A Novel Modification of Copper (II) Phthalocyanine Particles towards Electrophoretic Displays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
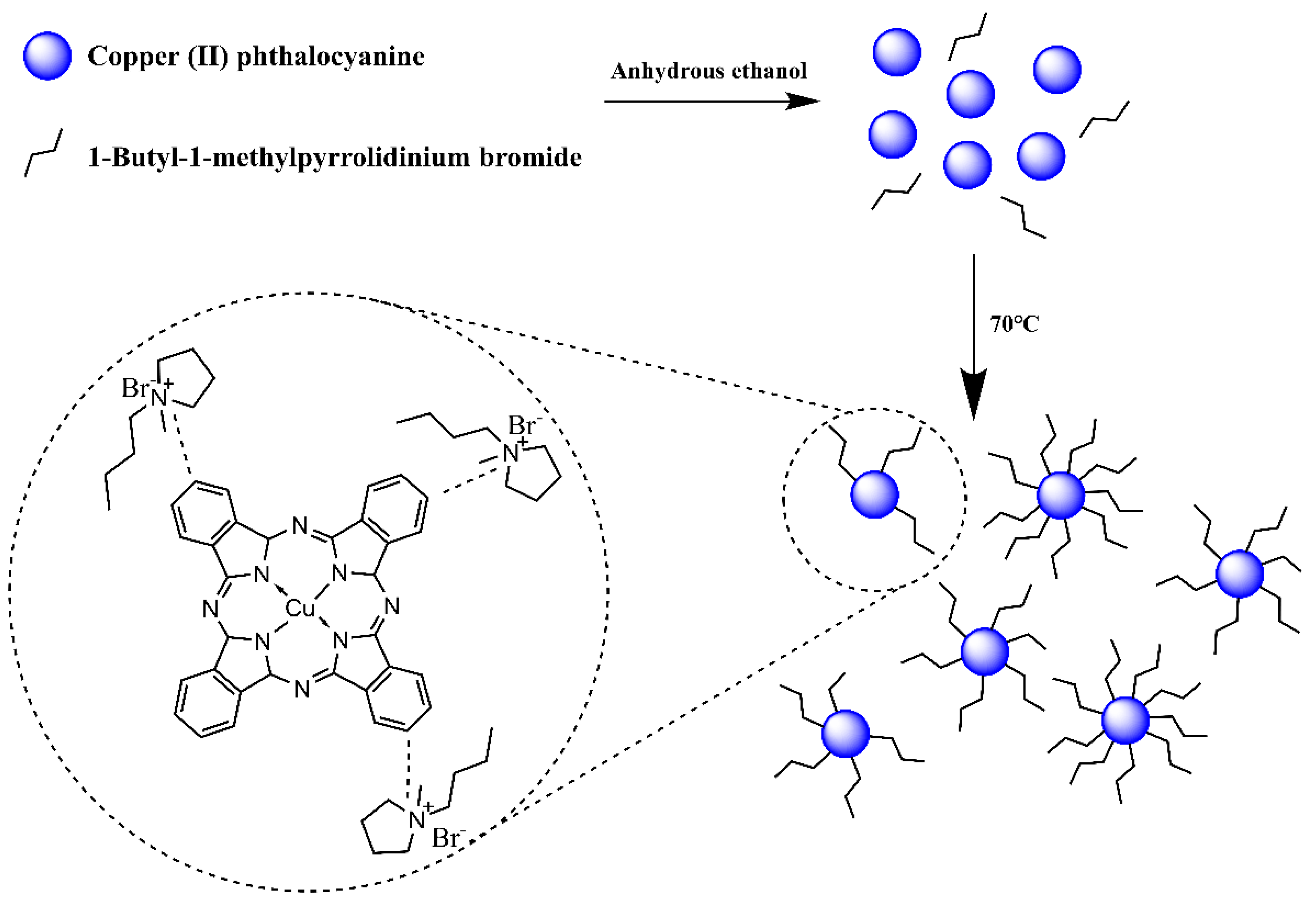
2.2. Surface Modification of CP with Mono Ionic Liquids
2.3. Preparation of Electrophoretic Dispersion
2.4. EPD Cell and EPD Experimental Platform
2.5. Instruments and Characterization
3. Results and Discussion
3.1. Energy Dispersive Spectroscopy (EDS) and Scanning Electron Microscope (SEM)
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Thermogravimetric Analysis (TGA)
3.4. Particle Size and Zeta Potential
3.5. Stability of Electrophoretic Dispersion
3.6. Display, Luminance, and Chromaticity Coordinate of an EPD Cell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.F.; Yi, Z.C.; de Rooij, N.; Zhou, G.F.; Gravina, R. Editorial: Modeling and Applications of Optoelectronic Devices for Access Networks. Front. Phys. 2021, 9, 678269. [Google Scholar] [CrossRef]
- Chen, Y. Flexible active-matrix electronic ink display. Nature 2003, 423, 136. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.J.; Yi, Z.C.; Zhao, Y.M.; Zeng, W.B.; Zhou, G.F. Design of Driving Waveform Based on Overdriving Voltage for Shortening Response Time in Electrowetting Displays. Front. Phys. 2021, 9, 642682. [Google Scholar] [CrossRef]
- Graham-Rowe, D. Electronic paper rewrites the rulebook for displays. Nat. Photonics 2007, 1, 248–251. [Google Scholar] [CrossRef]
- He, W.Y.; Yi, Z.C.; Shen, S.T.; Huang, Z.Y.; Zhou, G.F. Driving Waveform Design of Electrophoretic Display Based on Optimized Particle Activation for a Rapid Response Speed. Micromachines 2020, 11, 498. [Google Scholar] [CrossRef]
- Park, L.S.; Park, J.W.; Choi, H.Y.; Han, Y.S.; Kwon, Y.; Choi, H.S. Fabrication of charged particles for electrophoretic display. Curr. Appl. Phys. 2006, 6, 644–648. [Google Scholar] [CrossRef]
- Tan, T.F.; Wang, S.R.; Bian, S.G.; Li, X.G.; An, Y.; Liu, Z.J. Novel synthesis and electrophoretic response of low density TiO–TiO2–carbon black composite. Appl. Surf. Sci. 2010, 256, 6932–6935. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, T.F.; Qi, P.; Wang, S.R.; Bian, S.G.; Li, X.G.; An, Y.; Liu, Z.J. Preparation and surface encapsulation of hollow TiO nanoparticles for electrophoretic displays. Appl. Surf. Sci. 2011, 257, 3499–3503. [Google Scholar] [CrossRef]
- Li, G.X.; Meng, S.X.; Feng, Y.Q. Preparation of electrophoretic nanoparticles for electronic paper. Mater. Res. Innov. 2016, 20, 545–551. [Google Scholar] [CrossRef]
- Ota, I.; Ohnishi, J.; Yoshiyama, M. Electrophoretic image display (EPID) panel. Proc. IEEE 1973, 61, 832–836. [Google Scholar] [CrossRef]
- Werts, M.P.L.; Badila, M.; Brochon, C.; Hébraud, A.; Hadziioannou, G. Titanium DioxidePolymer Core–Shell Particles Dispersions as Electronic Inks for Electrophoretic Displays. Chem. Mater. 2009, 20, 1292–1298. [Google Scholar] [CrossRef]
