Electrochemical Detection of Alpha-Fetoprotein Based on Black Phosphorus Nanosheets Modification with Iron Ions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. Synthesis of BPNSs
2.3. Synthesis of Fe3+/BPNSs
2.4. MTT Assay
2.5. Electrochemical Characterization
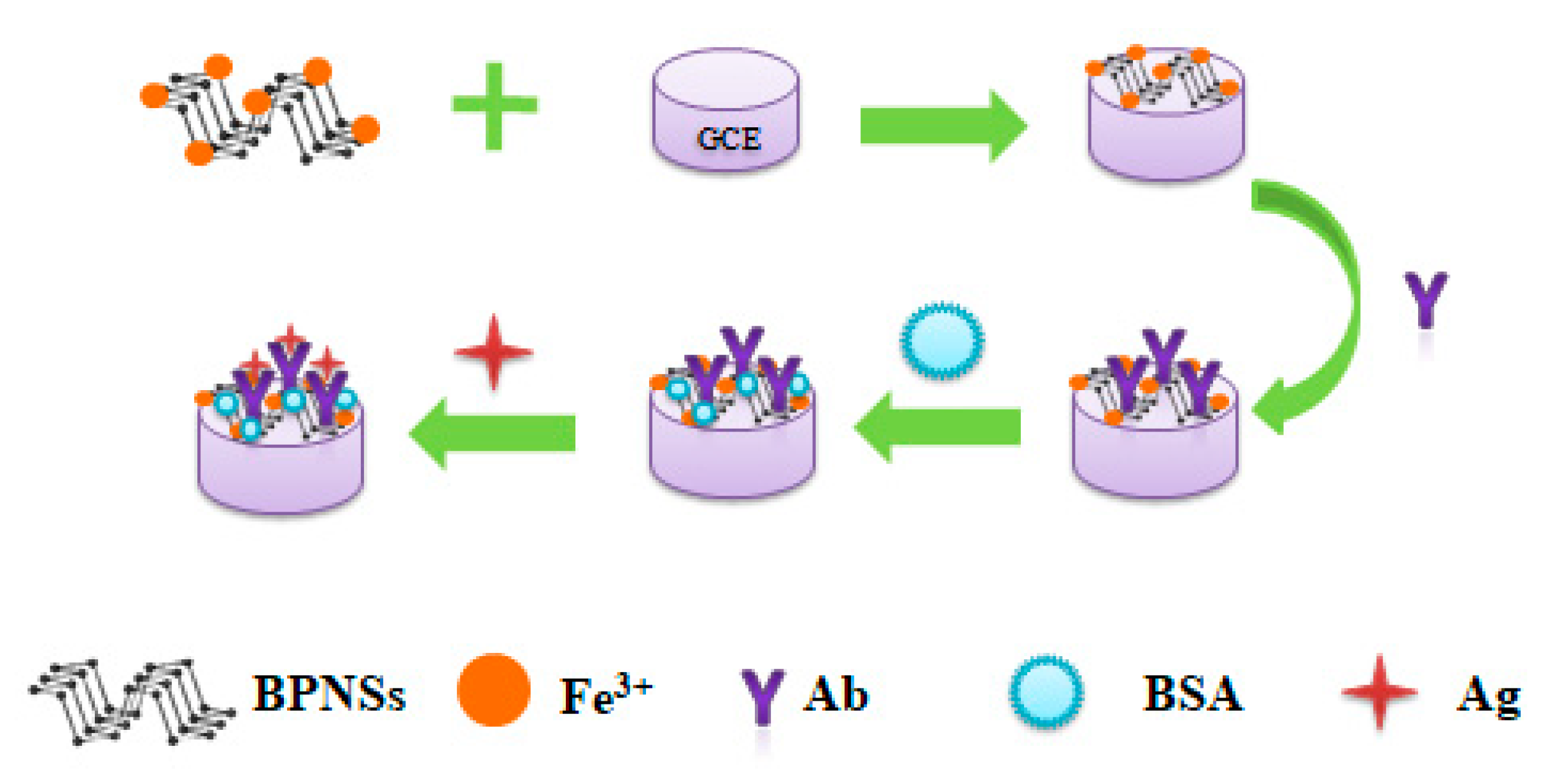
2.6. Detection of Alpha-Fetoprotein
3. Results and Discussion
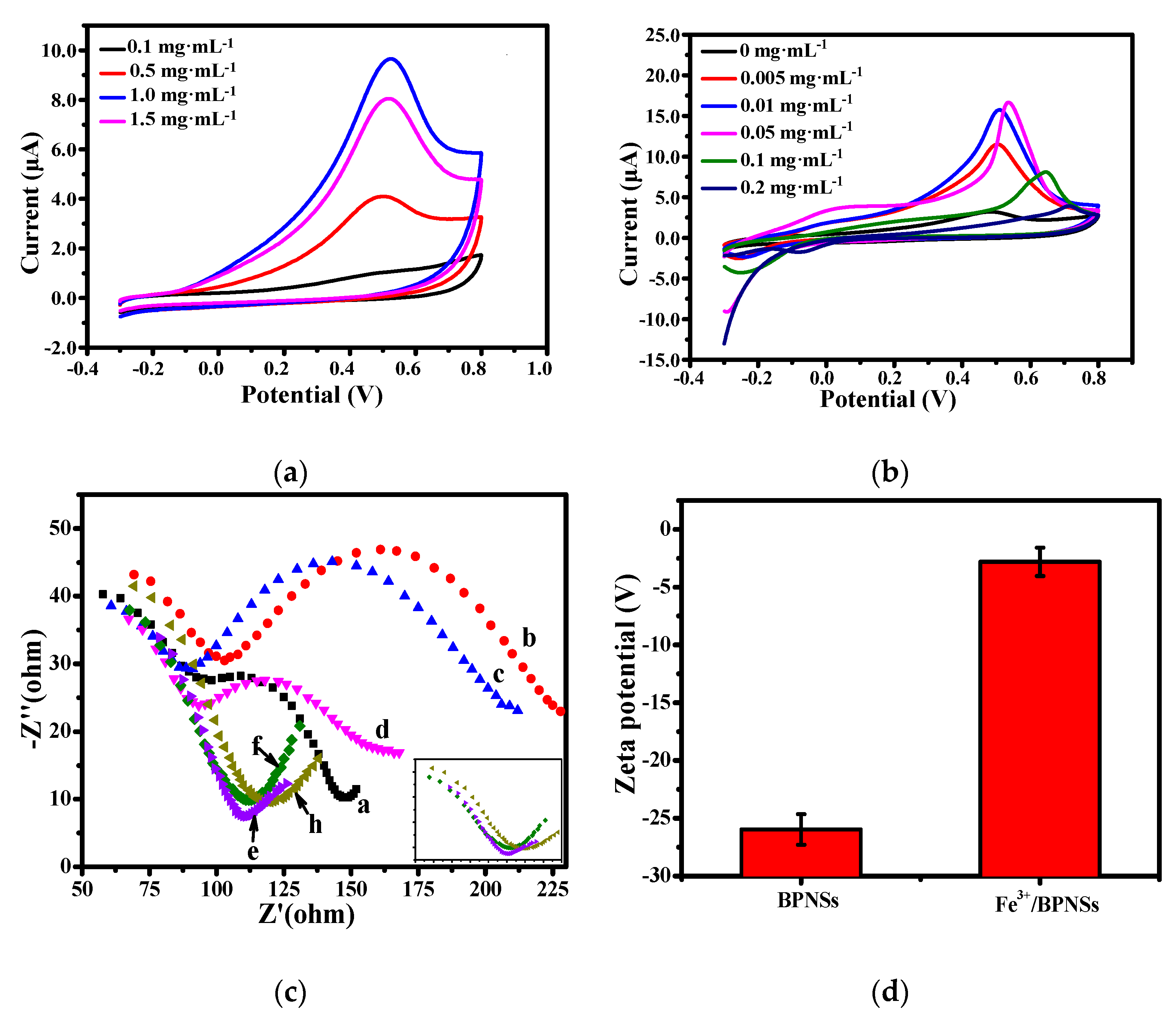
3.1. Characterization of BPNSs and Fe3+/BPNSs
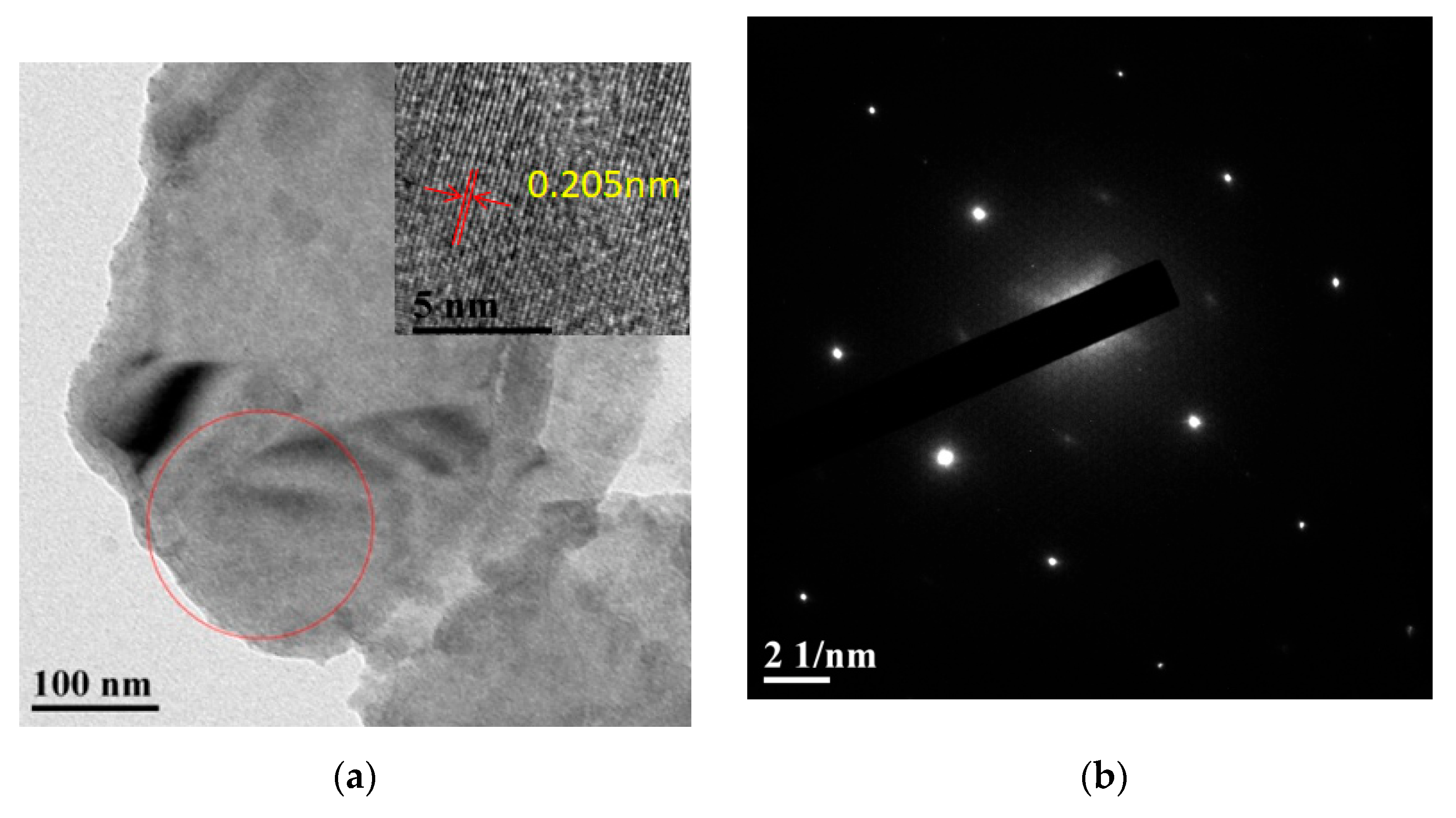
3.1.1. Structural Analysis
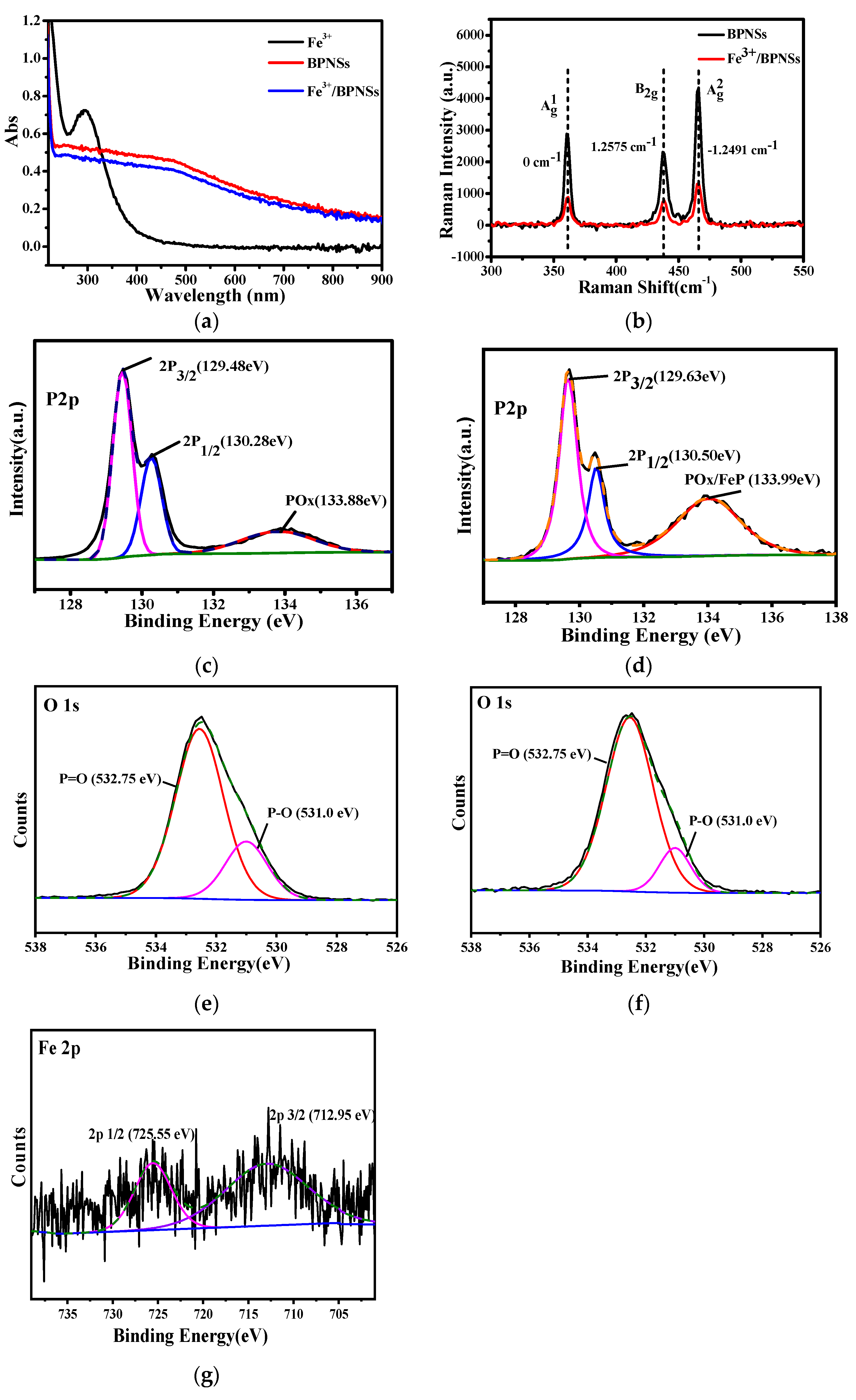
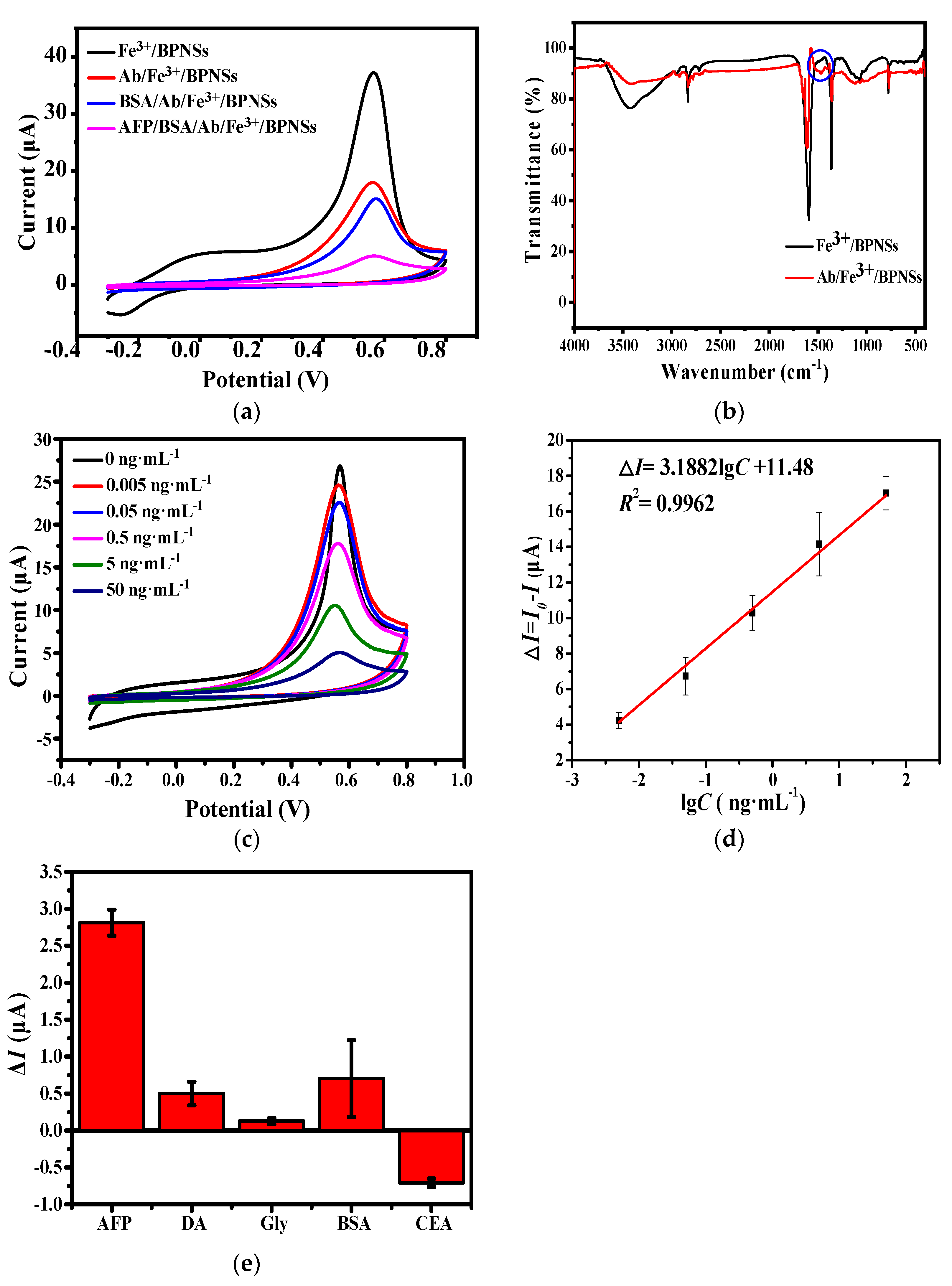
3.1.2. Spectroscopy Analysis
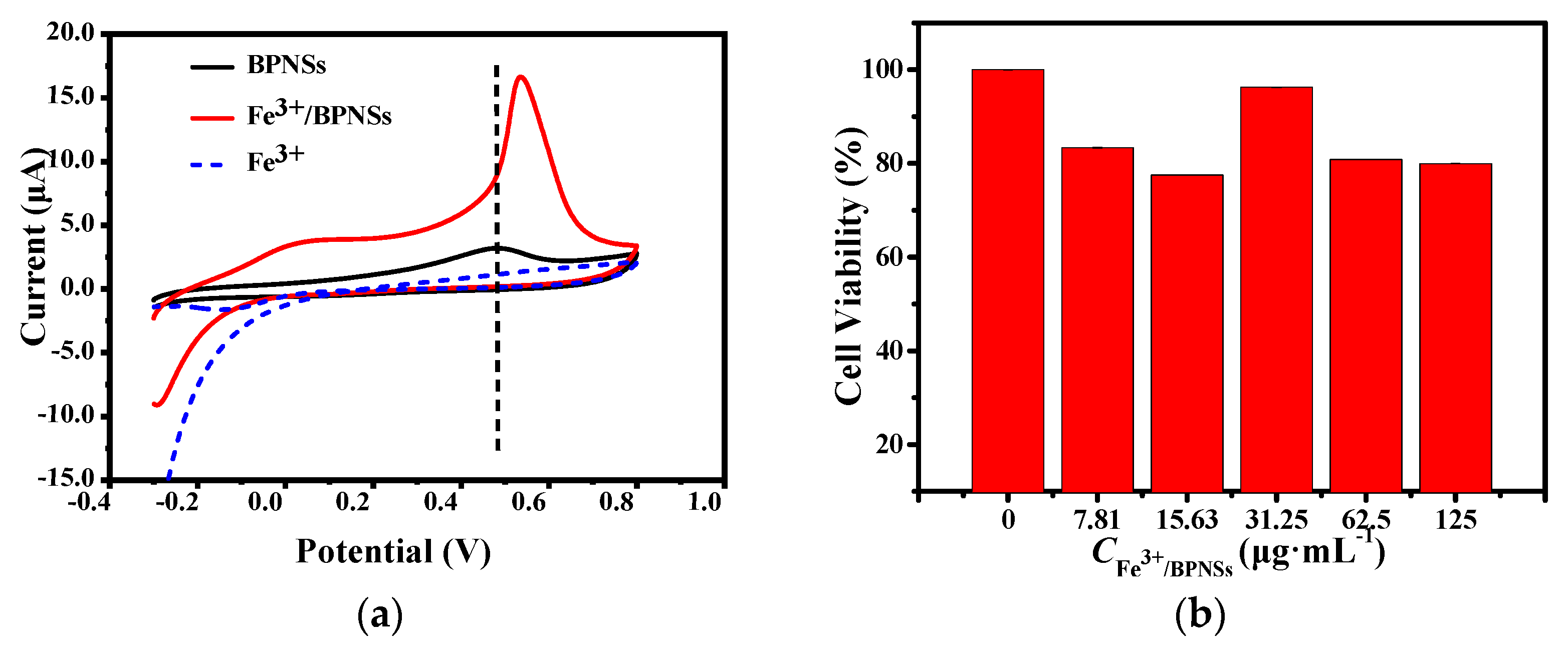
3.1.3. Electrochemical Analysis of Fe3+/BPNSs
3.1.4. Cytotoxicity of Fe3+/BPNSs
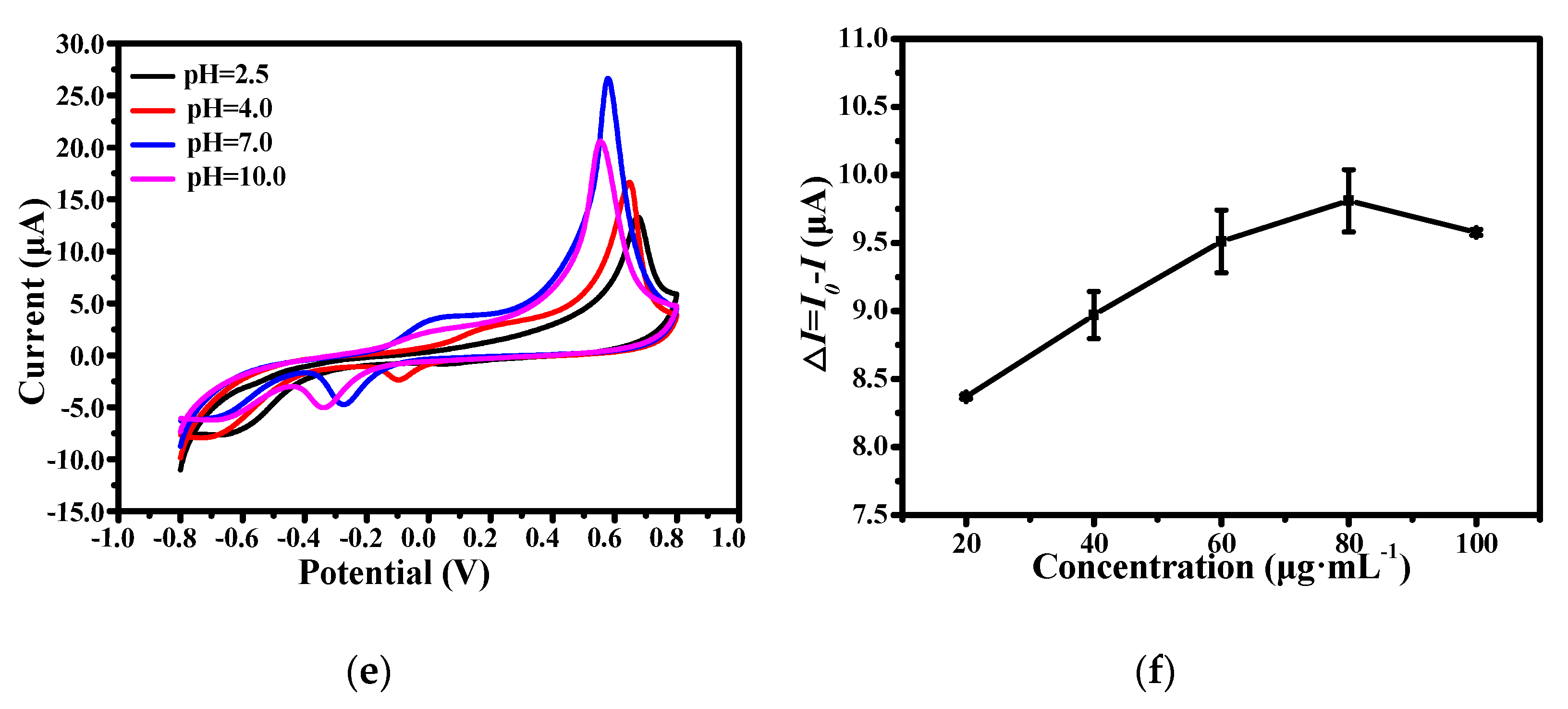
3.2. Optimization of Experimental Conditions
3.3. Electrochemical Determination of Alpha-Fetoprotein
3.4. Selectivity of the Electrochemical Immunosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, X.; Wang, X.; Li, P.; Chen, S.; Hong, C. Electrochemical immunosensor using artificial enzyme-induced metallization for the ultra-sensitive detection of alpha fetoprotein. Sens. Actuators B Chem. 2021, 344, 130258. