Microfluidics for High Pressure: Integration on GaAs Acoustic Biosensors with a Leakage-Free PDMS Based on Bonding Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
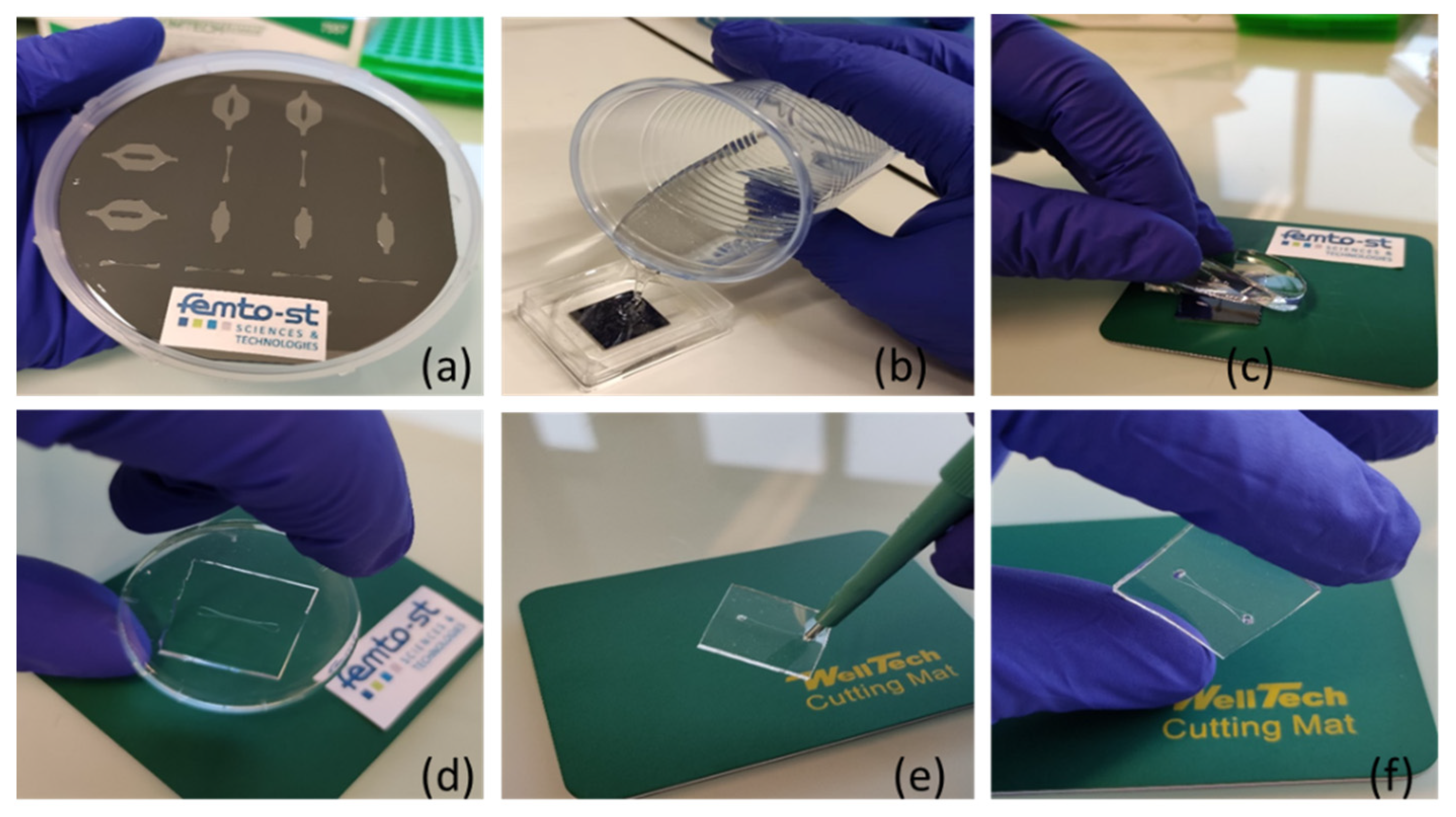
2.2. Methods and Equipment
3. Results
3.1. SEM Characterization
3.2. Activation–Characterization of PDMS and GaAs/SiO2 Surfaces-Interfaces
3.3. Optimization of the Bonding Microfluidic Channel with GaAs Substrate
3.3.1. Test with Plasma O2 and Annealing
3.3.2. Bonding Strength Evaluation
3.3.3. Leakage Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, S.; Hadwen, B.; Brown, C. Thin-film-transistor digital microfluidics for high valuein vitrodiagnostics at the point of need. Lab Chip 2021, 21, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Caen, O.; Vrignon, J.; Zonta, E.; El Harrak, Z.; Nizard, P.; Baret, J.C.; Taly, V. High throughput single cell counting in droplet-based microfluidics. Sci. Rep. 2017, 7, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neužil, P.; Giselbrecht, S.; Länge, K.; Huang, T.-J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discovery 2012, 11, 620–632. [Google Scholar] [CrossRef]
- Chen, S.; Shamsi, M.-H. Biosensors-on-chip: A topical review. J. Micromech. Microeng. 2017, 27, 083001. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.-R.; Weigl, B.-H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.-G.; Kupitz, C.; Fromme, P.; Ros, A. Crystallization of the large membrane protein complex photosystem i in a microfluidic channel. ACS Nano 2013, 7, 10534–10543. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Qin, J.; Lin, B. Electrophoretic separations on microfluidic chips. J. Chromatogr. A 2008, 1184, 542–559. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.-E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Liu, K.-K.; Wu, R.-G.; Chuang, Y.-J.; Khoo, H.-S.; Huang, S.-H.; Tseng, F.-G. Microfluidic systems for biosensing. Sensors 2010, 10, 6623–6661. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Liu, L.; Xu, Y.; Wang, L.; Chen, L.; Yan, S.; Shui, Z.; Wangf, Z.; Li, S. A highly efficient preconcentration route for rapid and sensitive detection of endotoxin based on an electrochemical biosensor. Analyst 2020, 145, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, L.; Huang, F.; Wang, S.; Wang, L.; Liu, N.; Lin, J. A capillary biosensor for rapid detection of Salmonella using Fe-nanocluster amplification and smart phone imaging. Biosens. Bioelectron. 2019, 127, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; de Vittorio, M. GHz AlN-based multiple mode SAW temperature sensor fabricated on PEN substrate. Sens. Actuators A Phys 2020, 315, 112268. [Google Scholar] [CrossRef]
