Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monotheca buxifolia Extract Preparation
2.2. Biosynthesis of Zinc Oxide Nanoparticles (ZnO-NPs)
2.3. Characterization of Biosynthesized ZnO-NPs
2.4. Antibacterial Assay
2.4.1. Collection of Bacteria
2.4.2. Preparation of Antibiotic Discs Coated with ZnO-NPs
2.4.3. Agar Well Diffusion Assay for ZnO-NPs
2.4.4. Disc Diffusion Assay for ZnO-NPs Coated and Non-Coated Antibiotics
2.5. Antifungal Assay
2.6. Anti-Leishmanial Assay
2.7. Anti-Alzheimer’s Assay
2.8. Protein Kinase Inhibition Assay
2.9. Anti-Diabetic Assay
2.9.1. α-Amylase Inhibition Assay
2.9.2. α-Glucosidase Inhibition Assay
2.10. Biocompatibility Studies
3. Results
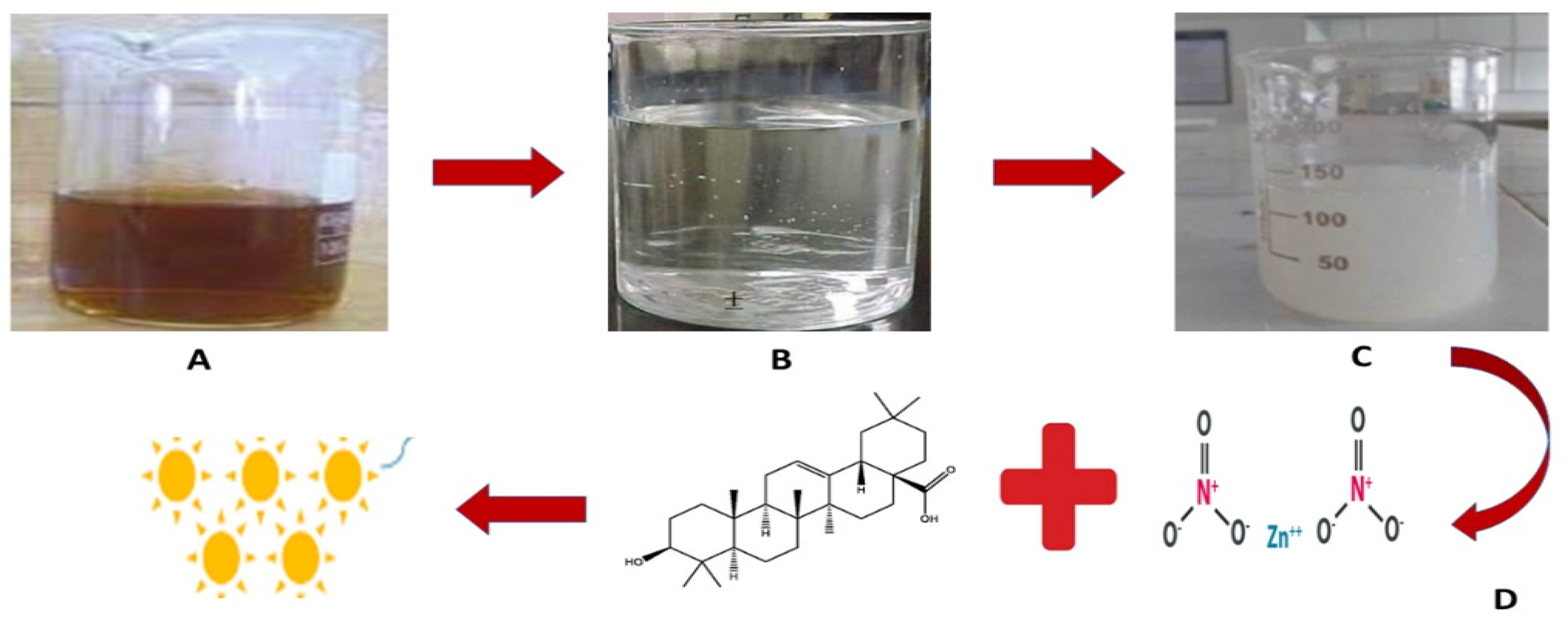
3.1. Synthesis of Zinc Oxide Nanoparticles Using Monotheca buxifolia Extract
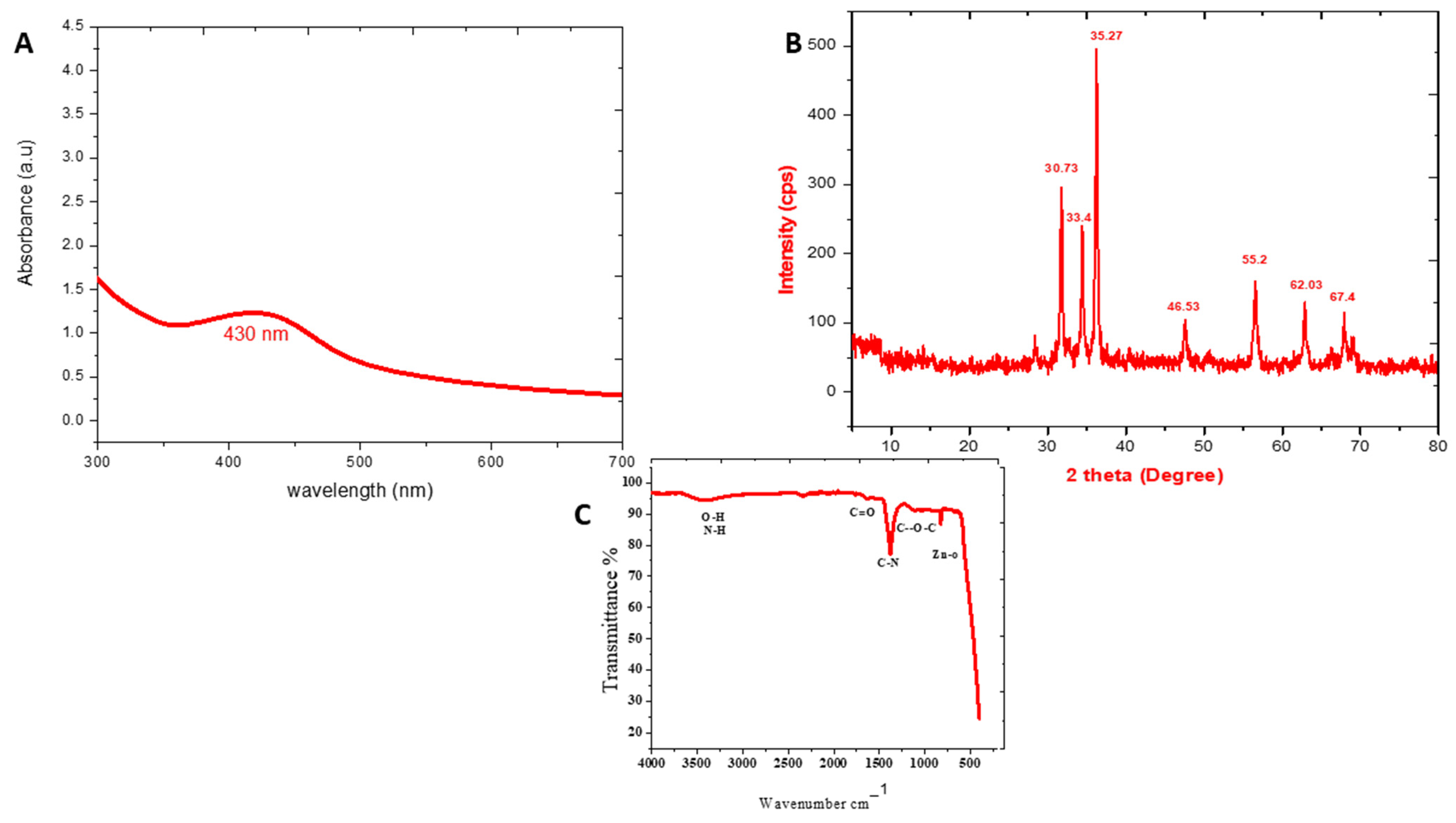
3.2. UV-Analysis
3.3. XRD Analysis
3.4. FTIR Analysis
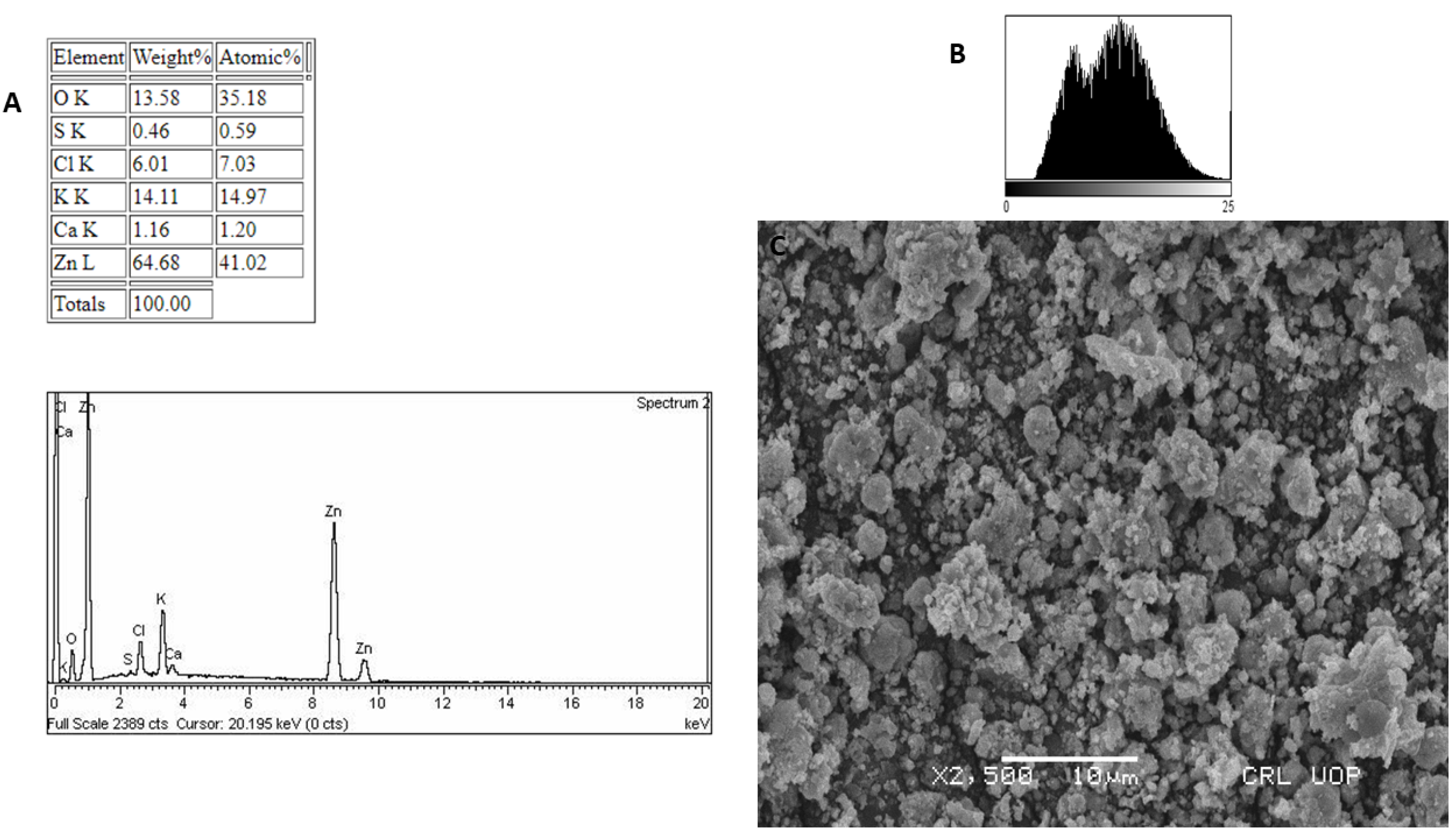
3.5. EDX and SEM Analysis
3.6. Antibacterial Activity of ZnO-NPs
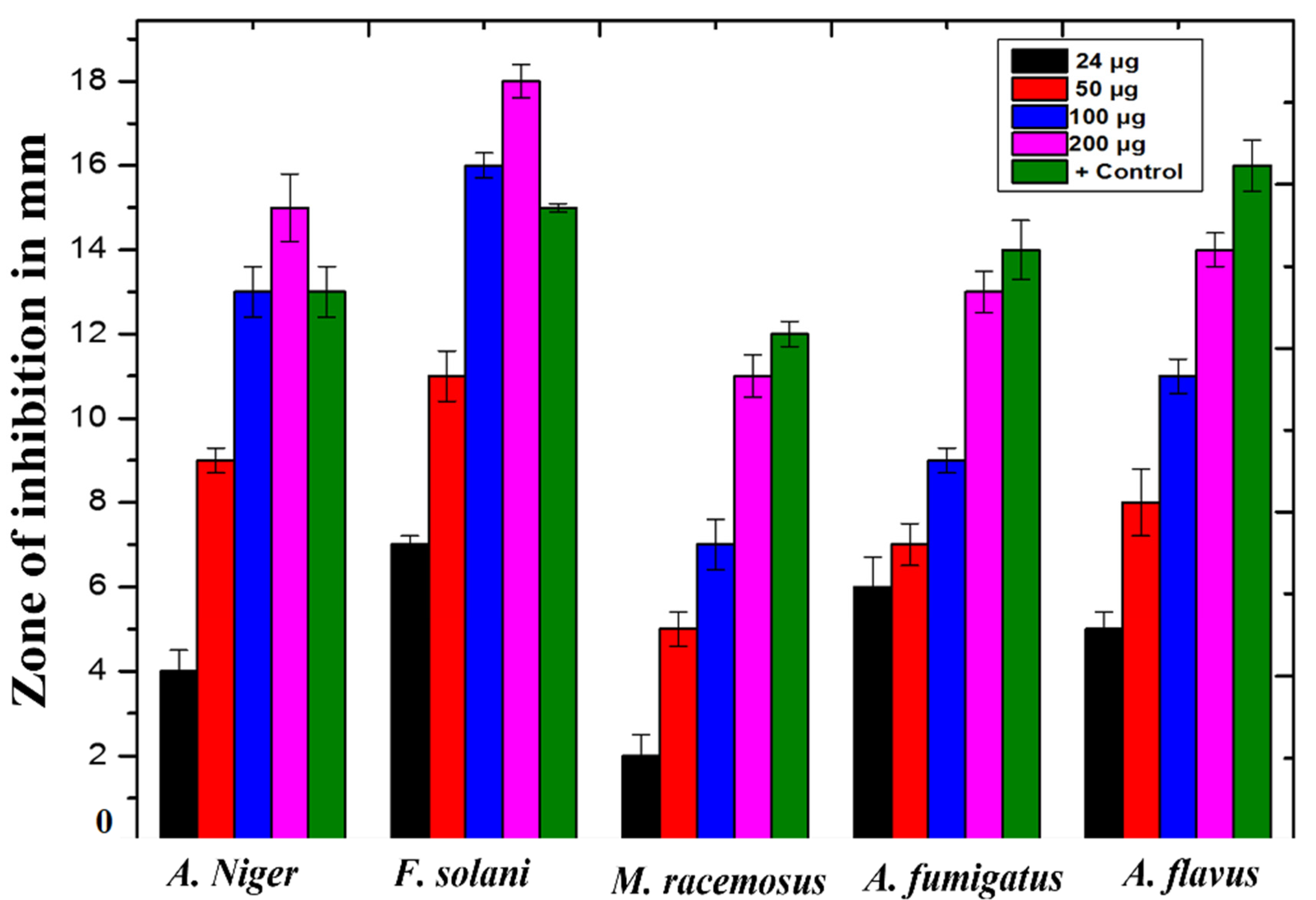
3.7. Antifungal Activity
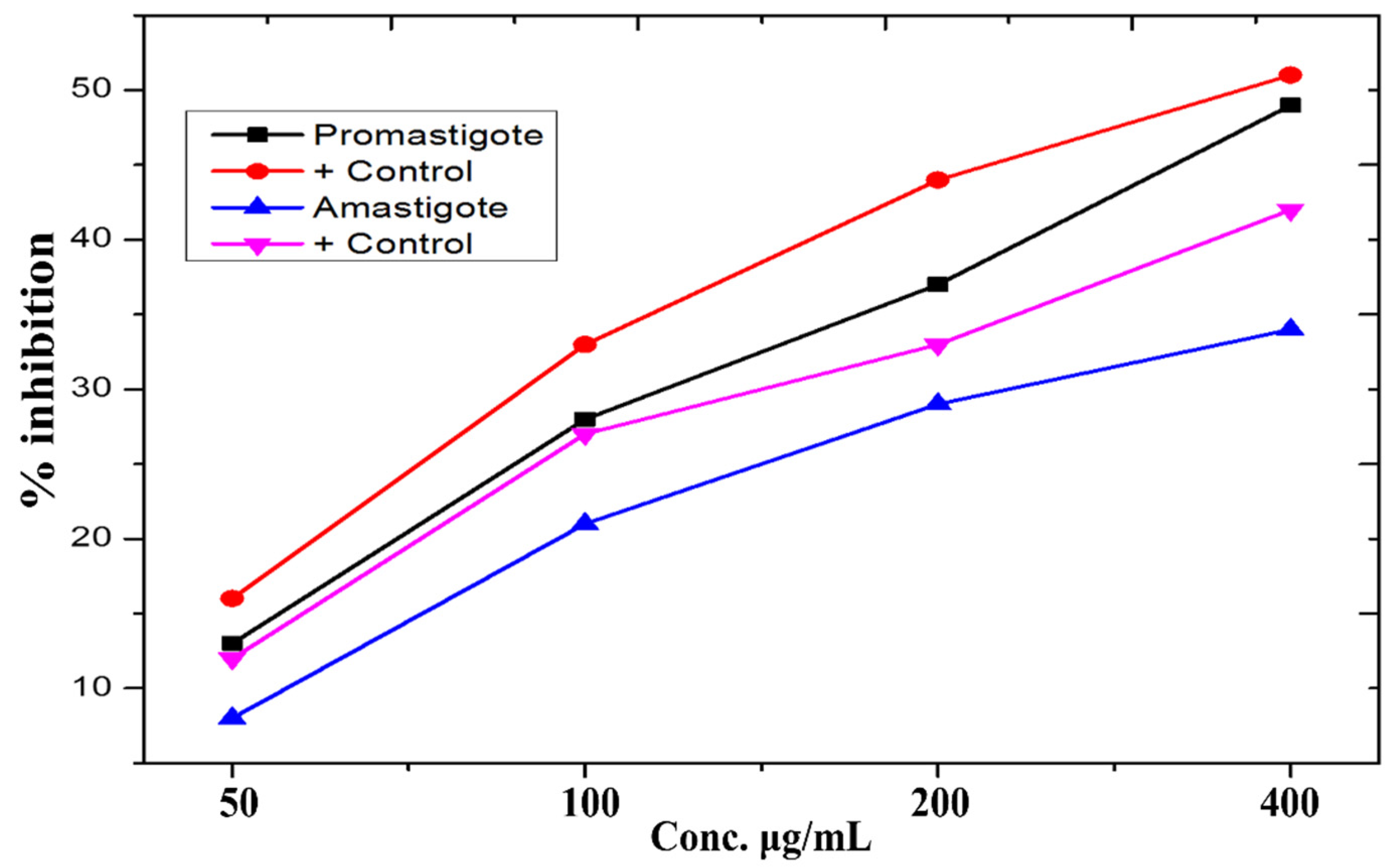
3.8. Anti-Leishmanial Activity
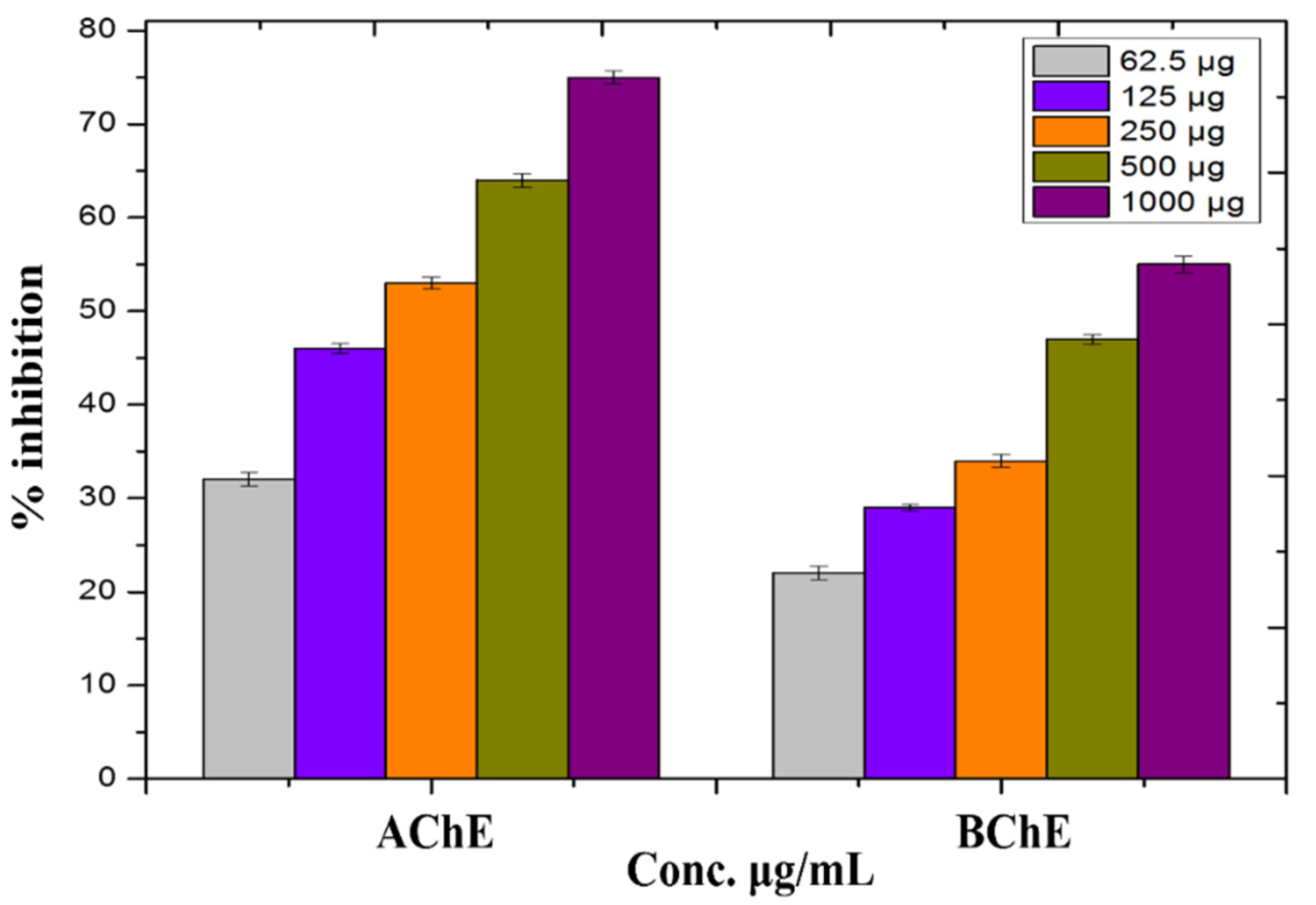
3.9. In Vitro Anti-Alzheimer’s Activity
3.10. Protein Kinase Inhibition Potential of Biosynthesized ZnO-NPs
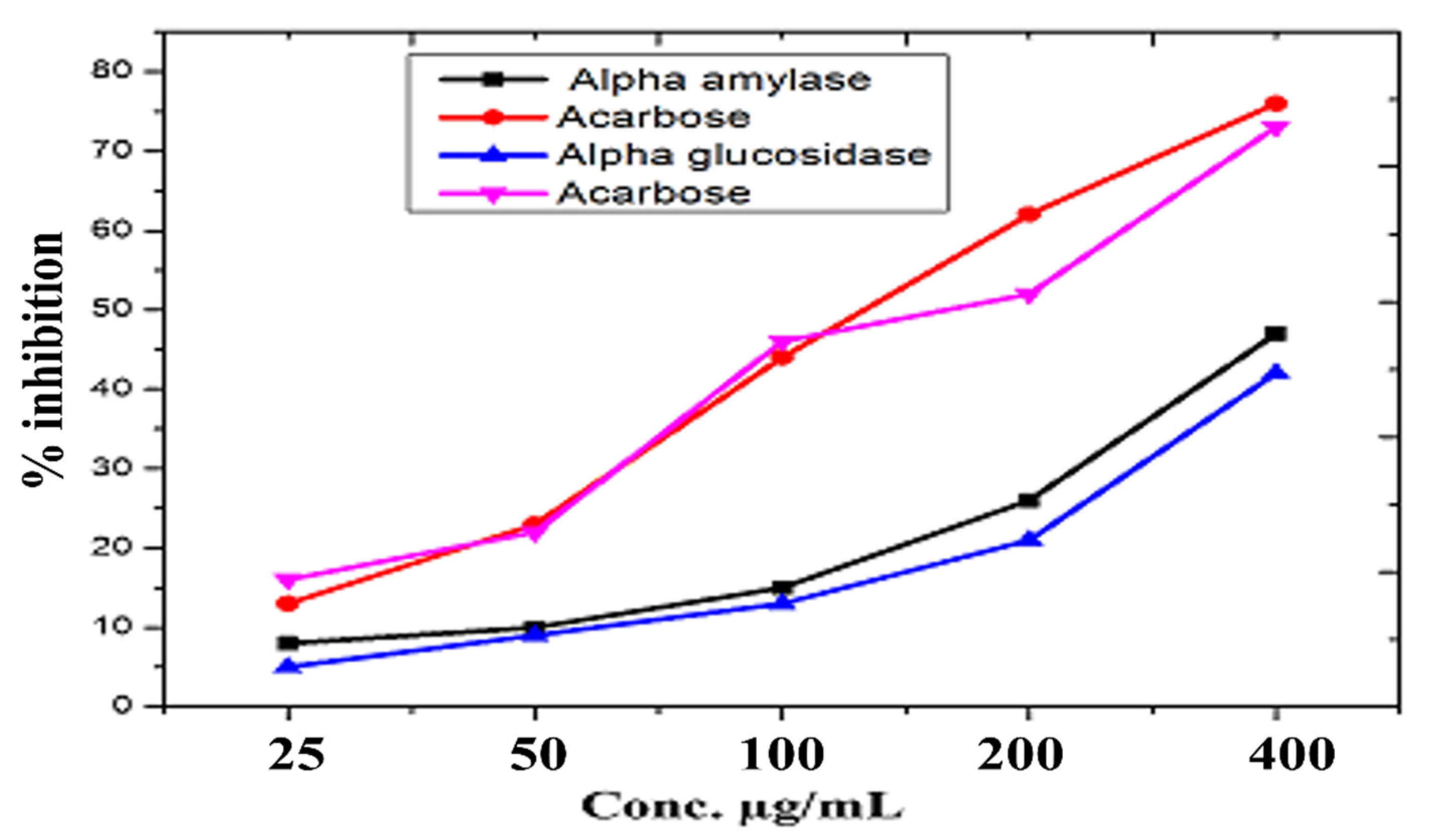
3.11. Anti-Diabetic Activity
3.12. In Vitro Biocompatibility Study
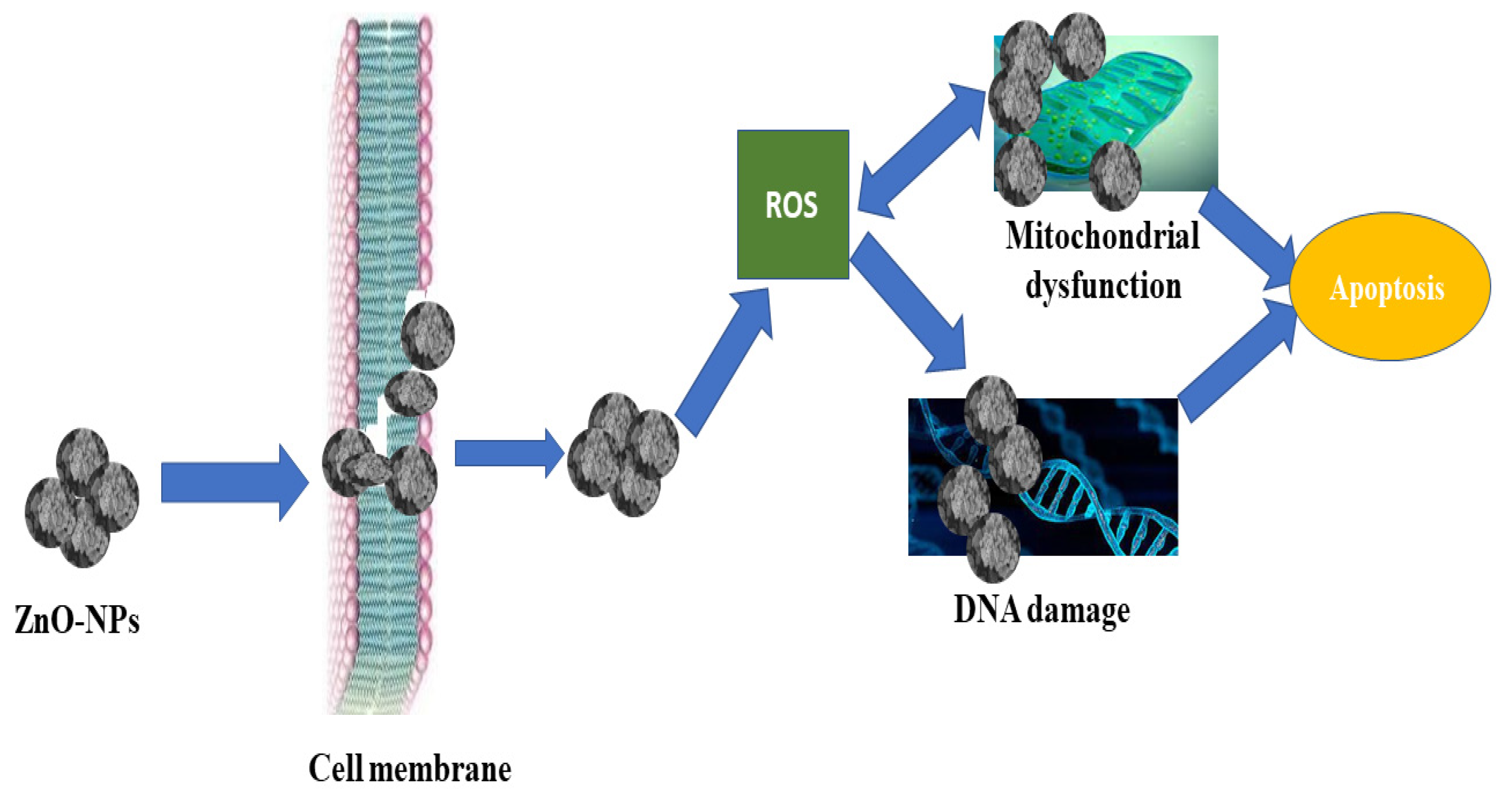