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Wang, J.; Wang, J. Dual-recognition immune-co-chemical ECL-sensor based on Ti, Mg@N-CDs-induced and novel signal-sensing units Poly(DVB-co-PBA)-reported for alpha-fetoprotein detection. Sens. Actuators B Chem. 2021, 346, 130548. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wu, D.; Ma, H.; Pang, X.; Fan, D.; Wei, Q.; Du, B. Ultrasensitive label-free electrochemical immunosensor based on multifunctionalized graphene nanocomposites for the detection of alpha fetoprotein. Sci. Rep. 2017, 7, 42361. [Google Scholar] [CrossRef]
- Chang, J.; Gao, N.; Dai, P.; Zhu, Z.; You, H.; Han, W.; Li, L. Facile engineered polymeric microdevice via co-coupling of phenylboronic acid and Protein A for oriented antibody immobilization enables substantial signal enhancement for an enhanced fluorescence immunoassay. Sens. Actuators B Chem. 2021, 346, 130444. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Xu, D.; Liu, J.; Yu, R.; Jing, C.; Han, H.; Ma, W. Silver-amplified fluorescence immunoassay via aggregation-induced emission for detection of disease biomarker. Talanta 2021, 225, 121963. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bong, J.; Park, J.; Song, Z.; Pyun, J. Cesium lead bromide (CsPbBr3) perovskite quantum dot-based photosensor for chemiluminescence immunoassays. ACS Appl. Mater. Interface 2021, 13, 29392–29405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, C.; Liu, M.; Liu, F.; Biao, S.; Du, S.; Zhu, S.; Wang, H. Biominerized