Polysaccharide Layer-by-Layer Coating for Polyimide-Based Neural Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Polyimide Film Deposition and Plasma Treatment
2.2.2. LbL Thin Film Deposition by Dip Coating
2.2.3. LbL Thin Film Deposition by Spin Coating
2.2.4. PI and LbL Thin Film Characterization
2.2.5. Statistical Analysis
3. Results
3.1. Spin Coating Deposition and Plasma Treatment of PI Substrates
3.2. LbL Polysaccharide Deposition by Dip Coating
3.3. LbL Polysaccharide Deposition by Spin Coating
3.4. Comparison between PI Surface Properties and the Two LbL Deposition Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Micera, S.; Caleo, M.; Chisari, C.; Hummel, F.C.; Pedrocchi, A. Advanced Neurotechnologies for the Restoration of Motor Function. Neuron 2020, 105, 604–620. [Google Scholar] [CrossRef]
- Henry, J.D.; von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical Assessment of Social Cognitive Function in Neurological Disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Borton, D.; Micera, S.; Millán, J.D.R.; Courtine, G. Personalized Neuroprosthetics. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Cutrone, A.; Micera, S. Implantable Neural Interfaces and Wearable Tactile Systems for Bidirectional Neuroprosthetics Systems. Adv. Healthc. Mater. 2019, 8, e1801345. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.E.; Meng, E. A Review for the Peripheral Nerve Interface Designer. J. Neurosci. Methods 2020, 332, 108523. [Google Scholar] [CrossRef] [PubMed]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory Feedback Restoration in Leg Amputees Improves Walking Speed, Metabolic Cost and Phantom Pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and Technologies for Soft Implantable Neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef] [Green Version]
- Navarro, X.; Krueger, T.B.; Lago, N.; Micera, S.; Stieglitz, T.; Dario, P. A Critical Review of Interfaces with the Peripheral Nervous System for the Control of Neuroprostheses and Hybrid Bionic Systems. J. Peripher. Nerv. Syst. 2005, 10, 229–258. [Google Scholar] [CrossRef]
