Graphene-Based Polymer Composites for Flexible Electronic Applications
Abstract
:1. Introduction
2. Synthesis of Graphene and Its Derivatives
2.1. Synthesis of Graphene
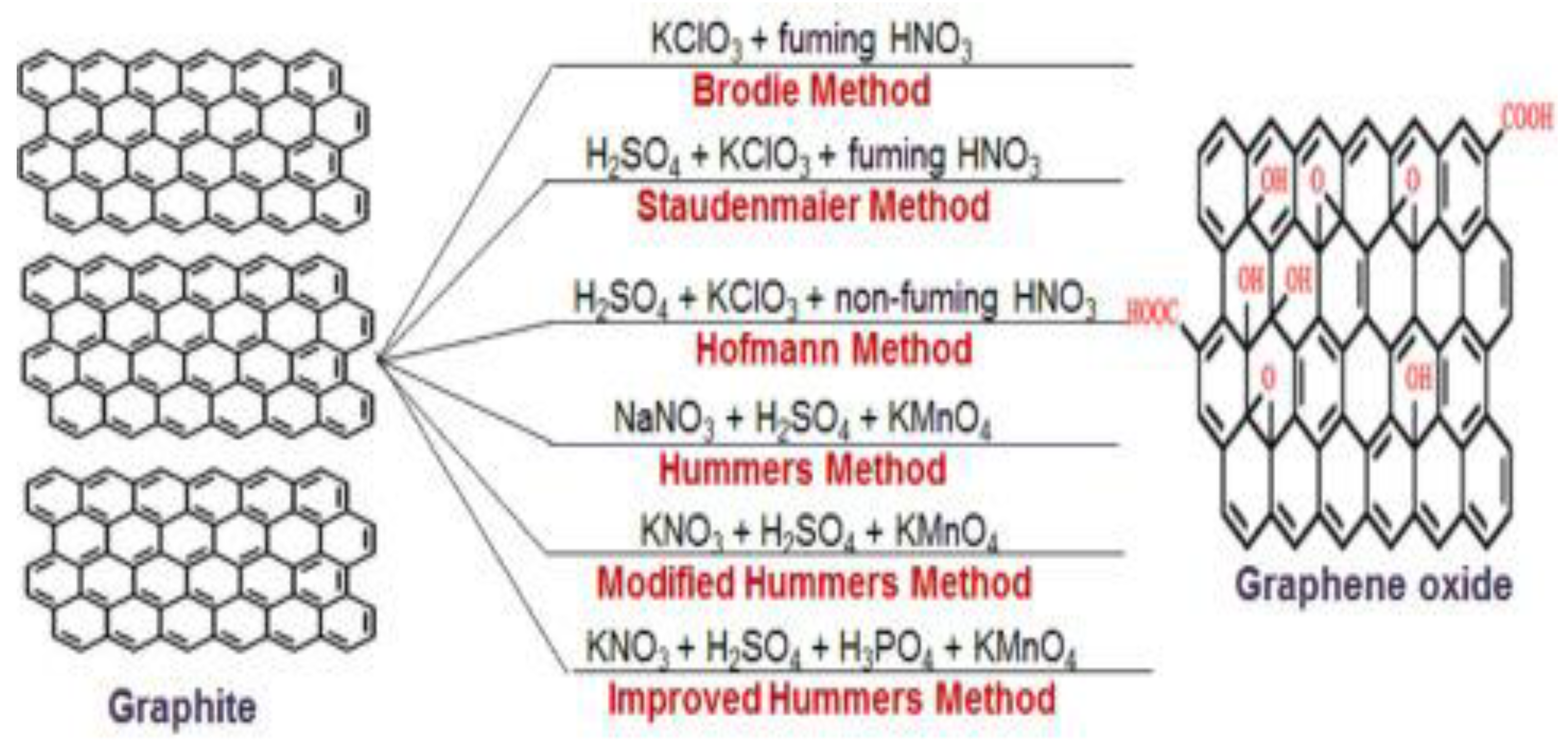
2.2. Synthesis of Graphene Oxide
2.3. Synthesis of Reduced Graphene Oxide
3. Graphene-Based Polymeric Composites for Flexible Electronics
3.1. Graphene-Based Electrodes in Solar Cells
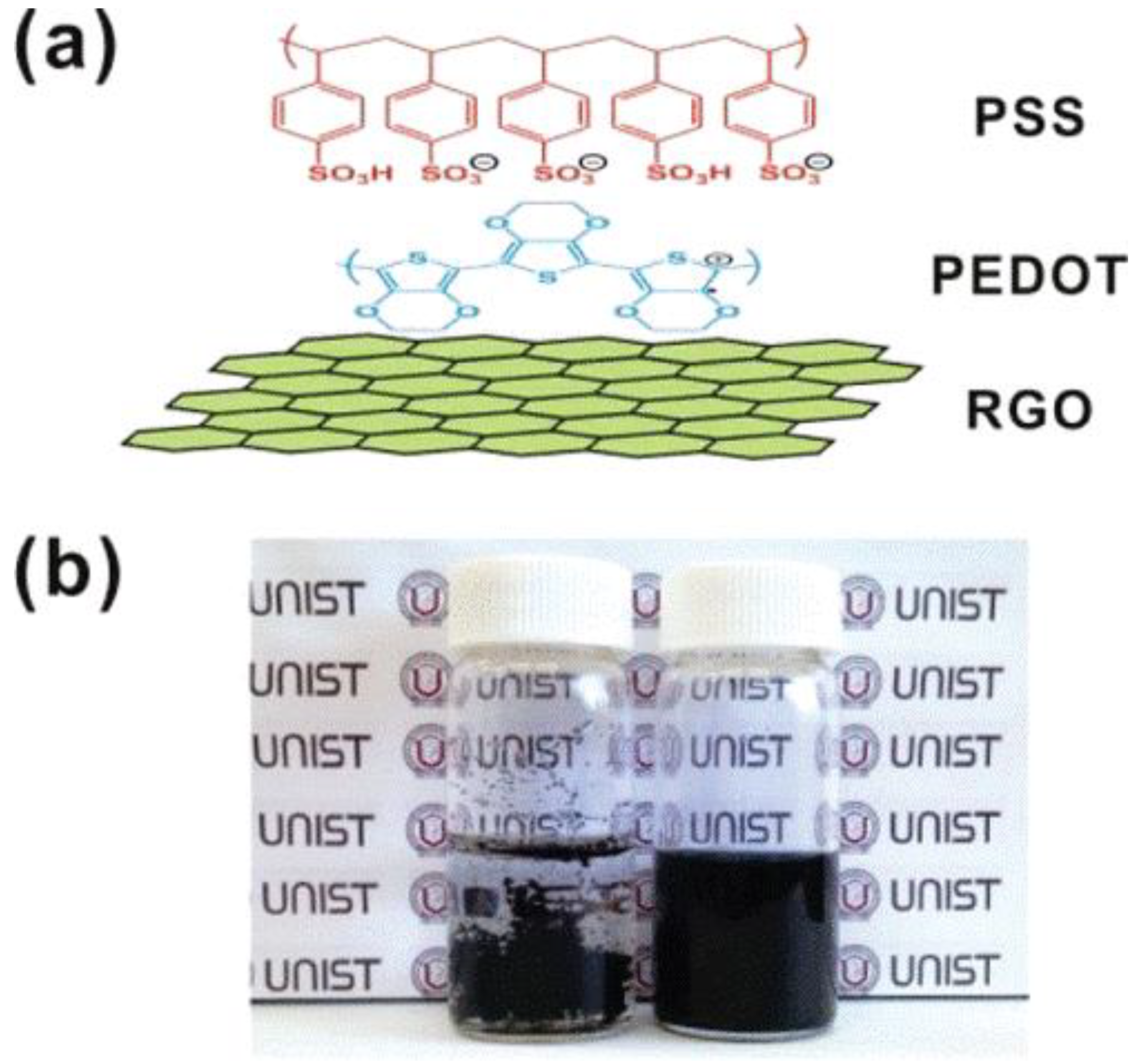
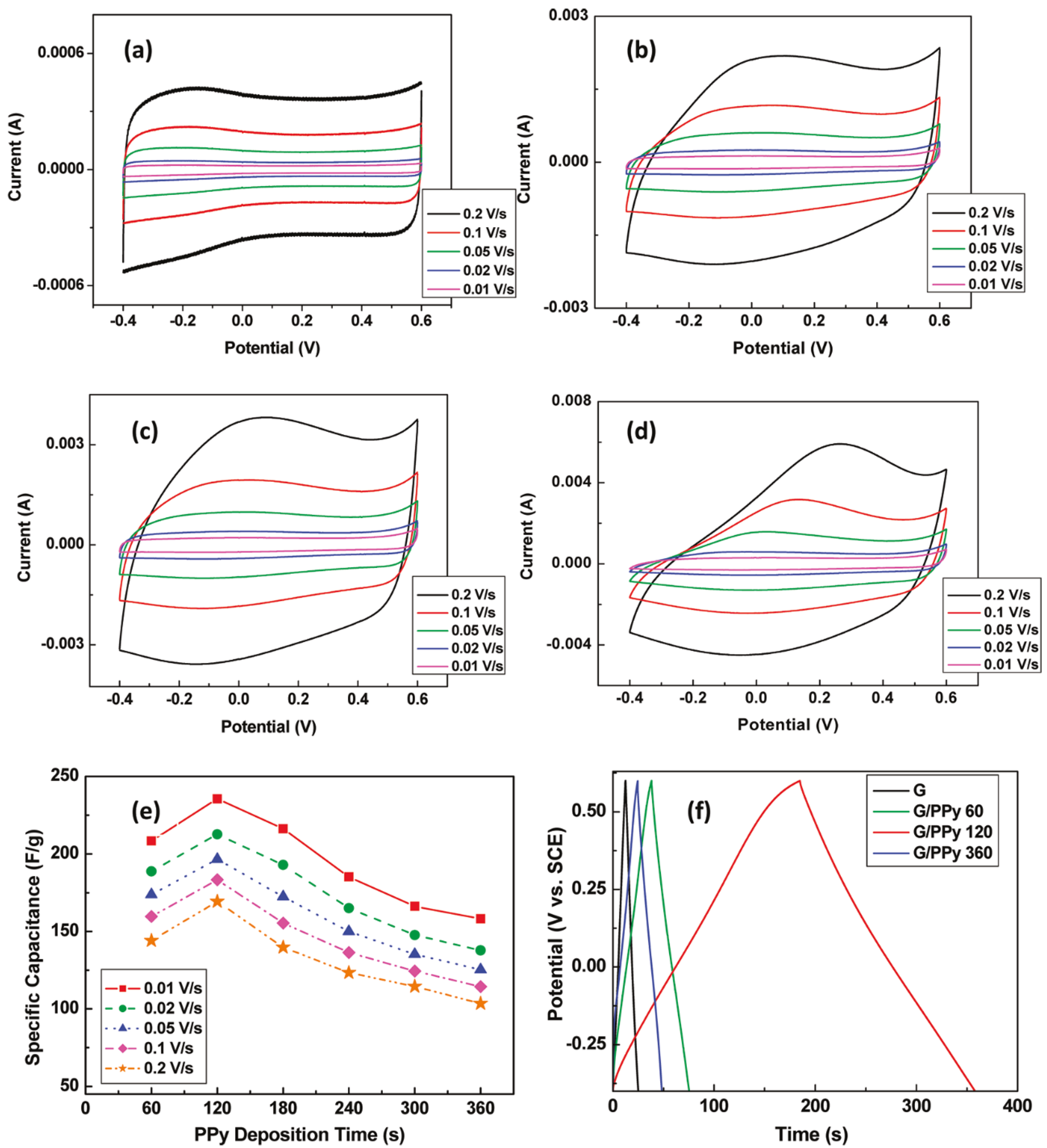
3.2. Graphene in Flexible Capacitors
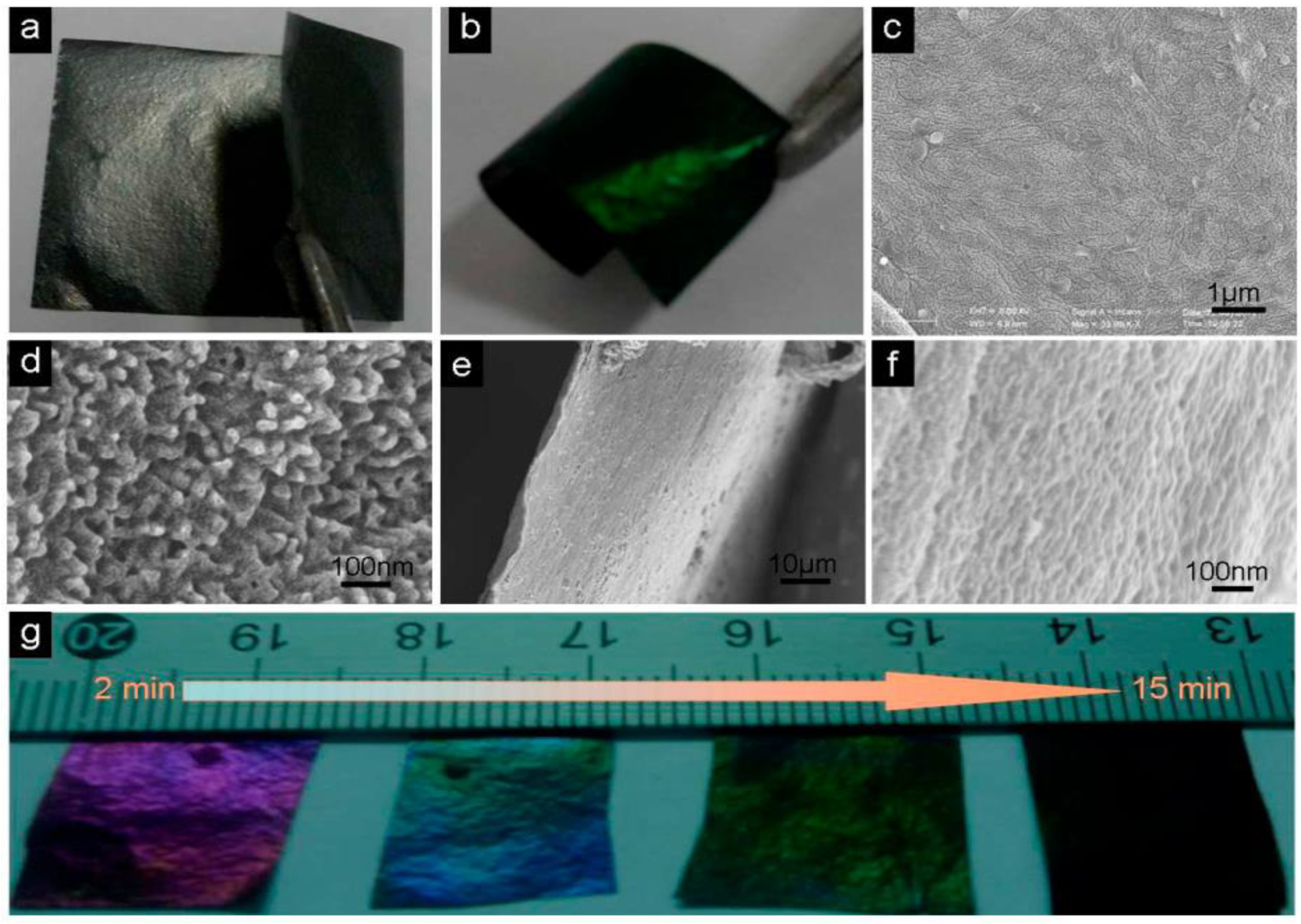
3.3. Graphene in Flexible Electronic Textiles
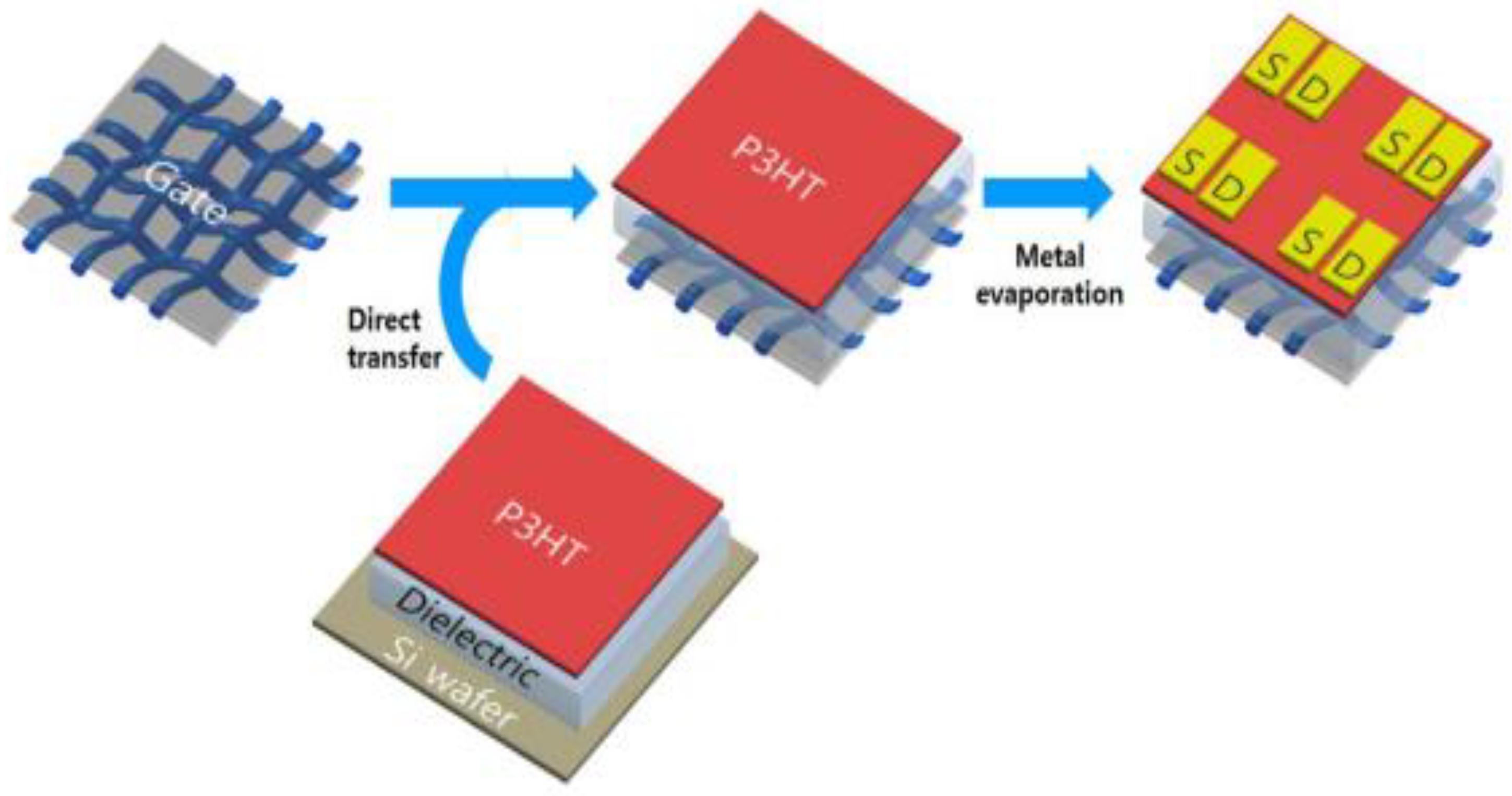
3.4. Graphene in Flexible Transistors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Srinivasan, C. Graphene—Mother of all graphitic materials. Curr. Sci. 2007, 92, 1338–1339. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Sahoo, S.; Das, S. Supersymmetric structure of fractional quantum Hall effect in graphene. Indian J. Pure Appl. Phys. 2009, 47, 186–191. [Google Scholar]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano. Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Luceño-Sánchez, J.A.; Maties, G.; Gonzalez-Arellano, C.; Diez-Pascual, A.M. Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate. Nanomaterials 2018, 8, 870. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M. Development of Graphene-Based Polymeric Nanocomposites: A Brief Overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly (Propylene Fumarate)/Polyethylene Glycol-Modified Graphene Oxide Biocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Sainz-Urruela, C.; Vallés, C.; Vera-López, S.; San Andrés, M.P. Tailorable Synthesis of Highly Oxidized Graphene Oxides via an Environmentally-Friendly Electrochemical Process. Nanomaterials 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.; Wang, B.; Lin, Y.; LI, Y.; Zhu, D.; Wu, J.; Xia, J.; Wang, B. Water proof mechanically robust multifuctional conformal sensors for underwater interactive human-machine interfaces. Adv. Int. Sys. 2021, 3, 2100056. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser fabrication of graphene-based flexible electronics. Adv. Mater. 2020, 32, e1901981. [Google Scholar] [CrossRef]
- Heo, S.; Ha, J.; Son, S.J.; Choi, I.S.; Lee, H.J. Instant, multiscale dry transfer printing by atomic diffusion control at heterogeneous interfaces. Sci. Adv. 2021, 7, 1872. [Google Scholar] [CrossRef] [PubMed]
- Zumeit, A.; Dahiya, S.; Christou, A.; Shakthivel, D.; Dahiya, R. Direct roll transfer printed silicon nanoribbon arrays based high-performance flexible electronics. NPJ Flex. Electron. 2021, 5, 18. [Google Scholar] [CrossRef]
