Identifying and Manipulating Giant Vesicles: Review of Recent Approaches
Abstract
1. Introduction
2. Identification Methods of GVs
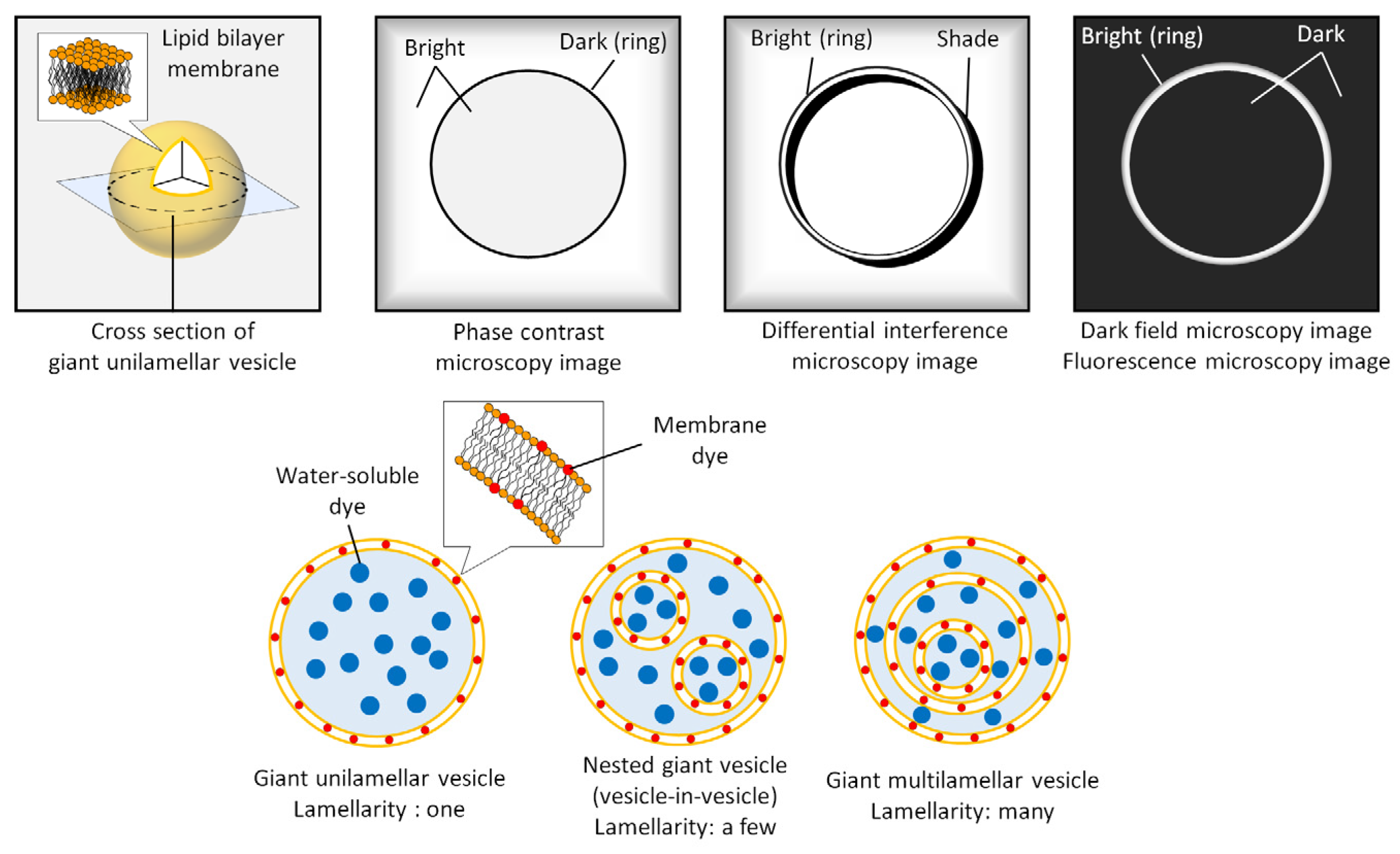
2.1. Morphology
2.2. Lamellarity
2.3. Membrane Bending Rigidity
2.4. Permeability and Intramembrane Ion Current
2.5. Other Properties
3. Manipulation Methods of GVs
3.1. Immobilization on a Substrate
3.2. Size Sorting and Purification
3.3. Microfluidic Manipulation
3.4. Micropipette Manipulation
3.5. Optical Trapping and Acoustic Trapping
3.6. Electric and Magnetic Field Application
3.7. Laser-Assisted Three-Dimensional (3D) Printing Inside of GUV
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Ashkenasy, G.; Hermans, T.M.; Otto, S.; Taylor, A.F. Systems chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. Artificial cells with emphasis on bioencapsulation in biotechnology. Biotech. Ann. Rev. 1995, 1, 267–295. [Google Scholar]
- Dimova, R.; Stano, P.; Marques, C.M.; Walde, P. Preparation methods for giant unilamellar vesicles. In The Giant Vesicle Book; Dimova, R., Marques, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–16. [Google Scholar]
- Sessa, G.; Weissmann, G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968, 9, 310–318. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef]
- Criado, M.; Keller, B.U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987, 224, 172–176. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Cellular mimics engineered from diblock copolymers. In Proceedings of the First Joint BMES/EMBS Conference: 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society, Atlanta, GA, USA, 13–16 October 1999; Volume 1, p. 75. [Google Scholar]
- Tanaka, S.; Takiguchi, K.; Hayashi, M. Repetitive stretching of giant liposomes utilizing the nematic alignment of confined actin. Commun. Phys. 2018, 1, 18. [Google Scholar] [CrossRef]
- Lach, S.; Yoon, S.M.; Grzybowski, B.A. Tactic, reactive, and functional droplets outside of equilibrium. Chem. Soc. Rev. 2016, 45, 4766–4796. [Google Scholar] [CrossRef]
- Sato, Y.; Takinoue, M. Creation of artificial cell-like structures promoted by microfluidics technologies. Micromachines 2019, 10, 216. [Google Scholar] [CrossRef]
- Robinson, T. Microfluidic handling and analysis of giant vesicles for use as artificial cells: A review. Adv. Biosyst. 2019, 3, 1800318. [Google Scholar] [CrossRef]
- Lyu, Y.; Peng, R.; Liu, H.; Kuai, H.; Mo, L.; Han, D.; Li, J.; Tan, W. Protocells programmed through artificial reaction networks. Chem. Sci. 2020, 11, 631–642. [Google Scholar] [CrossRef]
- Olivi, L.; Berger, M.; Creyghton, R.N.; De Franceschi, N.; Dekker, C.; Mulder, B.M.; Claassens, N.J.; ten Wolde, P.R.; van der Oost, J. Towards a synthetic cell cycle. Nat. Commun. 2021, 12, 4531. [Google Scholar] [CrossRef]
