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, Devices, and Systems of On-Skin Electrodes for Electrophysiological Monitoring and Human–Machine Interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.-W.; Kim, G.-T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Breathable and Stretchable Temperature Sensors Inspired by Skin. Sci. Rep. 2015, 5, 11505. [Google Scholar] [CrossRef] [Green Version]
- Usher, B. Human sweat as a culture medium for bacteria: A preliminary report. Arch. Dermatol. Syphilol. 1928, 18, 276–280. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Teufel, L.; Pipal, A.; Schuster, K.C.; Staudinger, T.; Redl, B. Material-dependent growth of human skin bacteria on textiles investigated using challenge tests and DNA genotyping. J. Appl. Microbiol. 2010, 108, 450–461. [Google Scholar] [CrossRef]
- Kwon, Y.-T.; Norton, J.J.S.; Cutrone, A.; Lim, H.-R.; Kwon, S.; Choi, J.J.; Kim, H.S.; Jang, Y.C.; Wolpaw, J.R.; Yeo, W.-H. Breathable, large-area epidermal electronic systems for recording electromyographic activity during operant conditioning of H-reflex. Biosens. Bioelectron. 2020, 165, 112404. [Google Scholar] [CrossRef]
- Liu, Y.; Norton, J.J.S.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.-I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sun, B.; Ling, Y.; Fei, Q.; Chen, Z.; Li, X.; Guo, P.; Jeon, N.; Goswami, S.; Liao, Y.; et al. Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 205–213. [Google Scholar] [CrossRef]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, T.; Wang, L.; Li, G.; Bai, N.; Li, C.; Lu, P.; Cai, M.; Wu, Z.; Lu, N.; et al. Epidermal electrodes with enhanced breathability and high sensing performance. Mater. Today Phys. 2020, 12, 100191. [Google Scholar] [CrossRef]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Du, C.; Zhang, A.; Li, L. Breath Figure Arrays: Unconventional Fabrications, Functionalizations, and Applications. Angew. Chem. Int. Ed. 2013, 52, 12240–12255. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; di Marzo, M.; Qiao, Y.M.; Tartarini, P. Effect of liquid-solid contact angle on droplet evaporation. Fire Saf. J. 1996, 27, 141–158. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wang, Z.; Bian, H.; Hou, Y.; Wang, F.; Xu, G.; Liu, B.; Liu, Y. Ultrahigh Oxidation Resistance and High Electrical Conductivity in Copper-Silver Powder. Sci. Rep. 2016, 6, 39650. [Google Scholar] [CrossRef] [Green Version]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-Dimensional Materials in Biosensing and Healthcare: From In Vitro Diagnostics to Optogenetics and Beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, E.-M.; Hirsch, T. Recent developments in carbon-based two-dimensional materials: Synthesis and modification aspects for electrochemical sensors. Microchim. Acta 2020, 187, 441. [Google Scholar] [CrossRef]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef]
- Carpena-Núñez, J.; Rao, R.; Maruyama, B. One-pot chemistry: Alkyne-assisted CNT growth enables in situ functionalization. MRS Bull. 2021, 46, 469–470. [Google Scholar] [CrossRef]


| Electrode Material | Manufacturing Method | Conductivity/ Resistivity | SNR/Contact Impedance | Stretchability | Thickness | Ref. |
|---|---|---|---|---|---|---|
| Silk sericin-CNT ink | Sericin extraction, Drop casting | 42.1 ± 1.8 S cm−1 | N/A | N/A | N/A | [55] |
| Functionalized conductive graphene (FCG) | AJP, Spin-coating, Photonic-sintering | 1.15 Ω | 9.5 dB SNR | 60% | 3.1 nm | [56] |
| CNT encapsulation onto textile surface | Laser patterning, Heat lamination | N/A | 3.4 × 104~ 1.4 × 107 Ω (@ 100 Hz) | 20–30% | 5–10 μm | [57] |
| Laser-induced porous graphene (LIG) | Direct laser patterning | 10.96 Ω/sq | ~17 kΩ (@ 100 Hz) | 60% | ~20 μm | [58] |
| PDMS-CB | Casting, Thin film lamination to curved surface | 1 × 10−10–1 × 10−3 S/m | 13–18 kΩ (@ 10–10,000 Hz) | N/A | 30–100 μm | [59] |
| Electrode Material | Manufacturing Method | Conductivity/ Resistivity | SNR/Contact Impedance | Stretchability | Thickness | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS/PEO | IJP | 84 Ω | N/A | <50% | 0.5–4 μm | [70] |
| PEDOT:PSS | Glyceroand andpolysorbate additive treatment | 70–140 S/cm | 7 × 102–3 × 105 Ω/cm2 (@ 10–105 Hz) | 90% | 20 μm | [71] |