- Wang, M.; Ma, C.; Uzabakiriho, P.C.; Chen, X.; Chen, Z.; Cheng, Y.; Wang, Z.; Zhao, G. Stencil Printing of Liquid Metal upon Electrospun Nanofibers Enables High-Performance Flexible Electronics. ACS Nano 2021, 15, 19364–19376. [Google Scholar] [CrossRef]
- Boehm, H.; Clauss, A.; Fischer, G.O.; Hofmann, U. In Surface Properties of Extremely Thin Graphite Lamellae. In Proceedings of the Fifth Conference on Carbon, University Park, PA, USA, 19–23 June 1961; pp. 73–80. [Google Scholar]
- Bhuyan, M.S.A.; Uddin, N.; Islam, M.; Biphasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Li, Z. Mechanisms of Liquid-Phase Exfoliation for the Production of Graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene Oxides Derivatives Prepared by an Electrochemical Approach: Correlation between Structure and Properties. Nanomaterials 2020, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R Soc. Lond. 1859, 14, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber Dtsch Chem Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Adetayo, A.; Runsewe, D. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open J. Compos. Mater. 2019, 9, 207–229. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Li, C.; Shi, G. Functional Composite Materials based on Chemically Converted Graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash reduction and patterning of graphene oxide and its polymer composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef]
- Fernandez-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Roldil, S.; Solis Fernandez, P.; Martinez-Alonso, A.; Tascon, J.M.D. Vitamin C as an ideal substitute for Hydrazine in the reduction of graphene oxide. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Periasamy, M.; Thirumalaikumar, M. Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic chemistry. J. Organomet. Chem. 2000, 609, 137–151. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Ayala, G.; Cortez-Valadez, M.; Mani-Gonzalez, P.G.; Hurtado, R.B.; Contreras-Rascón, J.I.; Carrillo-Torres, R.C.; Zayas, M.A.; Castillo, S.J.; Hernández-Martínez, A.R.; Flores-Acosta, M. Green synthesis of reduced graphene oxide using ball milling. Carbon Lett. 2017, 21, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M. Graphene-based Polymer Nanocomposites: Recent Advances. Polymers 2022, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y.; Tsai, J.-T.; Sun, C.-L. Synthesis of PEDOT-modified graphene composite materials as flexible electrodes for energy storage and conversion applications. Int. J. Hydrogen Energy 2012, 37, 13880–13886. [Google Scholar] [CrossRef]