- Peng, B.; Li, Y.; Li, J.; Bi, L.; Lu, H.P.; Xie, J.L.; Ren, X.L.; Cao, Y.H.; Wang, N.; Meng, X.W.; et al. Monodisperse light color nanoparticle ink toward chromatic electrophoretic displays. Nanoscale 2016, 8, 10917–10921. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Kim, C.W.; Cha, H.J.; Pal, U.; Kang, Y.S. Encapsulated-dye all-organic charged colored ink nanoparticles for electrophoretic image display. Adv. Mater. 2009, 21, 4987–4991. [Google Scholar] [CrossRef]
- Meng, X.W.; Qiang, L.; Wei, J.F.; Shi, H.T. Preparation of electrophoretic nanoparticles for electronic paper. J. Nanosci. Nanotechnol. 2014, 14, 1617–1630. [Google Scholar] [CrossRef]
- Li, J.J.; Deng, L.D.; Xing, J.F.; Dong, A.J.; Li, X.G. Preparation and characterization of TiO2-cationic hybrid nanoparticles as electrophoretic particles. Appl. Surf. Sci. 2012, 258, 3152–3157. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Zhang, T.Y.; Lai, S.F.; Liu, L.W.; He, W.Y.; Zhou, G.F.; Yi, Z.C. Driving Waveform Design with Rising Gradient and Sawtooth Wave of Electrowetting Displays for Ultra-Low Power Consumption. Micromachines 2020, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.J.; Yi, Z.C.; Zhou, X.C.; Zhao, Y.M.; Zhou, G.F. Design of Driving Waveform for Shortening Red Particles Response Time in Three-Color Electrophoretic Displays. Micromachines 2021, 12, 578. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Feng, Y.Q.; Li, X.G.; Li, X.X.; Bai, Y.; Tang, Q.M.; Gao, Y.D. Fabrication of diarylide yellow pigments/modified SiO2 core–shell hybrid composite particles for electrophoretic displays. Curr. Appl. Phys. 2012, 12, 259–265. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, C.A.; Ahn, S.D.; Kang, S.R.; Suh, K.S. Density compatibility of encapsulation of white inorganic TiO2 particles using dispersion polymerization technique for electrophoretic display. Synth. Met. 2004, 146, 197–199. [Google Scholar] [CrossRef]
- Kim, C.A.; Joung, M.J.; Ahn, S.D.; Kim, G.H.; Kang, S.Y.; You, I.K.; Oh, J.; Myoung, H.J.; Baek, K.H.; Suh, K.S. Microcapsules as an electronic ink to fabricate color electrophoretic displays. Synth. Met. 2005, 151, 181–185. [Google Scholar] [CrossRef]
- Comiskey, B.; Albert, J.D.; Yoshizawa, H.; Jacobson, J. An electrophoretic ink for all-printed reflective electronic displays. (cover story). Nature 1998, 394, 253–255. [Google Scholar] [CrossRef]
- Yi, Z.C.; Zeng, W.B.; Ma, S.M.; Feng, H.Q.; Zeng, W.J.; Shen, S.T.; Shui, L.L.; Zhou, G.F.; Zhang, C.F. Design of Driving Waveform Based on a Damping Oscillation for Optimizing Red Saturation in Three-Color Electrophoretic Displays. Micromachines 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kwon, Y.R.; Kim, S.K.; Noh, C.H.; Lee, J.Y. Electrophoretic Display of surface modified TiO2 driven by Poly (3,4-ethylenedioxythiophene) Electrode. Polym. Bull. 2007, 59, 331–338. [Google Scholar] [CrossRef]
- Wen, T.; Meng, X.W.; Li, Z.Y.; Ren, J.; Tang, F.Q. Pigment-based tricolor ink particles via mini-emulsion polymerization for chromatic electrophoretic displays. J. Mater. Chem. 2010, 20, 8112–8117. [Google Scholar] [CrossRef]