gold-Hemin@MOF composites with peroxidase-like and gold catalysis activities: A high-throughput colorimetric immunoassay for alpha-fetoprotein in blood by ELISA and gold-catalytic silver staining. Sens. Actuators B Chem. 2018, 266, 543–552. [Google Scholar] [CrossRef]
- Gan, N.; Jia, L.; Zheng, L. A novel sandwich electrochemical immunosensor based on the DNA-derived magnetic nanochain probes for alpha-fetoprotein. J. Autom. Methods Manag. Chem. 2011, 4, 957805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhao, C.; Zhang, C.; Wen, K.; Zhu, Y. Bi-directionally amplified ratiometric electrochemical aptasensor for the ultrasensitive detection of alpha-fetoprotein. Sens. Actuators B Chem. 2020, 323, 128666. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y.; Li, Y.; Zhang, Q.; Hu, Z.; Zhang, Y.; Hussain, E.; Yang, X.; Yu, D.; Yu, C. Polyphosphoric acid-induced perylene probe self-assembly and label-free fluorescence turn-on detection of alkaline phosphatase. Anal. Bioanal. Chem. 2017, 409, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Li, Y.; Wang, Y.; Zhang, Q.; Hussain, E.; Yang, M.; Shahzad, S.A.; Yu, D.; Yu, C. Nucleic acid-controlled quantum dots aggregation: A label-free fluorescence turn-on strategy for alkaline phosphatase detection. Talanta 