| PEDOT:PSS:DVS coated textile | Drop casting | 605.9 ± 28.4 S cm−1 | 1.5 × 102–1 × 105 Ω (@ 0.1–105 Hz) | <15% ± 0.4% | 100 nm | [72] |
| PEDOT:PSS, Waterborne polyurethane, D-sorbitol | Casting blended solution to mold | 100–600 S/cm | 82 kΩ cm2 (@ 10 Hz) | 43% | 20 μm | [73] |
| Self-adhesive conductive polymer (SACP) | Supermolecular solvent-doping | 1–37 S/cm | N/A | 700% | <150 μm | [74] |
| Conductive polymer polypyrrole (PPy) | In-situ polymerization | 18.5–24.7 Ω cm−2 (@ 1 Hz) | 28 Ω cm−2 | N/A | N/A | [75] |
| Type | Material | Structure | Adhesion Strength | Adhesive Durability | Other Characteristics | Ref. |
|---|---|---|---|---|---|---|
| Dry adhesive | Carbon Nanocomposite | Micropillar | ~1.3 N cm−2 | 30 attaching cycles | Underwater usable | [92] |
| Vinyl siloxane-PDMS | microfibrillar film | 1.8 N cm−2 | Bending, 300 cycles | Rough skin usable | [93] | |
| Graphene Coated fabric | Octopus-like patterned structure | 1.89 N cm−2 | N/A | Wet skin usable | [94] | |
| Ag/AgCl, TPU | Fructus Xanthii mimic structure | N/A | Bending, 5000 cycles | Hairy skin usable | [95] | |
| PSA | PDMS/PEIE/CNT composite | Thin film | 3 N cm−2 | 100 attaching cycles | Usable under sweat condition | [96] |
| PWS | Thin film | 0.41 N/cm | N/A | High resistance to motion artifact | [73] | |
| DMPA-DA | Adhesive protein | ~6.2 N cm−2 | N/A | Sweat resistant | [97] |
| Type | Material | Structure | Thickness | Young’s Modulus | Adhesion Force | SNR/Contact Impedance | Stretchability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Material modification | PEDOT:PSS, glycerol-silk fiber mat | Silk fiber mat structure | 20–30 μm | <3 MPa | N/A | ~90 kΩ (at 1 kHz) | ~250% | [112] |
| PEDOT:PSS, glycerol, polysorbate | Fibrous structure | 20 μm | 0.8 MPa | ~1.2 N/cm | 35.23 dB | 90% | [71] | |
| AgNW, PDMS, Triton X | a-PDMS matrix | N/A | 40 ± 5 kPa | ~25 N/m | ~20 kΩ (at 1 kHz) | 400% | [113] | |
| Structure modification | Au, parylene | Thin film structure | 300 nm | 20–150 kPa | N/A | 44 kΩ (at 1 kHz)/ 21.09 dB | 60% | [111] |
| PEDOT:PSS, graphene, SDS, BSL | Thin film structure | 100 nm | 640 kPa | N/A | 32 kΩ (at 100 Hz)/ 23 ± 0.7 dB | 40% | [80] | |
| PEDOT:PSS, Au | Tattoo structure | 600–1200 nm | 1 GPa | N/A | 294 kΩ (at 60 Hz, after 60 min) | 10% | [114] | |
| Graphene, PMMA | Tattoo structure | 463 nm | 20–150 kPa | N/A | 15.22 dB | 40% | [115] |
| Type | Material | Core Breathable Characteristics | Thickness | Breathability (WVTR a) | Ref. |
|---|---|---|---|---|---|
| Intrinsically breathable material substrates | Au, PI, elastomer, fabric | Composite of breathable elastomer and the fabric | ~1.1 mm | 0.31 mg cm−2 h−1 | [126] |
| Cellular silicone/elastomeric fabric | Composite of breathable elastomer and the fabric | Elastomer (~100 μm), fabric (~1 mm) | ~1 mg cm−2 h−1 | [121] | |
| Silbione, Ecoflex | Composite of breathable silicone and elastomer layer | 2 mm | 2 mg h−1 | [127] | |
| Post-processed Porous substrates | SEBS | Phase separation generated pores | ~100 μm | 20.6 mg cm−2 h−1 | [128] |
| AgNW, TPU | Breathable pores generated with breath figure method | 4.6 μm | 23 mg cm−2 h−1 | [119] | |
| Silbione/Ecoflex | Microperforated silicone layer | 88 μm | can be controlled | [129] | |
| Graphene, silicone elastomer sponge | Sugar templated porous silicone elastomer sponges | 500 μm | 18 mg cm−2 h−1 | [58] | |
| Substrate-free | Au Nanomesh | Breathable Nano mesh directly attached on skin | Several tens of nanometers | 5.95 mg h−1 | [24] |
| Au Nanomesh | Hydrophilically treated breathable Au nanomeshes design | 30 nm | N/A | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, E.; Choi, C.; Yeo, W.-H. Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices. Micromachines 2022, 13, 629. https://doi.org/10.3390/mi13040629
Kim H, Kim E, Choi C, Yeo W-H. Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices. Micromachines. 2022; 13(4):629. https://doi.org/10.3390/mi13040629
Chicago/Turabian StyleKim, Hyeonseok, Eugene Kim, Chanyeong Choi, and Woon-Hong Yeo. 2022. "Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices" Micromachines 13, no. 4: 629. https://doi.org/10.3390/mi13040629
APA StyleKim, H., Kim, E., Choi, C., & Yeo, W.-H. (2022). Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices. Micromachines, 13(4), 629. https://doi.org/10.3390/mi13040629