- Meng, X.W.; Tang, F.Q.; Peng, B.; Ren, J. Monodisperse Hollow Tricolor Pigment Particles for Electronic Paper. Nanoscale Res. Lett. 2009, 5, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.Y.; Wu, G.; Chen, H.Z. Stable titanium dioxide grafted with poly [N-(p-vinyl benzyl) phthalimide] composite particles in suspension for electrophoretic displays. Colloid Polym. Sci. 2011, 289, 401–407. [Google Scholar] [CrossRef]
- Yuan, J.J.; Zhou, S.X.; Wu, L.M.; You, B. Organic pigment particles coated with titania via sol-gel process. J. Mater. Chem. B 2006, 110, 388–394. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Feng, Y.Q.; Li, X.G.; Bai, Y.; Li, X.X.; An, J.; Lu, M. Surface modification of organic pigment particles for microencapsulated electrophoretic displays. Dyes Pigment. 2012, 92, 554–562. [Google Scholar] [CrossRef]
- Mürau, P.; Singer, B. The understanding and elimination of some suspension instabilities in an electrophoretic display. J. Appl. Phys. 1978, 49, 4820–4829. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhou, C.L. Properties of copper phthalocyanine blue (C.I. Pigment Blue 15:3) treated with poly(ethylene glycol)s. Dyes Pigment. 1997, 35, 123–130. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhao, X.P.; Guo, H.L.; Zheng, Q. Preparation and response behavior of blue electronic ink microcapsules. Opt. Mater. 2008, 30, 1268–1272. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, L.L.; Huang, Y.; Nan, X.Y.; Fan, Q.G.; Shao, J.Z. Preparation and characterization of Phthalocyanine Blue encapsulated with silane coupling agent for blue light curable inkjet printing of textiles. Dyes Pigment. 2017, 139, 453–459. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, K.; Zhhang, M.J.; Tian, L. Encapsulated phthalocyanine blue pigment with polymerisable dispersant for inkjet printing inks. Pigm. Resin. Technol. 2012, 41, 3–8. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.J.; Li, L.L.; Liu, Z.Y.; Jin, P.; Tang, C.C. Preparation of chromatic composite hollow nanoparticles containing mixed metal oxides for full-color electrophoretic displays. J. Mater. Chem. C 2016, 4, 5664–5670. [Google Scholar] [CrossRef]
- Kholghi Eshkalak, S.; Khatibzadeh, M.; Kowsari, E.; Chinnappan, A.; Ramakrishna, S. Application of ionic liquids as charge control agents of pigments and preparation of microcapsules as electronic inks through electrospraying. Opt. Mater. 2018, 84, 73–81. [Google Scholar] [CrossRef]
- Hu, Y.; Al-Shujaa, S.A.S.; Zhen, B.; Zhang, Y.P.; Li, X.G.; Feng, Y.Q. Blue nanocomposites coated with an ionic liquid polymer for electrophoretic displays. RSC Adv. 2021, 11, 20760–20768. [Google Scholar] [CrossRef]
- Prieve, D.C.; Yezer, B.A.; Khair, A.S.; Sides, P.J.; Schneider, J.W. Formation of Charge Carriers in Liquids. Adv. Colloid Interface Sci. 2017, 244, 21–35. [Google Scholar] [CrossRef]
- Li, D.; Le, Y.; Hou, X.Y.; Chen, J.F.; Shen, Z.G. Colored nanoparticles dispersions as electronic inks for electrophoretic display. Synth. Met. 2011, 161, 1270–1275. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Li, G.X.; Qin, S.C.; Feng, Y.Q.; Fang, S.; Meng, S.X. Preparation and Characterization of Microcapsule-Encapsulated Colored Electrophoretic Fluid in Trifluorotoluene System for Electrophoretic Display. J. Disp. Technol. 2016, 12, 1145–1151. [Google Scholar] [CrossRef]