2017, 169, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sreeramareddygari, M.; Devaramani, S.; Thippeswamy, R.; Kempahanumakkagari, S.; Surareungchai, W. Trending approaches in electrochemical sensing. Electrochemistry 2021, 16, 1–43. [Google Scholar]
- Zou, J.; Lan, X.W.; Zhao, G.Q.; Huang, Z.N.; Liu, Y.P.; Yu, J.G. Immobilization of 6-O-α-maltosyl-β-cyclodextrin on the surface of black phosphorus nanosheets for selective chiral recognition of tyrosine enantiomers. Microchim. Acta 2020, 187, 636. [Google Scholar] [CrossRef]
- Long, L.; Niu, X.; Yan, K.; Zhou, G.; Wang, J.; Wu, X.; Chu, P. Highly fluorescent and stable black phosphorus quantum dots in water. Small 2018, 14, 1803132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, G.; Zuo, X. Two-dimensional black phosphorus, an emerging anode material for lithium-ion batteries. Nano-Micro Lett. 2020, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Bellus, M.Z.; Yang, Z.; Zereshki, P.; Hao, J.; Lau, S.P.; Zhao, H. Efficient hole transfer from monolayer WS2 to ultrathin amorphous black phosphorus. Nanoscale Horiz. 2019, 4, 236–242. [Google Scholar] [CrossRef]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured aptamer-functionalised black phosphorus sensing platform for label-free detection of myoglobin, a cardiovascular disease biomarker. ACS Appl. Mater. Interface 2016, 8, 22860–22868. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Hussain, E.; Huang, X.; Zhang, Y.; Niu, N.; Shahzad, S.A.; Yu, C. Black phosphorus nanosheets based sensitive protease detection and inhibitor screening. Talanta 2019, 197, 270–276. [Google Scholar] [CrossRef]
- Sofer, Z.; Luxa, J.; Bouša, D.; Sedimidubský, D.; Lazar, P.; Hartman, T.; Hardtdegen, H.; Pumera, M. The covalent functionalization of layered black phosphorus by nucleophilic reagents. Angew. Chem. Int. Ed. 2017, 56, 9891–9896. [Google Scholar] [CrossRef]
- Lee, G.; Jung, S.; Jang, S.; Kim, J. Platinum-functionalized black phosphorus hydrogen sensors. Appl. Phys. Lett. 2017, 110, 242103. [Google Scholar] [CrossRef]
- Ramalingam, S.; Chand, R.; Singh, C.B.; Singh, A. Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid. Biosens. Bioelectron. 2019, 135, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, S.; Wang, Z.; Yang, Z.; Liu, F.; Xu, Y.; Wang, J.; Yi, Y.; Zhang, H.; Liao, L.; et al. Metal-ion-modified black phosphorus with enhanced stability and transistor performance. Adv. Mater. 2017, 29, 1703811. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ling, Z.; Ying, M.; Liu, M.; Wang, X.; Wang, X.; Cao, L.; Zhang, H.; Xu, G. Black phosphorus nanosheets for rapid microRNA detection. Nanoscale 2018, 