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In Proceedings of the AIP Conference Proceedings, Penang, Malaysia, 10–12 April 2018; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Faisal, S.; Khan, M.A.; Jan, H.; Shah, S.A.; Abdullah, A.; Shah, S.; Rizwan, M.; Wajidullah, W.; Akbar, M.T.; Redaina, R. Edible mushroom (Flammulina velutipes) as biosource for silver nanoparticles: From synthesis to diverse biomedical and environmental applications. Nanotechnology 2020, 32, 065101. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Khan, M.A.; Usman, H.; Shah, M.; Ansir, R.; Faisal, S.; Ullah, N.; Rahman, L. The Aquilegia pubiflora (Himalayan columbine) mediated synthesis of nanoceria for diverse biomedical applications. RSC Adv. 2020, 10, 19219–19231. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Chabattula, S.; Gupta, P.K.; Tripathi, S.K.; Gahtori, R.; Padhi, P.; Mahapatra, S.; Biswal, B.K.; Singh, S.K.; Dua, K.; Ruokolainen, J.; et al. Anticancer therapeutic efficacy of biogenic Am-ZnO nanoparticles on 2D and 3D tumor models. Mater. Today Chem. 2021, 22, 100618. [Google Scholar] [CrossRef]
- Borysiewicz, M.A.J.C. ZnO as a functional material, a review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Yayayürük, A.E.; Yayayürük, O.J.C.A.C. Recent Advances in Environmental Analysis Towards Green Nanomaterials. Curr. Anal. Chem. 2021, 17, 449–460. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.K.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef]