- Stano, P.; Luisi, P.L. Semi-synthetic minimal cells: Origin and recent developments. Curr. Opin. Biotechnol. 2013, 24, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Systems of creation: The emergence of life from nonliving matter. Acc. Chem. Res. 2012, 45, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.; Szostak, J.W. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Tamura, M.; Shohda, K.-I.; Toyota, T.; Suzuki, K.; Sugawara, T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 2011, 3, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, K.A.; Devaraj, N.K. Synthesis of lipid membranes for artificial cells. Nat. Rev. Chem. 2021, 5, 676–694. [Google Scholar] [CrossRef]
- Hermann, E.; Bleicken, S.; Subburaj, Y.; García-Sáez, A.J. Automated analysis of giant unilamellar vesicles using circular Hough transformation. Bioinformatics 2014, 30, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Roy, D.; Steinkühler, J.; Robinson, T.; Lipowsky, R.; Dimova, R. Super-Resolution Imaging of Highly Curved Membrane Structures in Giant Vesicles Encapsulating Molecular Condensates. Adv. Mater. 2022, 34, 2106633. [Google Scholar] [CrossRef]
- Roy, D.; Steinkühler, J.; Zhao, Z.; Lipowsky, R.; Dimova, R. Mechanical Tension of Biomembranes Can Be Measured by Super Resolution (STED) Microscopy of Force-Induced Nanotubes. Nano Lett. 2020, 20, 3185–3191. [Google Scholar] [CrossRef]
- Vorauer-Uhl, K.; Wagner, A.; Borth, N.; Katinger, H. Determination of liposome size distribution by flow cytometry. Cytometry 2000, 39, 166–171. [Google Scholar] [CrossRef]
- Sato, K.; Obinata, K.; Sugawara, T.; Urabe, I.; Yomo, T. Quantification of structural properties of cell-sized individual liposomes by flow cytometry. J. Biosci. Bioeng. 2006, 102, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Hosoi, T.; Sunami, T.; Toyota, T.; Fujinami, M.; Oguma, K.; Matsuura, T.; Suzuki, H.; Yomo, T. Population analysis of structural properties of giant liposomes by flow cytometry. Langmuir 2009, 25, 10439–10443. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Ishiodori, Y.; Hanczyc, M.M.; Wang, A.; Szostak, J.W.; Yomo, T. Using imaging flow cytometry to quantify and optimize giant vesicle production by water-in-oil emulsion transfer methods. Langmuir 2019, 35, 2375–2382. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of unilamellar vesicles using an inverted emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Bayley, H. Pore-forming proteins with built-in triggers and switches. Bioorg. Chem. 1995, 23, 340–354. [Google Scholar] [CrossRef]
- Noireaux, V.; Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674. [Google Scholar] [CrossRef]
- Shoji, K.; Kawano, R. Recent advances in liposome-based molecular robots. Micromachines 2020, 11, 788. [Google Scholar] [CrossRef]
- Xu, C.; Lu, P.; Gamal El-Din, T.M.; Pei, X.Y.; Johnson, M.C.; Uyeda, A.; Bick, M.J.; Xu, Q.; Jiang, D.; Bai, H.; et al. Computational design of transmembrane pores. Nature 2020, 585, 129–134. [Google Scholar] [CrossRef]
- Chiba, M.; Miyazaki, M.; Ishiwata, S.I. Quantitative analysis of the lamellarity of giant liposomes prepared by the inverted emulsion method. Biophys. J. 2014, 107, 346–354. [Google Scholar] [CrossRef]