11, 5060–5064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, N.; Tao, H.; Yan, C.; Huang, J.; Liu, T.; Roberston, A.W.; Texter, J.; Wang, J.; Sun, Z. Exfoliation of stable 2D black phosphorus for device fabrication. Chem. Mater. 2017, 29, 6445–6456. [Google Scholar] [CrossRef]
- Favron, A.; Gaufrès, E.; Fossard, F.; Heureux, A.P.; Tang, N.Y.; Lévesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Brajpuriya, R.; Shripathi, T. Investigation of Fe/Al interface as a function of annealing temperature using XPS. Appl. Surf. Sci. 2009, 255, 6149–6154. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Cang, R.; Lu, B.; Zhao, J.; Cai, Q. Highly selective oxidation of styrene over FeCl3- imidazolium ionic liquid grafted SBA-15. Catal. Lett. 2019, 149, 2994–2999. [Google Scholar] [CrossRef]
- Lu, T.; Hou, Y.; Wu, W.; Niu, M.; Li, W.; Ren, S. Catalytic oxidation of cellulose to formic acid in V(V)-Fe(III)-H2SO4 aqueous solution with O2. Fuel Process. Technol. 2018, 173, 197–204. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Huang, T.; Cheng, L.; Yao, X. Effects of coagulants on the catalytic properties of iron–manganese co-oxide filter films for ammonium and manganese removal from surface water. J. Clean. Prod. 2020, 242, 118494. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Neethirajan, S. Exploration of 2-dimensional bio-functionalized phosphorene nanosheets (black phosphorous) for label free haptoglobin electro-immunosensing applications. Nanotechnology 2018, 29, 135101. [Google Scholar] [CrossRef]
- Li, G.; Zeng, J.; Liu, H.; Ding, P.; Liang, J.; Nie, X.; Zhou, Z. A fluorometric aptamer nanoprobe for alpha-fetoprotein by exploiting the FRET between 5-carboxyfluorescein and palladium nanoparticles. Microchim. Acta 2019, 186, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, Z.; Sun, C.; Long, Y.; Zheng, H. Bifunctional antibody and copper-based metal-organic framework nanocomposites for colorimetric α-fetoprotein sensing. Microchim. Acta 2020, 187, 465. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, F.; Wang, Z.; Liang, Q. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ameer, H.; Chi, Y. Label-free and sensitive electrochemiluminescent immunosensor based on novel luminophores of Zn2SnO4 nanorods. Sens. Actuators B Chem. 2021, 337, 129761. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, M.; Guo, L.; Xie, Y.; Luo, R.; Chen, W.; Cheng, F.; Wang, L. A dual-signal self-checking photoelectrochemical immunosensor based on the sole composite of MIL-101(Cr) and CdSe quantum dots for the detection of α-fetoprotein. Biosens. Bioelectron. 2021, 189, 113389. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Pumera, M. Voltammetry of layered black phosphorus: Electrochemistry of multilayer phosphorene. ChemElectroChem 2015, 2, 324–327. [Google Scholar] [CrossRef]