- Moghaddas, S.M.T.H.; Elahi, B.; Javanbakht, V. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloys Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other In Vitro Properties. Oxidative Med. Cell. Longev. 2021, 2021, 4786227. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, J.S.; Ul-Haq, I.; Zia, M. Biological and phytochemicals properties of Monotheca buxifolia: An unexplored medicinal plant. Pharm. Chem. J. 2020, 54, 293–301. [Google Scholar] [CrossRef]
- Ali, J.S.; Saleem, H.; Mannan, A.; Zengin, G.; Mahomoodally, M.F.; Locatelli, M.; Abidin, S.A.Z.; Ahemad, N.; Zia, M. Metabolic fingerprinting, antioxidant characterization, and enzyme-inhibitory response of Monotheca buxifolia (Falc.) A. DC. extracts. BMC Complement. Med. Ther. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Khan, K.; Saleem, A.; Baig, M.M.F.A.; Rasul, A.; Abdel-Daim, M.M. Chemical characterization and anti-arthritic appraisal of Monotheca buxifolia methanolic extract in Complete Freund’s Adjuvant-induced arthritis in Wistar rats. Inflammopharmacology 2021, 29, 393–408. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Ebrahimy, O.; Sarani, M. Cytotoxic and antifungal studies of biosynthesized zinc oxide nanoparticles using extract of Prosopis farcta fruit. Green Chem. Lett. Rev. 2020, 13, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Akpomie, K.G.; Ghosh, S.; Gryzenhout, M.; Conradie, J. One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Hussain, S.A.; Marzoog, T.R.; Jabir, M.S. Synthesis, characterization and evaluation of anti-bacterial, anti-parasitic and anti-cancer activities of aluminum-doped zinc oxide nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3677–3693. [Google Scholar] [CrossRef]
- Imran, M.; Jan, H.; Faisal, S.; Shah, S.A.; Shah, S.; Khan, M.N.; Akbar, M.T.; Rizwan, M.; Jan, F.; Syed, S. In vitro examination of anti-parasitic, anti-Alzheimer, insecticidal and cytotoxic potential of Ajuga bracteosa Wallich leaves extracts. Saudi J. Biol. Sci. 2021, 28, 3031–3036. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Biogenic synthesis and characterization of antimicrobial and anti-parasitic zinc oxide (ZnO) nanoparticles using aqueous extracts of the Himalayan columbine (Aquilegia pubiflora). Oxidative Med. Cell. Longev. 2020, 7, 249. [Google Scholar]
- Silas, N.; Lawrence, R.S. Anti-diabetic property of green synthesized zinc-oxide nanoparticles from leaf extract of Chrysanthemum indicum Plant. Rasayan J. Chem. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anuf, A.R.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Salem, M.S.E.