- Cutrone, A.; del Valle, J.; Santos, D.; Badia, J.; Filippeschi, C.; Micera, S.; Navarro, X.; Bossi, S. A Three-Dimensional Self-Opening Intraneural Peripheral Interface (SELINE). J. Neural Eng. 2015, 12, 016016. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martínez, I.; Righi, M.; Santos, D.; Cutrone, A.; Bossi, S.; D’Amico, S.; del Valle, J.; Micera, S.; Navarro, X. Fascicular Nerve Stimulation and Recording Using a Novel Double-Aisle Regenerative Electrode. J. Neural Eng. 2017, 14, 046003. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Harreby, K.R.; Yoshida, K.; Boretius, T.; Stieglitz, T.; Jensen, W. Stimulation Selectivity of the “thin-Film Longitudinal Intrafascicular Electrode” (TfLIFE) and the “Transverse Intrafascicular Multi-Channel Electrode” (Time) in the Large Nerve Animal Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.; Boretius, T.; Andreu, D.; Azevedo-Coste, C.; Stieglitz, T.; Navarro, X. Comparative Analysis of Transverse Intrafascicular Multichannel, Longitudinal Intrafascicular and Multipolar Cuff Electrodes for the Selective Stimulation of Nerve Fascicles. J. Neural Eng. 2011, 8, 036023. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A Transverse Intrafascicular Multichannel Electrode (TIME) to Interface with the Peripheral Nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef]
- Goding, J.A.; Gilmour, A.D.; Aregueta-Robles, U.A.; Hasan, E.A.; Green, R.A. Living Bioelectronics: Strategies for Developing an Effective Long-Term Implant with Functional Neural Connections. Adv. Funct. Mater. 2018, 28, 1702969. [Google Scholar] [CrossRef]
- Lotti, F.; Ranieri, F.; Vadalà, G.; Zollo, L.; di Pino, G. Invasive Intraneural Interfaces: Foreign Body Reaction Issues. Front. Neurosci. 2017, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- de la Oliva, N.; Navarro, X.; del Valle, J. Time Course Study of Long-Term Biocompatibility and Foreign Body Reaction to Intraneural Polyimide-Based Implants. J. Biomed. Mater. Res. Part A 2018, 106, 746–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurth, S.; Capogrosso, M.; Raspopovic, S.; Gandar, J.; Federici, G.; Kinany, N.; Cutrone, A.; Piersigilli, A.; Pavlova, N.; Guiet, R.; et al. Long-Term Usability and Bio-Integration of Polyimide-Based Intra-Neural Stimulating Electrodes. Biomaterials 2017, 122, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Redolfi Riva, E.; Micera, S. Progress and Challenges of Implantable Neural Interfaces Based on Nature-Derived Materials. Bioelectron. Med. 2021, 7, 6. [Google Scholar] [CrossRef]