| Methods | Linear Range (ng·mL−1) | LOD (pg·mL−1) | Refs. |
|---|---|---|---|
| Fluorescence | 0.01–100 | 3 | [31] |
| Colorimetry | 0–20 | 35 | [32] |
| Electrochemistry | 0.01–100 | 3 | [33] |
| Electrochemiluminescent immunosensors | 0.001–50 | 3.4 | [34] |
| Photoelectrochemistry | 0.1–300 | 82 (cathode)/54 (anode) | [35] |
| Electrochemistry | 0.005–50 | 1.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, X.; Lin, J.; Zhuang, Y.; Han, Z.; Chen, J. Electrochemical Detection of Alpha-Fetoprotein Based on Black Phosphorus Nanosheets Modification with Iron Ions. Micromachines 2022, 13, 673. https://doi.org/10.3390/mi13050673
Chen Y, Chen X, Lin J, Zhuang Y, Han Z, Chen J. Electrochemical Detection of Alpha-Fetoprotein Based on Black Phosphorus Nanosheets Modification with Iron Ions. Micromachines. 2022; 13(5):673. https://doi.org/10.3390/mi13050673
Chicago/Turabian StyleChen, Yiyan, Xiaoping Chen, Jianwei Lin, Yafeng Zhuang, Zhizhong Han, and Jinghua Chen. 2022. "Electrochemical Detection of Alpha-Fetoprotein Based on Black Phosphorus Nanosheets Modification with Iron Ions" Micromachines 13, no. 5: 673. https://doi.org/10.3390/mi13050673