-D.; Mahfouz, A.Y.; Fathy, R.M. Fathy, The antibacterial and antihemolytic activities assessment of zinc oxide nanoparticles synthesized using plant extracts and gamma irradiation against the uro-pathogenic multidrug resistant Proteus vulgaris. BioMetals 2021, 34, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Abdullah; Jan, H.; Shah, S.A.; Shah, S.; Rizwan, M.; Zaman, N.; Hussain, Z.; Uddin, M.N.; Bibi, N.; et al. Bio-catalytic activity of novel Mentha arvensis intervened biocompatible magnesium oxide nanomaterials. Catalysts 2021, 11, 780. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. International Scholarly Research Notices. Int. Sch. Res. Not. 2012, 2012, 372505. [Google Scholar]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Song, Z.; Kelf, T.A.; Sanchez, W.H.; Roberts, M.S.; Rička, J.; Frenz, M.; Zvyagin, A.V. Characterization of optical properties of ZnO nanoparticles for quantitative imaging of transdermal transport. Biomed. Opt. Express 2011, 2, 3321–3333. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. 2019, 12, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Parra, M.R.; Haque, F.Z. Aqueous chemical route synthesis and the effect of calcination temperature on the structural and optical properties of ZnO nanoparticles. J. Mater. Res. Technol. 2014, 3, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Vidya, C.; Hiremath, S.; Chandraprabha, M.N.; Antonyraj, M.L.; Gopal, I.V.; Jain, A.; Bansal, K. Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int. J. Curr. Eng. Technol. 2013, 1, 118–120. [Google Scholar]