- Han, J.J.; Zhang, W.H.; Li, X.G.; Sun, C.; Shao, J.Z.; Feng, Y.Q. Encapsulation of modified copper phthalocyanine (CuPc) via miniemulsion polymerisation for electrophoretic display. Mater. Res. Innov. 2014, 19, 24–27. [Google Scholar] [CrossRef]
- Duan, Y.D.; Fu, N.Q.; Fang, Y.Y.; Li, X.N.; Liu, Q.P.; Zhou, X.W.; Lin, Y. Synthesis and formation mechanism of mesoporous TiO2 microspheres for scattering layer in dye-sensitized solar cells. Electrochim. Acta 2013, 113, 109–116. [Google Scholar] [CrossRef]
- Badila, M.; Hebraud, A.; Brochon, C.; Hadziioannou, G. Design of colored multilayered electrophoretic particles for electronic inks. ACS Appl. Mater. Interfaces 2011, 3, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.P.; Wu, G.; Dai, R.Y.; Qin, W.L.; Wang, M.; Chen, H.Z. Fine encapsulation of dual-particle electronic ink by incorporating block copolymer for electrophoretic display application. J. Colloid Interface Sci. 2012, 388, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Lee, J.H.; Singh, V.; Behrens, S.H. Surfactant mediated charging of polymer particles in a nonpolar liquid. J. Colloid Interface Sci. 2013, 392, 83–89. [Google Scholar] [CrossRef]
- Espinosa, C.E.; Guo, Q.; Singh, V.; Behrens, S.H. Particle charging and charge screening in nonpolar dispersions with nonionic surfactants. Langmuir 2010, 26, 16941–16948. [Google Scholar] [CrossRef]
- Sun, C.; Feng, Y.Q.; Zhang, B.; Li, X.G.; Shao, J.Z.; Han, J.J.; Chen, X. Preparation and application of microcapsule-encapsulated color electrophortic fluid in Isopar M system for electrophoretic display. Opt. Mater. 2013, 35, 1410–1417. [Google Scholar] [CrossRef]
- Duan, J.H.; Feng, Y.Q.; Yang, G.; Xu, W.L.; Li, X.G.; Liu, Y.; Zhao, J. Novel Synthesis and Characterization of Yellow Inorganic/Organic Composite Spheres for Electrophoretic Display. Ind. Eng. Chem. Res. 2009, 48, 1468–1475. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Daik, R. Applications of ionic liquids and their mixtures for preparation of advanced polymer blends and composites: A short review. Rev. Adv. Mater. Sci. 2015, 40, 45–59. [Google Scholar]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Liu, X.M.; Dong, H.F.; Zhang, X.P.; Zhang, S.J. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Song, Y.; Sun, N.J.; Zhao, N.N.; Li, M.X.; Qi, L.M. Ionic Liquid-Assisted Growth of Single-Crystalline Dendritic Gold Nanostructures with a Three-Fold Symmetry. Chem. Mater. 2008, 20, 3965–3972. [Google Scholar] [CrossRef]
- Meischein, M.; Fork, M.; Ludwig, A. On the Effects of Diluted and Mixed Ionic Liquids as Liquid Substrates for the Sputter Synthesis of Nanoparticles. Nanomaterials 2020, 10, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowsari, E.; Zare, A.; Ansari, V. Phosphoric acid-doped ionic liquid-functionalized graphene oxide/sulfonated polyimide composites as proton exchange membrane. Int. J. Hydrogen Energy 2015, 40, 13964–13978. [Google Scholar] [CrossRef]