- Aregueta-Robles, U.A.; Woolley, A.J.; Poole-Warren, L.A.; Lovell, N.H.; Green, R.A. Organic Electrode Coatings for Next-Generation Neural Interfaces. Front. Neuroeng. 2014, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righi, M.; Puleo, G.L.; Tonazzini, I.; Giudetti, G.; Cecchini, M.; Micera, S. Peptide-Based Coatings for Flexible Implantable Neural Interfaces. Sci. Rep. 2018, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wang, J.; Guan, S.; Zou, L.; Gao, L.; Li, H.; Fang, Y.; Wang, C. Anti-Fouling Peptide Functionalization of Ultraflexible Neural Probes for Long-Term Neural Activity Recordings in the Brain. Biosens. Bioelectron. 2021, 192, 113477. [Google Scholar] [CrossRef] [PubMed]
- Alves-Sampaio, A.; García-Rama, C.; Collazos-Castro, J.E. Biofunctionalized PEDOT-Coated Microfibers for the Treatment of Spinal Cord Injury. Biomaterials 2016, 89, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Mi, G.; Shi, D.; Bassous, N.; Hickey, D.; Webster, T.J. Nanotechnology and Nanomaterials for Improving Neural Interfaces. Adv. Funct. Mater. 2018, 28, 1700905. [Google Scholar] [CrossRef]
- Shen, W.; Karumbaiah, L.; Liu, X.; Saxena, T.; Chen, S.; Patkar, R.; Bellamkonda, R.V.; Allen, M.G. Extracellular Matrix-Based Intracortical Microelectrodes: Toward a Microfabricated Neural Interface Based on Natural Materials. Microsyst. Nanoeng. 2015, 1, 15010. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Lee, Y.J.; Heo, D.N.; Kwon, I.K.; Yun, K.S.; Kang, J.Y.; Lee, S.H. Functional Nerve Cuff Electrode with Controllable Anti-Inflammatory Drug Loading and Release by Biodegradable Nanofibers and Hydrogel Deposition. Sens. Actuators B Chem. 2015, 215, 133–141. [Google Scholar] [CrossRef]
- Shur, M.; Fallegger, F.; Pirondini, E.; Roux, A.; Bichat, A.; Barraud, Q.; Courtine, G.; Lacour, S.P. Soft Printable Electrode Coating for Neural Interfaces. ACS Appl. Bio Mater. 2020, 3, 4388–4397. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.G.; Chen, J.H.; Liu, K.T.; Luo, S.C. Engineering Antifouling Conducting Polymers for Modern Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 21294–21307. [Google Scholar] [CrossRef] [PubMed]
- Golabchi, A.; Wu, B.; Cao, B.; Bettinger, C.J.; Cui, X.T. Zwitterionic Polymer/Polydopamine Coating Reduce Acute Inflammatory Tissue Responses to Neural Implants. Biomaterials 2019, 225, 119519. [Google Scholar] [CrossRef]