- Stamm, W.E.; Hooton, T.M. Management of urinary tract infections in adults. N. Engl. J. Med. 1993, 329, 1328–1334. [Google Scholar] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Nejad, M.S.; Salari, S.; Almani, P.G.N. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotechnol. 2016, 10, 237–243. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Anjum, S.; Hano, C. Differential effects of in vitro cultures of Linum usitatissimum L.(Flax) on biosynthesis, stability, antibacterial and antileishmanial activities of zinc oxide nanoparticles: A mechanistic approach. RSC Adv. 2017, 7, 15931–15943. [Google Scholar] [CrossRef] [Green Version]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Bayrami, A.; Parvinroo, S.; Habibi-Yangjeh, A.; Rahim Pouran, S. Bio-extract-mediated ZnO nanoparticles: Microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 730–739. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Matthaei, S.; Stumvoll, M.; Kellerer, M.; Häring, H.U. Pathophysiology and pharmacological treatment of insulin resistance. Endocr. Rev. 2000, 21, 585–618. [Google Scholar]
- Siew, E.D.; Ikizler, T.A. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. In Seminars in Dialysis; Blackwell Publishing Ltd.: Oxford, UK, 2010; Volume 23, pp. 378–382. [Google Scholar]
- Faisal, S.; Jan, H.; Abdullah Alam, I.; Rizwan, M.; Hussain, Z.; Sultana, K.; Ali, Z.; Uddin, M.N. In Vivo Analgesic, Anti-Inflammatory, and Anti-Diabetic Screening of Bacopa monnieri-Synthesized Copper Oxide Nanoparticles. ACS Omega. 2022, 7, 4071–4082. [Google Scholar] [CrossRef] [PubMed]
- Al-Radadi, N.S.; Abdullah; Faisal, S.; Alotaibi, A.; Ullah, R.; Hussain, T.; Rizwan, M.; Saira; Zaman, N.; Iqbal, M.; et al. Zingiber officinale Driven Bioproduction of ZnO Nanoparticles and its Anti-inflammatory, Anti-diabetic, Anti-Alzheimer, Anti-oxidant, and Anti-microbial Applications. Inorg. Chem. Commun. 2022, 140, 109274. [Google Scholar] [CrossRef]

| Test Organisms | Activity of ZnO-NPs (mm) | Antibiotics | CLSI Standard Limit of Sensitivity (mm) | ZI of Non-Coated Antibiotics (mm) | ZI of ZnO-NPs Coated Antibiotics (mm) | Increase in the Potency of ZnO-NPs Coated Antibiotics (%) |
|---|---|---|---|---|---|---|
| E. coli | 14 ± 2.63 | Ciprofloxacin | 21 | 16 ± 0.9 | 19.2 ± 1.5 | 28.4 |
| Imipenem | 22 | 15 ± 1.0 | 18 ± 0.8 | 26.5 | ||
| Vancomycin | 19 | 13 ± 1.4 | 14 ± 1.0 | 20.2 | ||
| Amoxicillin-clavulanic acid | 18 | 10 ± 1.2 | 11.2 ± 1.2 | 22.6 | ||
| K. pneumoniae | 16 ± 2.78 | Ciprofloxacin | 21 | 15.5 ± 1.5 | 22.8 ± 0.4 | 29.0 |
| Imipenem | 22 | 14 ± 0.4 | 21 ± 1.5 | 25.2 | ||
| Vancomycin | 19 | 9 ± 1.1 | 12 ± 0.7 | 18.0 | ||
| Amoxicillin-clavulanic acid | 18 | 6 ± 0.6 | 11.6 ± 1.2 | 22.6 | ||
| P. aeruginosa | 13 ± 2.43 | Ciprofloxacin | 21 | 12.3 ± 0.9 | 18 ± 1.8 | 27.9 |
| Imipenem | 22 | 14 ± 0.6 | 21.3 ± 1.0 | 26.6 | ||