- McPhee, C.I.; Zoriniants, G.; Langbein, W.; Borri, P. Measuring the lamellarity of giant lipid vesicles with differential interference contrast microscopy. Biophys. J. 2013, 105, 1414–1420. [Google Scholar] [CrossRef]
- Karal, M.A.S.; Ahamed, M.K.; Ahmed, M.; Mahbubb, Z.B. Recent developments in the kinetics of ruptures of giant vesicles under constant tension. RSC Adv. 2021, 11, 29598. [Google Scholar] [CrossRef]
- Fa, N.; Marques, C.M.; Mendes, E.; Schröder, A.P. Rheology of giant vesicles: A micropipette study. Phys. Rev. Lett. 2004, 92, 108103. [Google Scholar] [CrossRef]
- Elias, M.; Dutoya, A.; Laborde, A.; Lecestre, A.; Montis, C.; Caselli, L.; Berti, D.; Lonetti, B.; Roux, C.; Joseph, P. Microfluidic characterization of biomimetic membrane mechanics with an on-chip micropipette. Micro Nano Eng. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Portet, T.; Dimova, R. A new method for measuring edge tensions and stability of lipid bilayers: Effect of membrane composition. Biophys. J. 2010, 99, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Ratanabanangkoon, P.; Gropper, M.; Merkel, R.; Sackmann, E.; Gast, A.P. Mechanics of Streptavidin-Coated Giant Lipid Bilayer Vesicles: A Micropipet Study. Langmuir 2003, 19, 1054–1062. [Google Scholar] [CrossRef][Green Version]
- Méléard, P.; Pott, T. Overview of a quest for bending elasticity measurement. Adv. Planar Lipid Bilayers Liposomes 2013, 17, 55–75. [Google Scholar]
- Drabik, D.; Przybyło, M.; Chodaczek, G.; Iglič, A.; Langner, M. The modified fluorescence based vesicle fluctuation spectroscopy technique for determination of lipid bilayer bending properties. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 244–252. [Google Scholar] [CrossRef]
- Faizi, H.A.; Reeves, C.J.; Georgiev, V.N.; Vlahovska, P.M.; Dimova, R. Fluctuation spectroscopy of giant unilamellar vesicles using confocal and phase contrast microscopy. Soft Matter 2020, 16, 8996–9001. [Google Scholar] [CrossRef]
- Boyd, M.A.; Kamat, N.P. Visualizing tension and growth in model membranes using optical dyes. Biophys. J. 2018, 115, 1307–1315. [Google Scholar] [CrossRef]
- Colom, A.; Derivery, E.; Soleimanpour, S.; Tomba, C.; Molin, M.D.; Sakai, N.; González-Gaitán, M.; Matile, S.; Roux, A. A fluorescent membrane tension probe. Nat. Chem. 2018, 10, 1118–1125. [Google Scholar] [CrossRef]
- Hasan, M.; Yamazaki, M. Elementary Processes and Mechanisms of Interactions of Antimicrobial Peptides with Membranes—Single Giant Unilamellar Vesicle Studies. In Antimicrobial Peptides; Matsuzaki, K., Ed.; Springer: Singapore, 2019; pp. 17–32. [Google Scholar]
- Robinson, T.; Kuhn, P.; Eyer, K.; Dittrich, P.S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics 2013, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Osaki, T.; Takeuchi, S.; Toyota, T. Hydrodynamic accumulation of small molecules and ions into cell-sized liposomes against a concentration gradient. Commun. Chem. 2020, 3, 32. [Google Scholar] [CrossRef]