- Trel’Ová, D.; Salgarella, A.R.; Ricotti, L.; Giudetti, G.; Cutrone, A.; Šrámková, P.; Zahoranová, A.; Chorvát, D.; Haško, D.; Canale, C.; et al. Soft Hydrogel Zwitterionic Coatings Minimize Fibroblast and Macrophage Adhesion on Polyimide Substrates. Langmuir 2019, 35, 1085–1099. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Mano, J.F. Biomimetic Extracellular Environment Based on Natural Origin Polyelectrolyte Multilayers. Small 2016, 12, 4308–4342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xing, M.; Li, B. Biomimetic Layer-by-Layer Self-Assembly of Nanofilms, Nanocoatings, and 3D Scaffolds for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1641. [Google Scholar] [CrossRef] [Green Version]
- Riva, E.R.; Desii, A.; Sinibaldi, E.; Ciofani, G.; Piazza, V.; Mazzolai, B.; Mattoli, V. Gold Nanoshell/Polysaccharide Nanofilm for Controlled Laser-Assisted Tissue Thermal Ablation. ACS Nano 2014, 8, 5552–5563. [Google Scholar] [CrossRef]
- Redolfi Riva, E.; Pastoriza-Santos, I.; Lak, A.; Pellegrino, T.; Pérez-Juste, J.; Mattoli, V. Plasmonic/Magnetic Nanocomposites: Gold Nanorods-Functionalized Silica Coated Magnetic Nanoparticles. J. Colloid Interface Sci. 2017, 502, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Okamura, Y.; Takeoka, S. Ubiquitous Transference of a Free-Standing Polysaccharide Nanosheet with the Development of a Nano-Adhesive Plaster. Adv. Mater. 2007, 19, 3549–3553. [Google Scholar] [CrossRef]
- Fujie, T.; Furutate, S.; Niwa, D.; Takeoka, S. A Nano-Fibrous Assembly of Collagen-Hyaluronic Acid for Controlling Cell-Adhesive Properties. Soft Matter 2010, 6, 4672–4676. [Google Scholar] [CrossRef]
- Fujie, T.; Matsutani, N.; Kinoshita, M.; Okamura, Y.; Saito, A.; Takeoka, S. Adhesive, Flexible, and Robust Polysaccharide Nanosheets Integrated for Tissue-Defect Repair. Adv. Funct. Mater. 2009, 19, 2560–2568. [Google Scholar] [CrossRef]
- Fujie, T.; Saito, A.; Kinoshita, M.; Miyazaki, H.; Ohtsubo, S.; Saitoh, D.; Takeoka, S. Dual Therapeutic Action of Antibiotic-Loaded Nanosheets for the Treatment of Gastrointestinal Tissue Defects. Biomaterials 2010, 31, 6269–6278. [Google Scholar] [CrossRef] [PubMed]
- Riva, E.R.; Desii, A.; Sartini, S.; la Motta, C.; Mazzolai, B.; Mattoli, V. PMMA/Polysaccharides Nanofilm Loaded with Adenosine Deaminase Inhibitor for Targeted Anti-Inflammatory Drug Delivery. Langmuir 2013, 29, 13190–13197. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-Film Enhanced Chitosan Nerve Guides for Long-Distance Regeneration of Peripheral Nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Cobianchi, S.; Geuna, S.; Barwig, C.; Freier, T.; Udina, E.; Navarro, X. Tubulization with Chitosan Guides for the Repair of Long Gap Peripheral Nerve Injury in the Rat. Microsurgery 2015, 35, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned Microchannel Polymer-Nanotube Composites for Peripheral Nerve Regeneration: Small Molecule Drug Delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hung, H.C.; Sun, F.; Bai, T.; Zhang, P.; Nowinski, A.K.; Jiang, S. Achieving Low-Fouling Surfaces with Oppositely Charged Polysaccharides via LBL Assembly. Acta Biomater. 2016, 40, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Xue, J.; Qian, B.; Chen, H.; Zhu, Y.; Lan, M. Preparation and Antifouling Property of Polyurethane Film Modified by Chondroitin Sulfate. Appl. Surf. Sci. 2017, 394, 403–413. [Google Scholar] [CrossRef]
- Mohan, T.; Kargl, R.; Tradt, K.E.; Kulterer, M.R.; Braćić, M.; Hribernik, S.; Stana-Kleinschek, K.; Ribitsch, V. Antifouling Coating of Cellulose Acetate Thin Films with Polysaccharide Multilayers. Carbohydr. Polym. 2015, 116, 149–158. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Schulz, U.; Munzert, P.; Kaiser, N. Surface Modification of PMMA by DC Glow Discharge and Microwave Plasma Treatment for the Improvement of Coating Adhesion. Surf. Coat. Technol. 2001, 142–144, 507–511. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, Y.; Wantabe, Y. Effect of Oxygen Plasma Etching on Adhesion between Polyimide Films and Metal. Thin Solid Films 1996, 290–291, 367–369. [Google Scholar] [CrossRef]
- Meddeb, A.B.; Ounaies, Z.; Lanagan, M. Enhancement of Electrical Properties of Polyimide Films by Plasma Treatment. Chem. Phys. Lett. 2016, 649, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Markutsya, S.; Tsukruk, V.V. Compliant, Robust, and Truly Nanoscale Free-Standing Multilayer Films Fabricated Using Spin-Assisted Layer-by-Layer Assembly. Adv. Mater. 2004, 16, 157–161. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Pires, R.A.; Reis, R.L.; Alves, N.M. Layer-by-Layer Films Based on Catechol-Modified Polysaccharides Produced by Dip- and Spin-Coating onto Different Substrates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1412–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juang, R.S.; Su, X.; Lee, I.C. Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems. Polymers 2021, 13, 2070. [Google Scholar] [CrossRef]