- Lacour, V.; Elie-Caille, C.; Leblois, T.; Dubowski, J.J. Regeneration of a thiolated and antibody functionalized GaAs (001) surface using wet chemical processes. Biointerphases 2016, 11, 019302. [Google Scholar] [CrossRef] [Green Version]
- Chawich, J.; Hassen, W.M.; Elie-Caille, C.; Leblois, T.; Dubowski, J.J. Regenerable ZnO/GaAs bulk acousticwave biosensor for detection of Escherichia coli in “complex” biological medium. Biosensors 2021, 11, 145. [Google Scholar] [CrossRef]
- Marquez, D.T.; Chawich, J.; Hassen, W.M.; Moumanis, K.; DeRosa, M.C.; Dubowski, J.J. Polymer Brush-GaAs Interface and Its Use as an Antibody-Compatible Platform for Biosensing. ACS Omega 2021, 6, 7286–7295. [Google Scholar] [CrossRef]
- Joint, F.; Abadie, C.; Vigneron, P.B.; Boulley, L.; Bayle, F.; Isac, N.; Cavanna, A.; Cambril, E.; Herth, E. GaAs manufacturing processes conditions for micro- and nanoscale devices. J. Manuf. Process. 2020, 60, 666–672. [Google Scholar] [CrossRef]
- Bienaime, A.; Liu, L.; Elie-Caille, C.; Leblois, T. Design and microfabrication of a lateral excited gallium arsenide biosensor. Eur. Phys. J. Appl. Phys. EDP Sci. 2012, 57, 21003. [Google Scholar] [CrossRef]
- Voznyy, O.; Dubowski, J.-J. Adsorption kinetics of hydrogen sulfide and thiols on GaAs (001) surfaces in a vacuum. J. Phys. Chem. C 2008, 112, 3726–3733. [Google Scholar] [CrossRef]
- Voznyy, O.; Dubowski, J.J. pH-Dependent Photocorrosion of GaAs/AlGaAs Quantum Well Microstructures. J. Phys. Chem. C. 2016, 120, 26129–26137. [Google Scholar]
- Lowe, A.M.; Ozer, B.H.; Wiepz, G.J.; Bertics, P.J.; Abbott, N.L. Engineering of PDMS surfaces for use in microsystems for capture and isolation of complex and biomedically important proteins: Epidermal growth factor receptor as a model system. Lab Chip 2008, 8, 1357–1364. [Google Scholar] [CrossRef] [Green Version]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Antfolk, M.; Antfolk, C.; Lilja, H.; Laurell, T.; Augustsson, P. A single inlet two-stage acoustophoresis chip enabling tumor cell enrichment from white blood cells. Lab Chip 2015, 15, 2102–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbansky, A.; Lenshof, A.; Dykes, J.; Laurell, T.; Scheding, S. Affinity-bead-mediated enrichment of CD8+ lymphocytes from peripheral blood progenitor cell products using acoustophoresis. Micromachines 2016, 7, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, N.C.; Yue, C.Y.; Lam, Y.C.; Tan, Y.L. Thermal bonding of PMMA: Effect of polymer molecular weight. Microsyst. Technol. 2010, 16, 487–491. [Google Scholar] [CrossRef]
- Kersey, L.; Ebacher, V.; Bazargan, V.; Wang, R.; Stoeber, B. The effect of adhesion promoter on the adhesion of PDMS to different substrate materials. Lab Chip 2009, 9, 1002–1004. [Google Scholar] [CrossRef]