- Sugiyama, H.; Osaki, T.; Takeuchi, S.; Toyota, T. Role of Negatively Charged Lipids Achieving Rapid Accumulation of Water-Soluble Molecules and Macromolecules into Cell-Sized Liposomes against a Concentration Gradient. Langmuir 2022, 38, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Tabuchi, M.; Toyota, T.; Sakurai, T.; Hosoi, T.; Nomoto, T.; Nakatani, K.; Fujinami, M.; Kanzaki, R. Giant vesicles functionally expressing membrane receptors for an insect pheromone. Chem. Commun. 2014, 50, 2958–2961. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Nitta, M.; Sakamoto, M.; Shoji, A.; Sugawara, M. Alamethicin Channels as a Signal Transduction Element in an Immobilized Single Giant Unilamellar Vesicle. Sens. Mater. 2021, 33, 171–180. [Google Scholar] [CrossRef]
- Velasco-Olmo, A.; Ormaetxea Gisasola, J.; Martinez Galvez, J.M.; Vera Lillo, J.; Shnyrova, A.V. Combining patch-clamping and fluorescence microscopy for quantitative reconstitution of cellular membrane processes with Giant Suspended Bilayers. Sci. Rep. 2019, 9, 7255. [Google Scholar] [CrossRef]
- Biltonen, R.L.; Lichtenberg, D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Yandrapalli, N.; Petit, J.; Bäumchen, O.; Robinson, T. Surfactant-free production of biomimetic giant unilamellar vesicles using PDMS-based microfluidics. Commun. Chem. 2021, 4, 100. [Google Scholar] [CrossRef]
- Lavaisse, L.M.; Hollmann, A.; Nazareno, M.A.; Disalvo, E.A. Zeta potential changes of Saccharomyces cerevisiae during fermentative and respiratory cycles. Colloids Surf. B Biointerfaces 2019, 174, 63–69. [Google Scholar] [CrossRef]
- Jimbo, T.; Sakuma, Y.; Urakami, N.; Ziherl, P.; Imai, M. Role of Inverse-Cone-Shape Lipids in Temperature-Controlled Self-Reproduction of Binary Vesicles. Biophys. J. 2016, 110, 1551–1562. [Google Scholar]
- Loftus, A.F.; Noreng, S.; Hsieh, V.L.; Parthasarathy, R. Robust measurement of membrane bending moduli using light sheet fluorescence imaging of vesicle fluctuations. Langmuir 2013, 29, 14588–14594. [Google Scholar] [CrossRef]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, D.; Vezy, C.; Viallat, A.; Bassereau, P.; Nassoy, P. Mimicking cell/extracellular matrix adhesion with lipid membranes and solid substrates: Requirements, pitfalls and proposals. J. Phys. Condens. Matter 2004, 16, S2427. [Google Scholar] [CrossRef]
- Van Lengerich, B.; Rawle, R.J.; Boxer, S.G. Covalent attachment of lipid vesicles to a fluid-supported bilayer allows observation of DNA-mediated vesicle interactions. Langmuir 2010, 26, 8666–8672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guttenberg, Z.; Lorz, B.; Sackmann, E.; Boulbitch, A. First-order transition between adhesion states in a system mimicking cell-tissue interaction. Europhys. Lett. 2001, 54, 826–832. [Google Scholar] [CrossRef]
- Wang, T.; Ingram, C.; Weisshaar, J.C. Model lipid bilayer with facile diffusion of lipids and integral membrane proteins. Langmuir 2010, 26, 11157–11164. [Google Scholar] [CrossRef]
- Lira, R.B.; Steinkühler, J.; Knorr, R.L.; Dimova, R.; Riske, K.A. Posing for a picture: Vesicle immobilization in agarose gel. Sci. Rep. 2016, 6, 25254. [Google Scholar] [CrossRef]