- Hao, L.; Chao, X.; Zou, D.B.; Ling, W.; Ying, T.K. Hydrothermal synthesis of hollow MoS2 microspheres in ionic liquids/water binary emulsions. Mater. Lett. 2008, 62, 3558–3560. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis. Nanomaterials 2021, 11, 2030. [Google Scholar] [CrossRef]
- Julio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos de Almeida, T.; Fonte, P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Mendez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; Moustafa, Y.M.; Sabeela, N.I.; Tawfeek, A.M.; Al-Lohedan, H.A.; Hashem, A.I. Synthesis of New Magnetic Crosslinked Poly (Ionic Liquid) Nanocomposites for Fast Congo Red Removal from Industrial Wastewater. Nanomaterials 2019, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Livi, S.; Duchet, J.; Gerard, J.F. Ionic Liquids-Containing Silica Microcapsules: A Potential Tunable Platform for Shaping-Up Epoxy-Based Composite Materials? Nanomaterials 2020, 10, 881. [Google Scholar] [CrossRef]
- Zornio, C.F.; Livi, S.; Duchet-Rumeau, J.; Gerard, J.F. Ionic Liquid-Nanostructured Poly(Methyl Methacrylate). Nanomaterials 2019, 9, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorini, L.; Santaniello, T.; Borghi, F.; Saettone, P.; Comes Franchini, M.; Generali, G.; Milani, P. Eco-Friendly Supercapacitors Based on Biodegradable Poly(3-Hydroxy-Butyrate) and Ionic Liquids. Nanomaterials 2020, 10, 2062. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman characteristic group frequencies table and charts. Proteomics 2005, 108, 1–347. [Google Scholar]
- RIES; Herman, E. Microelectrophoresis Measurements on Polymeric Flocculants Alone and in Excess with Model Colloids. Nature 1970, 226, 72–73. [Google Scholar] [CrossRef]







| Sample | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| CP | 932.90 | 32.42 |
| CP-IL | 1138.37 | 49.91 |
| Ionic Liquids | Chemical Constitution | Side Chain Length | Zeta Potential (mV) | Electrophoretic Mobility (m2 v−1 s−1) |
|---|---|---|---|---|
| 1-Butyl-3-methylimidazolium bromide |  | 4C | 41.60 | 9 × 10−10 |
| 1-Butyl-1-methylpyrrolidinium bromide |  | 4C | 49.91 | 1.04 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Z.; Chen, Q.; Ye, C.; Zhang, J.; Gao, Q.; Liu, L.; Yang, J.; Pan, X.; Miao, Y.; et al. A Novel Modification of Copper (II) Phthalocyanine Particles towards Electrophoretic Displays. Micromachines 2022, 13, 880. https://doi.org/10.3390/mi13060880
Wang Y, Zhang Z, Chen Q, Ye C, Zhang J, Gao Q, Liu L, Yang J, Pan X, Miao Y, et al. A Novel Modification of Copper (II) Phthalocyanine Particles towards Electrophoretic Displays. Micromachines. 2022; 13(6):880. https://doi.org/10.3390/mi13060880
Chicago/Turabian StyleWang, Yao, Zhi Zhang, Qun Chen, Caihong Ye, Jiahao Zhang, Qingguo Gao, Liming Liu, Jianjun Yang, Xinjian Pan, Yu Miao, and et al. 2022. "A Novel Modification of Copper (II) Phthalocyanine Particles towards Electrophoretic Displays" Micromachines 13, no. 6: 880. https://doi.org/10.3390/mi13060880