- Gonzalez-Gallardo, C.-L.; Díaz Díaz, A.; Casanova-Moreno, J.-R. Improving plasma bonding of PDMS to gold-patterned glass for electrochemical microfluidic applications. Microfluid. Nanofluid. 2021, 25, 20. [Google Scholar] [CrossRef]
- Han, Y.; Jiao, Z.; Zhao, J.; Chao, Z.; You, Z. A simple approach to fabricate multi-layer glass microfluidic chips based on laser processing and thermocompression bonding. Microfluid. Nanofluid. 2021, 25, 77. [Google Scholar] [CrossRef]
- Lee, K.S.; Ram, R.J. Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip 2009, 9, 1618–1624. [Google Scholar] [CrossRef]
- Du, L.; Allen, M.-G. Silica hermetic packages based on laser patterning and localized fusion bonding. In Proceedings of the IEEE Micro Electro Mechanical Systems, Belfast, UK, 21–25 January 2018. [Google Scholar]
- Du, L.; Allen, M.-G. CMOS Compatible Hermetic Packages Based on Localized Fusion Bonding of Fused Silica. J. Microelectromech. Syst. 2019, 28, 656–665. [Google Scholar] [CrossRef]
- Jeong, S.H.; Zhang, S.; Hjort, K.; Hilborn, J.; Wu, Z. PDMS-based elastomer tuned soft, stretchable, and sticky for epidermal electronics. Adv. Mater. 2016, 28, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Liu, H.; Zhu, Y.; Cai, B.; Jin, Y.; Wei, Y.; Zhou, F.; Liu, W.; Guo, S. An improved bulk acoustic waves chip based on a PDMS bonding layer for high-efficient particle enrichment. Microfluid. Nanofluid. 2018, 22, 32. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Tserepi, A.; Pavli, P.; Argitis, P.; Sanopoulou, M.; Misiakos, K. A low temperature surface modification assisted method for bonding plastic substrates. J. Micromech. Microeng. 2009, 19, 015007. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, P.; Liu, K.; Escobedo, C. A Versatile Bonding Method for PDMS and SU-8 and Its Application towards a Multifunctional Microfluidic Device. Micromachines 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Shen, J.; Zhou, M. Rapid fabrication of a four-layer PMMA- based microfluidic chip using CO2 -laser micromachining and thermal bonding. J. Micromech. Microeng. 2016, 26, 107001. [Google Scholar] [CrossRef]
- Liu, P.; Lv, Z.; Sun, B.; Gao, Y.; Qi, W.; Xu, Y.; Chen, L.; Wang, L.; Ge, C.; Li, S. A universal bonding method for preparation of microfluidic biosensor. Microfluid. Nanofluid. 2021, 25, 43. [Google Scholar] [CrossRef]
- Koklu, A.; Wustoni, S.; Musteata, V.E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.-P.; Inal, S. Micro fluidic Integrated Organic Electrochemical Transistor with a Nanoporous Membrane for Amyloid-β Detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef]