- Misawa, N.; Motegi, T.; Tero, R. Electroformation from patterned single-layered supported lipid bilayers for formation of giant vesicles with narrow size distribution. Appl. Phys. Express 2014, 7, 117001. [Google Scholar] [CrossRef]
- Ushiyama, R.; Koiwai, K.; Suzuki, H. Plug-and-play microfluidic production of monodisperse giant unilamellar vesicles using droplet transfer across Water–Oil interface. Sens. Actuators. B Chem. 2022, 355, 131281. [Google Scholar] [CrossRef]
- Karal, M.A.S.; Rahman, M.; Ahamed, M.; Shibly, S.U.A.; Ahmed, M.; Shakil, M. Low-cost non-electromechanical technique for the purification of giant unilamellar vesicles. Euro. Biophys. J. 2019, 48, 349–359. [Google Scholar] [CrossRef]
- Tamba, Y.; Terashima, H.; Yamazaki, M. A membrane filtering method for the purification of giant unilamellar vesicles. Chem. Phys. Lipids 2011, 164, 351–358. [Google Scholar] [CrossRef]
- Zhu, T.F.; Szostak, J.W. Preparation of large monodisperse vesicles. PLoS ONE 2009, 4, e5009. [Google Scholar] [CrossRef] [PubMed]
- Witek, M.A.; Freed, I.M.; Soper, S.A. Cell separations and sorting. Anal. Chem. 2019, 92, 105–131. [Google Scholar] [CrossRef]
- Kazayama, Y.; Teshima, T.; Osaki, T.; Takeuchi, S.; Toyota, T. Integrated microfluidic system for size-based selection and trapping of giant vesicles. Anal. Chem. 2016, 88, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Yandrapalli, N.; Robinson, T. Ultra-high capacity microfluidic trapping of giant vesicles for high-throughput membrane studies. Lab Chip 2019, 19, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Tivony, R.; Fletcher, M.; Al Nahas, K.; Keyser, U.F. A microfluidic platform for sequential assembly and separation of synthetic cell models. ACS Synth. Biol. 2021, 10, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Lee, S.; Bassereau, P.; Baroud, C.N. Trapping and release of giant unilamellar vesicles in microfluidic wells. Soft Matter 2014, 10, 5878–5885. [Google Scholar] [CrossRef] [PubMed]
- Ganzinger, K.A.; Merino-Salomón, A.; García-Soriano, D.A.; Butterfield, A.N.; Litschel, T.; Siedler, F.; Schwille, P. FtsZ reorganization facilitates deformation of giant vesicles in microfluidic traps. Angew. Chem. Int. Ed. 2020, 59, 21372–21376. [Google Scholar] [CrossRef]
- Deshpande, S.; Spoelstra, W.K.; Van Doorn, M.; Kerssemakers, J.; Dekker, C. Mechanical division of cell-sized liposomes. ACS Nano 2018, 12, 2560–2568. [Google Scholar] [CrossRef]
- Kumar, D.; Schroeder, C.M. Nonlinear Transient and Steady State Stretching of Deflated Vesicles in Flow. Langmuir 2021, 37, 13976–13984. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ault, J.T.; Stone, H.A. Flow-Driven Rapid Vesicle Fusion via Vortex Trapping. Langmuir 2015, 31, 7178–7182. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.B.; Robinson, T.; Dimova, R.; Riske, K.A. Highly efficient protein-free membrane fusion: A giant vesicle study. Biophys. J. 2019, 116, 79–91. [Google Scholar] [CrossRef]