- Marudova, M.; Bodurov, I.; Sotirov, S.; Uzunova, Y.; Pilicheva, B.; Avramova, I.; Viraneva, A.; Vlaeva, I.; Exner, G.; Yovcheva, T. Nanostructured Polyelectrolyte Multilayer Drug Delivery Systems for Buccal Administration. Bulg. Chem. Commun. 2016, 48, 468–474. [Google Scholar]
- Lee, Y.M.; Park, D.K.; Choe, W.S.; Cho, S.M.; Han, G.Y.; Park, J.; Yoo, P.J. Spin-Assembled Layer-by-Layer Films of Weakly Charged Polyelectrolyte Multilayer. J. Nanosci. Nanotechnol. 2009, 9, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.A.; Sirviö, J.A.; Mathew, A.P.; Oksman, K. Environmental Friendly and Sustainable Gas Barrier on Porous Materials: Nanocellulose Coatings Prepared Using Spin- and Dip-Coating. Mater. Des. 2016, 93, 19–25. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-Gel Synthesis of Calcium Hydroxyapatite Thin Films on Quartz Substrate Using Dip-Coating and Spin-Coating Techniques. J. Sol-Gel Sci. Technol. 2014, 71, 437–446. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Neoh, K.G.; Kang, E.T. Dextran- and Chitosan-Based Antifouling, Antimicrobial Adhesion, and Self-Polishing Multilayer Coatings from PH-Responsive Linkages-Enabled Layer-by-Layer Assembly. ACS Sustain. Chem. Eng. 2018, 6, 3916–3926. [Google Scholar] [CrossRef]
- Martins, A.F.; Vlcek, J.; Wigmosta, T.; Hedayati, M.; Reynolds, M.M.; Popat, K.C.; Kipper, M.J. Chitosan/Iota-Carrageenan and Chitosan/Pectin Polyelectrolyte Multilayer Scaffolds with Antiadhesive and Bactericidal Properties. Appl. Surf. Sci. 2020, 502, 144282. [Google Scholar] [CrossRef]
- Boddohi, S.; Kipper, M.J. Engineering Nanoassemblies of Polysaccharides. Adv. Mater. 2010, 22, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-Based Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.C.; Choi, H.; Kikionis, S.; Seo, J.; Youn, W.; Ioannou, E.; Han, S.Y.; Cho, H.; Roussis, V.; Choi, I.S. Fabrication and Characterization of Neurocompatible Ulvan-Based Layer-by-Layer Films. Langmuir 2020, 36, 11610–11617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.R.; Ma, J.; Liu, B.F.; Xu, Q.Y.; Cui, F.Z. Layer-by-Layer Assembly of Polyelectrolyte Films Improving Cytocompatibility to Neural Cells. J. Biomed. Mater. Res. Part A 2007, 81, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, J.; Xue, C.; Li, Z.; Ding, F.; Yang, Y.; Gu, X. Chitosan/Silk Fibroin-Based, Schwann Cell-Derived Extracellular Matrix-Modified Scaffolds for Bridging Rat Sciatic Nerve Gaps. Biomaterials 2014, 35, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Samorezov, J.E.; Khan, S.A.; Alsberg, E. Electrospun Chitosan-Alginate Nanofibers with in Situ Polyelectrolyte Complexation for Use as Tissue Engineering Scaffolds. Tissue Eng. Part A 2011, 17, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Gonzalez, I.; Macedo, W.A.A.; Nascimento, V.F.; Moraes, A.M.; Beppu, M.M.; Cotta, M.A. Hyaluronan/Chitosan Nanofilms Assembled Layer-by-Layer and Their Antibacterial Effect: A Study Using Staphylococcus Aureus and Pseudomonas Aeruginosa. Colloids Surf. B Biointerfaces 2016, 141, 499–506. [Google Scholar] [CrossRef]
- Nascimento, V.; França, C.; Hernández-Montelongo, J.; Machado, D.; Lancellotti, M.; Cotta, M.; Landers, R.; Beppu, M. Influence of PH and Ionic Strength on the Antibacterial Effect of Hyaluronic Acid/Chitosan Films Assembled Layer-by-Layer. Eur. Polym. J. 2018, 109, 198–205. [Google Scholar] [CrossRef]
- Hernández-Montelongo, J.; Nascimento, V.F.; Murillo, D.; Taketa, T.B.; Sahoo, P.; de Souza, A.A.; Beppu, M.M.; Cotta, M.A. Nanofilms of Hyaluronan/Chitosan Assembled Layer-by-Layer: An Antibacterial Surface for Xylella Fastidiosa. Carbohydr. Polym. 2016, 136, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Johnson, R.W.; Desai, T.A. XPS and AFM Analysis of Antifouling PEG Interfaces for Microfabricated Silicon Biosensors. Biosens. Bioelectron. 2004, 20, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.M.; Meng, F.N.; Quan, M.; Ding, K.; Dang, Y.; Gong, Y.K. Quantitative Fabrication, Performance Optimization and Comparison of PEG and Zwitterionic Polymer Antifouling Coatings. Acta Biomater. 2017, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, S.N. Bio-Inspired Strategies for Designing Antifouling Biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Riva, E.R.; Sinibaldi, E.; Grillone, A.F.; del Turco, S.; Mondini, A.; Li, T.; Takeoka, S.; Mattoli, V. Enhanced in Vitro Magnetic Cell Targeting of Doxorubicin-Loaded Magnetic Liposomes for Localized Cancer Therapy. Nanomaterials 2020, 10, 2104. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, J.J. Experimental Methods for Investigating Protein Adsorption Kinetics at Surfaces. Q. Rev. Biophys. 1994, 27, 41–105. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.G.; Ostuni, E.; Liang, M.N.; Meluleni, G.; Kim, E.; Yan, L.; Pier, G.; Warren, H.S.; Whitesides, G.M. Polymeric Thin Films That Resist the Adsorption of Proteins and the Adhesion of Bacteria. Langmuir 2001, 17, 1225–1233. [Google Scholar] [CrossRef]