- Liang, D.; Huo, Y.; Kang, Y.; Wang, K.X.; Gu, A.; Tan, M.; Yu, Z.; Li, S.; Jia, J.; Bao, X.; et al. Optical Absorption Enhancement in Freestanding GaAs Thin Film Nanopyramid Arrays. Adv. Energy Mater. 2012, 2, 1254–1260. [Google Scholar] [CrossRef]
- Sun, B.-Y.; Kumar, V.; Adesida, I.; Rogers, J.-A. Buckled Ribbons of GaAs for Flexible Electronics Buckled and Wavy Ribbons of GaAs for High-Performance Electronics on Elastomeric Substrates. Adv. Mater. 2006, 18, 2857–2862. [Google Scholar] [CrossRef]
- Norouzi, A.-R.; Nikfarjam, A.; Hajghassem, H. PDMS—PMMA bonding improvement using SiO 2 intermediate layer and its application in fabricating gas micro valves. Microsyst. Technol. 2018, 24, 2727–2736. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Suehara, S.; Suga, T. Room temperature wafer level glass/glass bonding. Sens. Actuator A-Phys 2006, 127, 31–36. [Google Scholar] [CrossRef]
- Fuard, D.; Tzvetkova-Chevolleau, T.; Decossas, S.; Tracqui, P.; Schiavone, P. Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectron. Eng. 2008, 85, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Zahid, A.; Dai, B.; Hong, R.; Zhang, D. Optical properties study of silicone polymer PDMS substrate surfaces modified by plasma treatment. Mater. Res. Express 2017, 4, 105301. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, R.; Lee, N.Y. Chemically robust succinimide group-assisted irreversible bonding of poly(dimethylsiloxane)–thermoplastic microfluidic devices at room temperature. Analyst 2020, 145, 6887–6894. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Tian, Y.; Wu, B.; Wang, S.; Zhang, H. Glass-on-LiNbO3 heterostructure formed via a two-step plasma activated low-temperature direct bonding method. Appl. Surf. Sci. 2018, 459, 621–629. [Google Scholar] [CrossRef]
- Takigawa, R.; Higurashi, E.; Asano, T. Room-temperature wafer bonding of LiNbO3 and SiO2 using a modified surface activated bonding method. Jpn. J. Appl. Phys. 2018, 57, 06HJ12. [Google Scholar] [CrossRef]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS – PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on Surface Wettability of Poly (Dimethyl) Siloxane (PDMS) and Glass Under Oxygen-Plasma Treatment and Correlation With Bond Strength. J. Microelectromechanical. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Heat and pressure-resistant room temperature irreversible sealing of hybrid PDMS—thermoplastic micro fl uidic devices via carbon—nitrogen covalent bonding and its application in a continuous-flow. RSC Adv. 2020, 10, 16502. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Wen, X.; Takahashi, S.; Hatakeyama, K.; Kamei, K.-I. Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures. Micromachines 2021, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, R.; Yu, H.; Li, G.; Xu, K.; Tien, N.C.; Roberts, R.C.; Li, D. Inkjet-printed microelectrodes on PDMS as biosensors for functionalized microfluidic systems. Lab Chip 2015, 15, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Cai, D.; Ruan, X.; Cai, A. Research on the selective adhesion characteristics of polydimethylsiloxane layer. AIP Adv. 2018, 8, 095004. [Google Scholar] [CrossRef] [Green Version]
- Chow WW, Y.; Lei, K.F.; Shi, G.; Li, W.J.; Huang, Q. Micro Fluidic Channel Fabrication by PDMS-Interface Bonding. Smart Mater. Struct. 2006, 15, S112. [Google Scholar] [CrossRef] [Green Version]
- Hammami, S.; Sylvestre, A.; Jomni, F.; Jean-Mistral, C. Electrical Conduction in Dielectric Elastomer Transducers. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 17–25. [Google Scholar] [CrossRef]
- Schneider, S.; Brás, E.J.; Schneider, O.; Schlünder, K.; Loskill, P. Facile Patterning of Thermoplastic Elastomers and Robust Bonding to Glass and Thermoplastics for Microfluidic Cell Culture and Organ-on-Chip. Micromachines 2021, 12, 575. [Google Scholar] [CrossRef]








| Sample | RMS Average Roughness Rq (pm) | Average Roughness Ra (pm) |
|---|---|---|
| GaAs/SiO2 | 359 | 552 |
| GaAs | 56 | 90 |
| GaAs/SiO2 after plasma O2 | 293 | 441 |
| PDMS | 1441 | not reported |
| PDMS treated by plasma O2 | 40,031 | not reported |
| Sample | Pressure (Bar) | Method to Bon to PMDS | Ref |
|---|---|---|---|
| GaAs | ≤8.5 | plasma O2, SiO2, annealed | This work |
| Glass | 5.1 | plasma oxygen ICP | [50] |
| PDMS | 6.7 | plasma oxygen RIE | [49] |
| SU-8 | 1.5 | plasma oxygen, small amount of PEIE and temperature | [38] |
| Glass/Au | 2.38 | plasma oxygen and narrow electrode | [28] |
| TPE | 4.7 | plasma oxygen and thermal bonding | [58] |
| PMMA | 2.5 | plasma oxygen | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammami, S.; Oseev, A.; Bargiel, S.; Zeggari, R.; Elie-Caille, C.; Leblois, T. Microfluidics for High Pressure: Integration on GaAs Acoustic Biosensors with a Leakage-Free PDMS Based on Bonding Technology. Micromachines 2022, 13, 755. https://doi.org/10.3390/mi13050755
Hammami S, Oseev A, Bargiel S, Zeggari R, Elie-Caille C, Leblois T. Microfluidics for High Pressure: Integration on GaAs Acoustic Biosensors with a Leakage-Free PDMS Based on Bonding Technology. Micromachines. 2022; 13(5):755. https://doi.org/10.3390/mi13050755
Chicago/Turabian StyleHammami, Saber, Aleksandr Oseev, Sylwester Bargiel, Rabah Zeggari, Céline Elie-Caille, and Thérèse Leblois. 2022. "Microfluidics for High Pressure: Integration on GaAs Acoustic Biosensors with a Leakage-Free PDMS Based on Bonding Technology" Micromachines 13, no. 5: 755. https://doi.org/10.3390/mi13050755