- Somasundar, A.; Ghosh, S.; Mohajerani, F.; Massenburg, L.N.; Yang, T.; Cremer, P.S.; Velegol, D.; Sen, A. Positive and negative chemotaxis of enzyme-coated liposome motors. Nat. Nanotechnol. 2019, 14, 1129–1134. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Needham, D. Quantitative optical microscopy and micromanipulation studies on the lipid bilayer membranes of giant unilamellar vesicles. Chem. Phys. Lipids 2014, 181, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Karlsson, M.; Sinclair, J.; Sott, K.; Orwar, O. Nanotube−Vesicle Networks with Functionalized Membranes and Interiors. J. Am. Chem. Soc. 2003, 125, 374–378. [Google Scholar] [CrossRef]
- Jesorka, A.; Stepanyants, N.; Zhang, H.; Ortmen, B.; Hakonen, B.; Orwar, O. Generation of phospholipid vesicle-nanotube networks and transport of molecules therein. Nat. Protoc. 2011, 6, 791–805. [Google Scholar] [CrossRef]
- Ali Doosti, B.; Fjällborg, D.; Kustanovich, K.; Jesorka, A.; Cans, A.S.; Lobovkina, T. Generation of interconnected vesicles in a liposomal cell model. Sci. Rep. 2020, 10, 14040. [Google Scholar] [CrossRef]
- Wick, R.; Angelova, M.I.; Walde, P.; Luisi, P.L. Microinjection into giant vesicles and light microscopy investigation of enzyme-mediated vesicle transformations. Chem. Biol. 1996, 3, 105–111. [Google Scholar] [CrossRef]
- Bucher, P.; Fischer, A.; Luisi, P.L.; Oberholzer, T.; Walde, P. Giant vesicles as biochemical compartments: The use of microinjection techniques. Langmuir 1998, 14, 2712–2721. [Google Scholar] [CrossRef]
- Lefrançois, P.; Goudeau, B.; Arbault, S. Dynamic monitoring of a bi-enzymatic reaction at a single biomimetic giant vesicle. Analyst 2020, 145, 7922–7931. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.J.; Liu, K.K.; Chan, V. Thermal effect on a viscously deformed liposome in a laser trap. Annal. Biomed. Eng. 2003, 31, 354–362. [Google Scholar] [CrossRef]
- Spyratou, E.; Mourelatou, E.A.; Georgopoulos, A.; Demetzos, C.; Makropoulou, M.; Serafetinides, A.A. Line optical tweezers: A tool to induce transformations in stained liposomes and to estimate shear modulus. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 35–42. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yoshikawa, K. Optical transport of a single cell-sized liposome. Appl. Phys. Lett. 2001, 79, 4598–4600. [Google Scholar] [CrossRef]
- Kulin, S.; Kishore, R.; Helmerson, K.; Locascio, L. Optical manipulation and fusion of liposomes as microreactors. Langmuir 2003, 19, 8206–8210. [Google Scholar] [CrossRef]
- Inaba, T.; Ishijima, A.; Honda, M.; Nomura, F.; Takiguchi, K.; Hotani, H. Formation and maintenance of tubular membrane projections require mechanical force, but their elongation and shortening do not require additional force. J. Mol. Biol. 2005, 348, 325–333. [Google Scholar] [CrossRef]
- Bolognesi, G.; Friddin, M.S.; Salehi-Reyhani, A.; Barlow, N.E.; Brooks, N.J.; Ces, O.; Elani, Y. Sculpting and fusing biomimetic vesicle networks using optical tweezers. Nat. Commun. 2018, 9, 1882. [Google Scholar] [CrossRef]