- Liu, J.; Lee, M.L. Permanent Surface Modification of Polymeric Capillary Electrophoresis Microchips for Protein and Peptide Analysis. Electrophoresis 2006, 27, 3533–3546. [Google Scholar] [CrossRef]
- Richardson, R.R.; Miller, J.A.; Reichert, W.M. Polyimides as Biomaterials: Preliminary Biocompatibility Testing. Biomaterials 1993, 14, 627–635. [Google Scholar] [CrossRef]
- Mortazavi, M.; Nosonovsky, M. A Model for Diffusion-Driven Hydrophobic Recovery in Plasma Treated Polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-Fouling Coatings of Poly(Dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D.Y. A Materials Roadmap to Functional Neural Interface Design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.; Vallejo-Giraldo, C.; Syed, O.; Green, R. Considerations for Hydrogel Applications to Neural Bioelectronics. J. Mater. Chem. B 2019, 7, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Zhou, H.; Li, T.; Li, C.; Duan, Y.Y. Polyethylene Glycol-Containing Polyurethane Hydrogel Coatings for Improving the Biocompatibility of Neural Electrodes. Acta Biomater. 2012, 8, 2233–2242. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shih, J.; Zhu, J.; Kotov, N.A. Layered Nanocomposites from Gold Nanoparticles for Neural Prosthetic Devices. Nano Lett. 2012, 12, 3391–3398. [Google Scholar] [CrossRef] [Green Version]


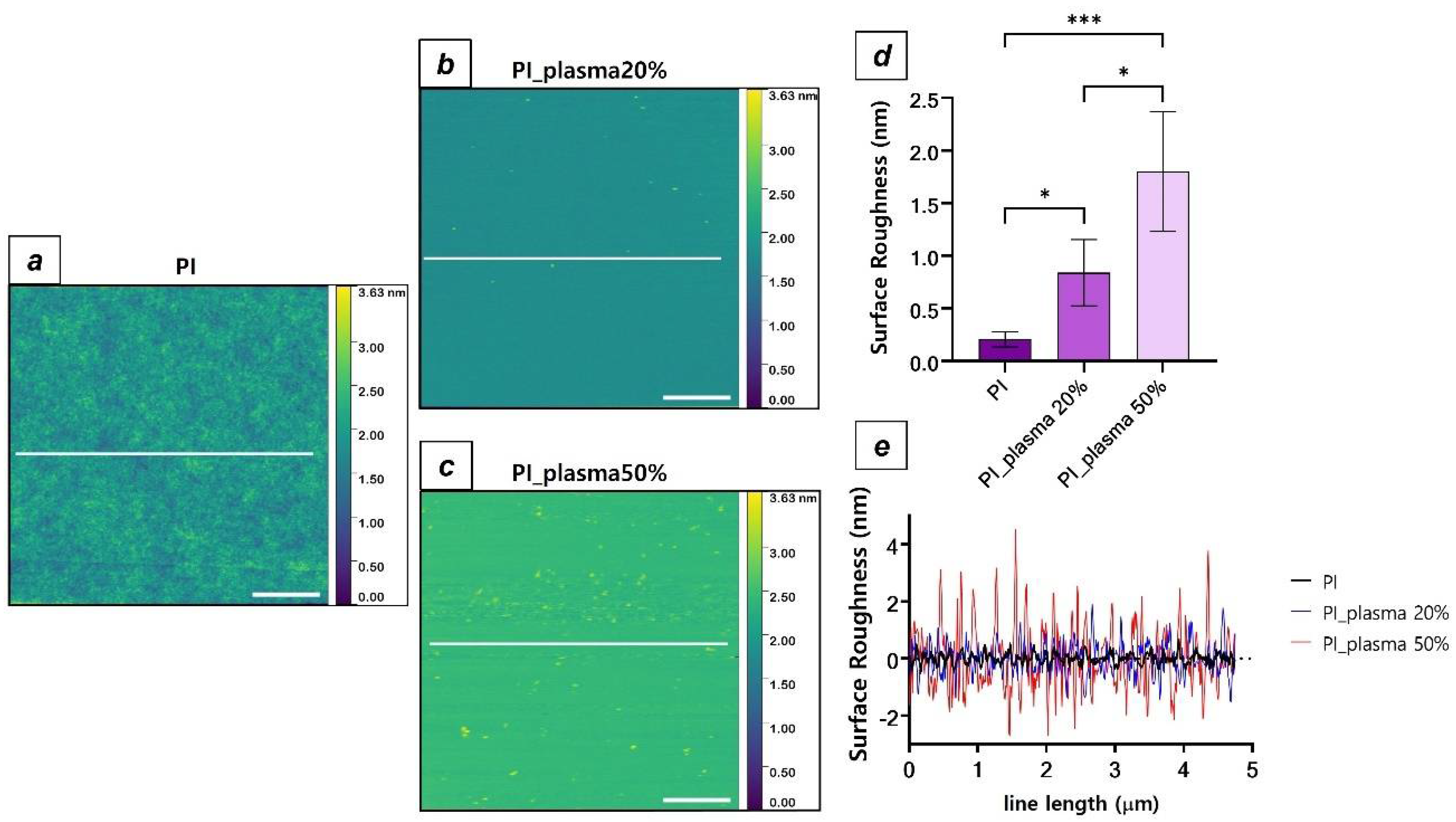

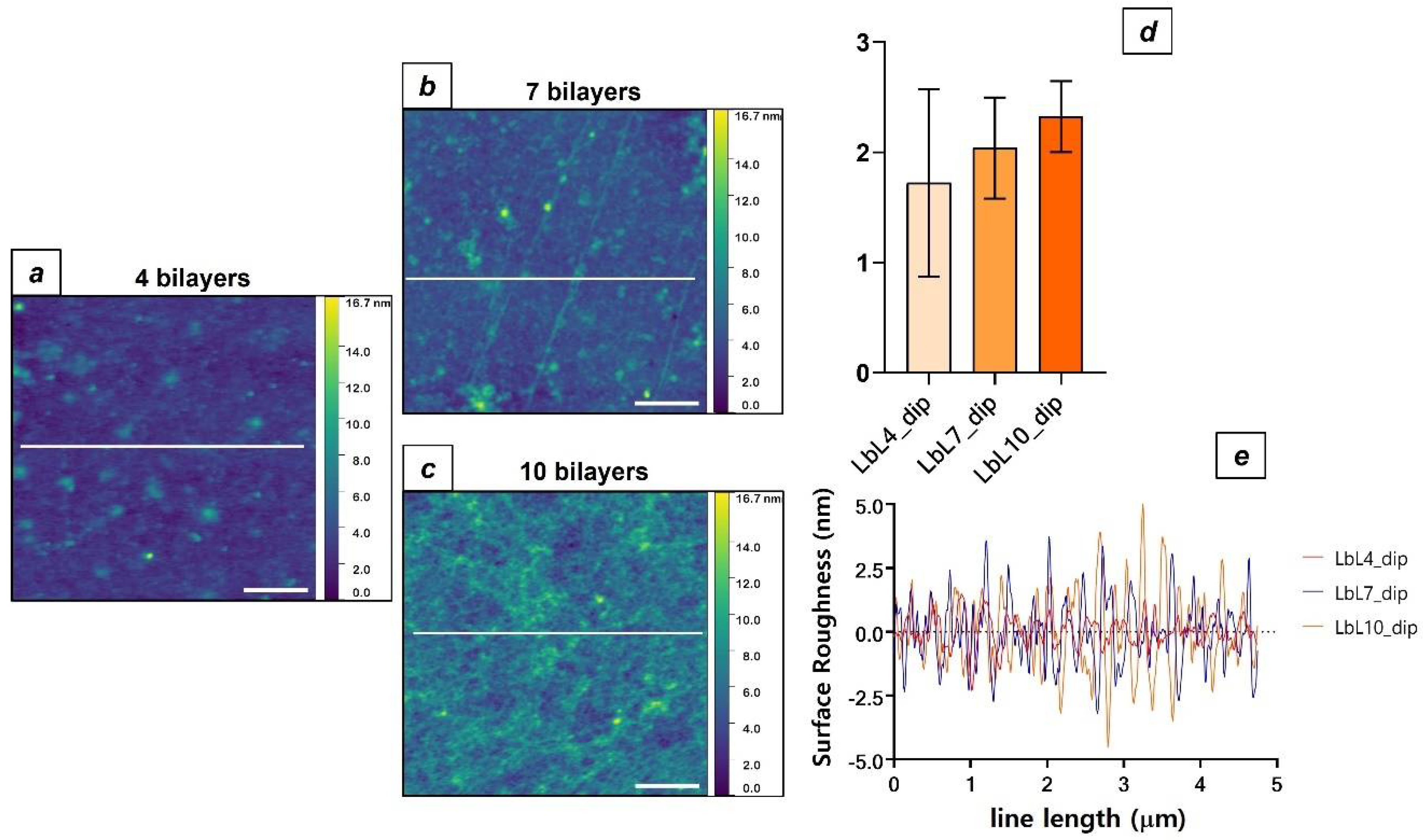
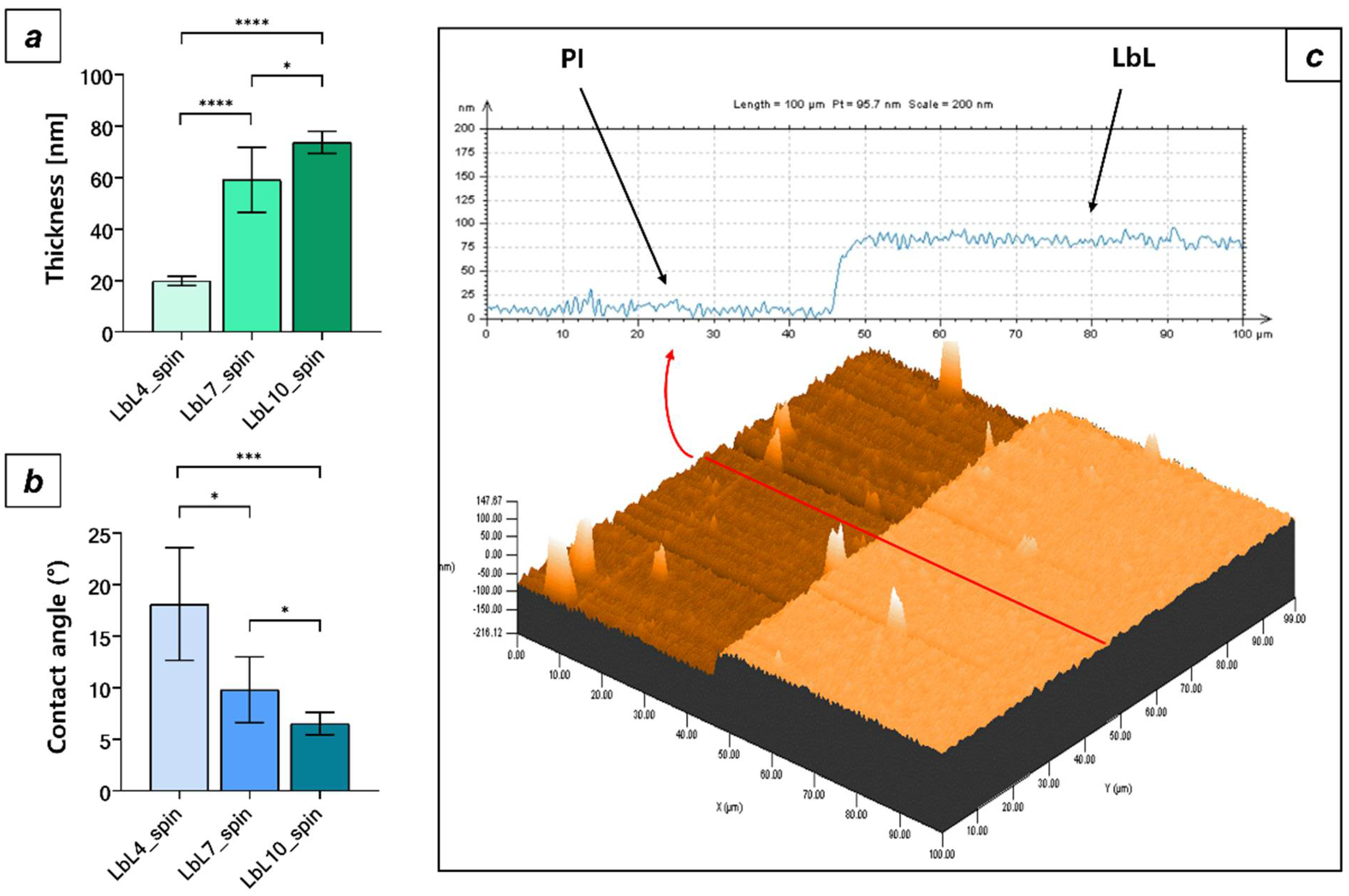

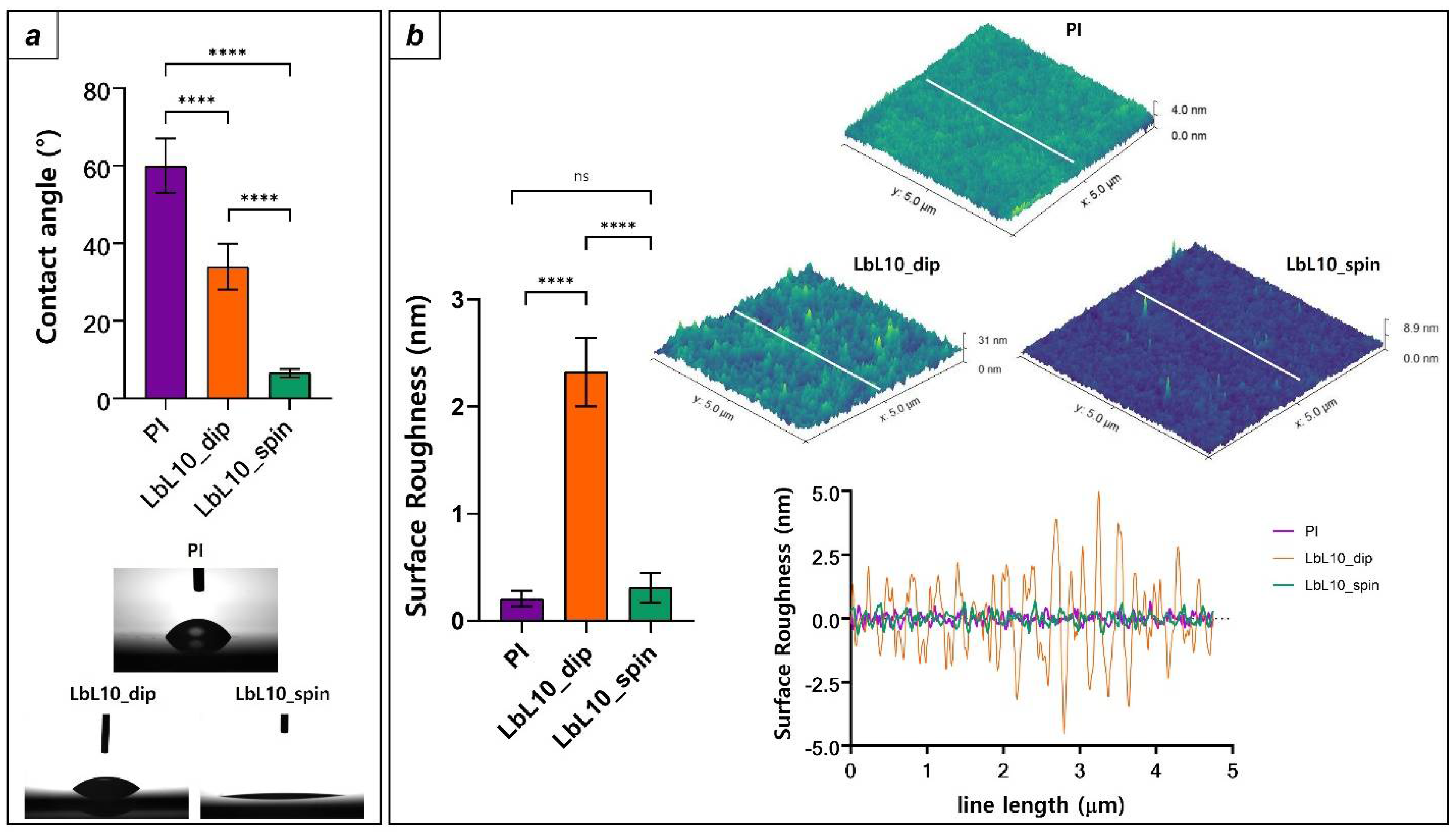
| Sample Type | Contact Angle (°) | Ra (nm) |
|---|---|---|
| PI | 60.01 ± 7.02 | 0.2 ± 0.07 |
| LbL10_dip | 34.03 ± 5.9 | 2.32 ± 0.32 |
| LbL10_spin | 6.5 ± 1.1 | 0.3 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redolfi Riva, E.; D’Alessio, A.; Micera, S. Polysaccharide Layer-by-Layer Coating for Polyimide-Based Neural Interfaces. Micromachines 2022, 13, 692. https://doi.org/10.3390/mi13050692
Redolfi Riva E, D’Alessio A, Micera S. Polysaccharide Layer-by-Layer Coating for Polyimide-Based Neural Interfaces. Micromachines. 2022; 13(5):692. https://doi.org/10.3390/mi13050692
Chicago/Turabian StyleRedolfi Riva, Eugenio, Angela D’Alessio, and Silvestro Micera. 2022. "Polysaccharide Layer-by-Layer Coating for Polyimide-Based Neural Interfaces" Micromachines 13, no. 5: 692. https://doi.org/10.3390/mi13050692