| Vancomycin | 19 | 8 ± 1.3 | 10 ± 0.5 | 13.8 | ||
| Amoxicillin-clavulanic acid | 18 | 10.4 ± 0.6 | 15.6 ± 0.9 | 21.4 | ||
| S. aureus | 18 ± 2.74 | Ciprofloxacin | 21 | 14.4 ± 0.9 | 22 ± 1.2 | 29.5 |
| Imipenem | 22 | 16 ± 0.4 | 21.6 ± 0.9 | 22.5 | ||
| Vancomycin | 19 | 9 ± 1.4 | 14.3 ± 0.7 | 26.9 |
| S.NO | Concentration (µg/mL) | % Hemolysis |
|---|---|---|
| 1 | 400 | 1.63 ± 0.21 |
| 2 | 200 | 1.23 ± 0.35 |
| 3 | 100 | 0.82 ± 0.38 |
| 4 | 50 | 0.42 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Shah, S.; Faisal, S.; Gul, S.; Khan, S.; Abdullah; Shah, S.A.; Shah, W.A. Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications. Micromachines 2022, 13, 668. https://doi.org/10.3390/mi13050668
Khan MI, Shah S, Faisal S, Gul S, Khan S, Abdullah, Shah SA, Shah WA. Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications. Micromachines. 2022; 13(5):668. https://doi.org/10.3390/mi13050668
Chicago/Turabian StyleKhan, Muhammad Ishaq, Sumaira Shah, Shah Faisal, Safia Gul, Shahzar Khan, Abdullah, Sajjad Ali Shah, and Wajid Ali Shah. 2022. "Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications" Micromachines 13, no. 5: 668. https://doi.org/10.3390/mi13050668
APA StyleKhan, M. I., Shah, S., Faisal, S., Gul, S., Khan, S., Abdullah, Shah, S. A., & Shah, W. A. (2022). Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications. Micromachines, 13(5), 668. https://doi.org/10.3390/mi13050668