- Shiomi, H.; Tsuda, S.; Suzuki, H.; Yomo, T. Liposome-based liquid handling platform featuring addition, mixing, and aliquoting of femtoliter volumes. PLoS ONE 2014, 9, e101820. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamaura, D.; Komiya, M.; Sugawara, M.; Mitsumori, Y.; Niwano, M.; Hirano-Iwata, A.; Edamatsu, K.; Sadgrove, M. Optical transport of sub-micron lipid vesicles along a nanofiber. Opt. Express 2020, 28, 38527–38538. [Google Scholar] [CrossRef]
- Pong, M.; Umchid, S.; Guarino, A.J.; Lewin, P.A.; Litniewski, J.; Nowicki, A.; Wrenn, S.P. In vitro ultrasound-mediated leakage from phospholipid vesicles. Ultrasonics 2006, 45, 133–145. [Google Scholar] [CrossRef]
- Dolatmoradi, A.; El-Zahab, B. Thermally-assisted ultrasonic separation of giant vesicles. Lab Chip 2016, 16, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, L.; Du, H.; Li, M.; Mu, W.; Drinkwater, B.W.; Han, X.; Mann, S. Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays. Chem. Sci. 2019, 10, 9446–9453. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.; Tian, L.; Franklin, A.; Wang, X.; Han, X.; Mann, S.; Drinkwater, B.W. Acoustic deformation for the extraction of mechanical properties of lipid vesicle populations. Phys. Rev. E 2019, 99, 063002. [Google Scholar] [CrossRef] [PubMed]
- Pereno, V.; Lei, J.; Carugo, D.; Stride, E. Microstreaming inside model cells induced by ultrasound and microbubbles. Langmuir 2020, 36, 6388–6398. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Bezlyepkina, N.; Jordö, M.D.; Knorr, R.L.; Riske, K.A.; Staykova, M.; Vlahovska, P.M.; Yamamoto, T.; Yang, P.; Lipowsky, R. Vesicles in electric fields: Some novel aspects of membrane behavior. Soft Matter 2009, 5, 3201–3212. [Google Scholar] [CrossRef]
- Korlach, J.; Reichle, C.; Müller, T.; Schnelle, T.; Webb, W.W. Trapping, deformation, and rotation of giant unilamellar vesicles in octode dielectrophoretic field cages. Biophys. J. 2005, 89, 554–562. [Google Scholar] [CrossRef]
- Knorr, R.L.; Staykova, M.; Gracia, R.S.; Dimova, R. Wrinkling and electroporation of giant vesicles in the gel phase. Soft Matter 2010, 6, 1990–1996. [Google Scholar] [CrossRef]
- Sugahara, K.; Morimoto, Y.; Takamori, S.; Takeuchi, S. A dynamic microarray device for pairing and electrofusion of giant unilamellar vesicles. Sens. Actuators B Chem. 2020, 311, 127922. [Google Scholar] [CrossRef]
- Terasawa, H.; Nishimura, K.; Suzuki, H.; Matsuura, T.; Yomo, T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. USA 2012, 109, 5942–5947. [Google Scholar] [CrossRef]
- Miyakawa, S.; Uesugi, K.; Morishima, K. A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell. Micromachines 2018, 9, 331. [Google Scholar] [CrossRef]
- Saito, A.C.; Ogura, T.; Fujiwara, K.; Murata, S.; Nomura, S.I.M. Introducing micrometer-sized artificial objects into live cells: A method for cell–giant unilamellar vesicle electrofusion. PLoS ONE 2014, 9, e106853. [Google Scholar] [CrossRef]
- Desai, S.P.; Vahey, M.D.; Voldman, J. Electrically addressable vesicles: Tools for dielectrophoresis metrology. Langmuir 2009, 25, 3867–3875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, Y.; Delaney, W.F.; Darroudi, T.; Wang, P. Microwave measurement of giant unilamellar vesicles in aqueous solution. Sci. Rep. 2018, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Nat. C 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, S.; Kurashima, H.; Abe, H. High-Magnetic-Field Effects on Liposomes and Black Membranes of Dipalmitoylphosphatidylcholin: Magnetoresponses in Membrane Potential and Magnetofusion. J. Phys. Chem. B 2000, 104, 5657–5660. [Google Scholar] [CrossRef]
- Suzuki, K.; Toyota, T.; Sato, K.; Iwasaka, M.; Ueno, S.; Sugawara, T. Characteristic curved structure derived from collagen-containing tubular giant vesicles under static magnetic field. Chem. Phys. Lett. 2007, 440, 286–290. [Google Scholar] [CrossRef]
- Bacri, J.C.; Cabuil, V.; Cebers, A.; Ménager, C.; Perzynski, R. Flattening of ferro-vesicle undulations under a magnetic field. Europhys. Lett. 1996, 33, 235–240. [Google Scholar] [CrossRef]
- Veloso, S.R.; Andrade, R.G.; Castanheira, E.M. Magnetoliposomes: Recent advances in the field of controlled drug delivery. Expert Opin. Drug Deliv. 2021, 18, 1323–1334. [Google Scholar] [CrossRef]
- Komatsu, D.; Fujiwara, K.; Nomura, S.I. Construction of a remote-controlled supramolecular micro-crawler. In ECAL 2013: The Twelfth European Conference on Artificial Life; MIT Press: Cambridge, MA, USA, 2013; pp. 208–209. [Google Scholar]
- Li, Q.; Li, S.; Zhang, X.; Xu, W.; Han, X. Programmed magnetic manipulation of vesicles into spatially coded prototissue architectures arrays. Nat. Commun. 2020, 11, 232. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Abele, T.; Messer, T.; Jahnke, K.; Hippler, M.; Bastmeyer, M.; Wegener, M.; Göpfrich, K. Two-Photon 3D Laser Printing Inside Synthetic Cells. Adv. Mater. 2022, 34, 2106709. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Jahnke, K.; Abele, T.; Göpfrich, K. Printing and Erasing of DNA-Based Photoresists Inside Synthetic Cells. Adv. Funct. Mater. 2022, 2200762. [Google Scholar] [CrossRef]
- Villar, G.; Graham, A.D.; Bayley, H. A tissue-like printed material. Science 2013, 340, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Mukwaya, V.; Mann, S.; Dou, H. Chemical communication at the synthetic cell/living cell interface. Commun. Chem. 2021, 4, 161. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyota, T.; Zhang, Y. Identifying and Manipulating Giant Vesicles: Review of Recent Approaches. Micromachines 2022, 13, 644. https://doi.org/10.3390/mi13050644
Toyota T, Zhang Y. Identifying and Manipulating Giant Vesicles: Review of Recent Approaches. Micromachines. 2022; 13(5):644. https://doi.org/10.3390/mi13050644
Chicago/Turabian StyleToyota, Taro, and Yiting Zhang. 2022. "Identifying and Manipulating Giant Vesicles: Review of Recent Approaches" Micromachines 13, no. 5: 644. https://doi.org/10.3390/mi13050644
APA StyleToyota, T., & Zhang, Y. (2022). Identifying and Manipulating Giant Vesicles: Review of Recent Approaches. Micromachines, 13(5), 644. https://doi.org/10.3390/mi13050644