- Luceño Sánchez, J.A.; Peña Capilla, R.; Díez-Pascual, A.M. High-Performance PEDOT: PSS/hexamethylene diisocyanatefunctionalized graphene oxide nanocomposites: Preparation and properties. Polymers 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Pascual, A.M.; Luceño-Sanchez, J.A.; Peña-Capilla, R.; Garcia-Diaz, P. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lang, Y.M.; Zhan, H. Organic Photovoltaic Devices Using Highly Flexible Reduced Graphene Oxide Films as Transparent Electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef]
- Konios, D.; Petridis, C.; Kakavelakis, G.; Sygletou, M.; Savva, K.; Stratakis, E.; Kymakis, E. Reduced graphene oxide micromesh electrodes for large area, flexible, organic photovoltaic devices. Adv. Funct. Mater. 2015, 15, 2213–2221. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Wang, G.; Yang, A.; Tao, X.; Liu, X. A Transparent, Flexible, Low-Temperature, and Solution-Processible Graphene Composite Electrode. Adv. Funct. Mater. 2010, 20, 2893–2902. [Google Scholar] [CrossRef]
- Jo, K.; Lee, T.; Choi, H.J.; Park, J.H.; Lee, D.J.; Lee, D.W.; Kim, B.-S. Stable aqueous dispersion of reduced graphene nanosheets via non-covalent functionalization with conducting polymers and application in transparent electrodes. Langmuir 2011, 27, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.F.; Matos, C.F.; Gonçalves, L.C.; Salvatierra, R.V.; Cava, C.E.; Zarbin, A.J.G.; Roman, L.S. Water based, solution-processable, transparent and flexible graphene oxide composite as electrodes in organic solar cell application. J. Phys. D Appl. Phys. 2016, 49, 105106. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Chang, S.; Zhou, X.; Kong, J.; Palacios, T.; Gradečak, S. Flexible graphene electrode-based organic photovoltaics with record-high efficiency. Nano Lett. 2014, 14, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the quantum capacitance of graphene. Nature Nanotechnol. 2009, 4, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Gan, M.; Fu, S.; Dai, W.; Zhou, T.; Sun, X.; Wang, H.; Wang, H. Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J. Power Sour. 2015, 299, 347–355. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Flexible graphene-polyaniline composite paper for high-performance supercapacitor. Ener. Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Sun, H.; She, P.; Xu, K.; Shang, Y.; Yin, S.; Liu, Z. A self-standing nanocomposite foam of polyaniline@reduced graphene oxide for flexible super-capacitors. Synthetic Metals 2015, 209, 68–73. [Google Scholar] [CrossRef]
- Kumar, N.A.; Choi, H.J.; Shin, Y.R.; Chang, D.W.; Dai, L.; Baek, J.B. Polyaniline grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, Y.; Hu, C.; Hu, Y.; Dong, Z.; Chen, N.; Zhang, Z.; Qu, L. Spinning fabrication of graphene/polypyrrole composite fibers for all-solid-state, flexible fibriform supercapacitors. J. Mater. Chem. A 2014, 2, 12355–12360. [Google Scholar] [CrossRef]
- Davies, A.; Audette, P.; Farrow, B.; Hassan, F.; Chen, Z.; Choi, J.Y.; Yu, A. Graphene-based flexible supercapacitors: Pulse-electropolymerization of polypyrrole on freestanding graphene films. J. Phys. Chem. C. 2011, 115, 17612–17620. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Lu, X.; Xie, C.-M.; Wan, G.-J.; Zhang, H.-P.; Youhong, T. Flexible, free-standing TiO2-graphene-polypyrrole composite flims as electrodes for supercapacitors. J. Phys. Chem. C 2015, 119, 3903–3910. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light-induced graphene from phenolic resin: A new approach for directly writing graphene based electrochemical devices on various substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, W.; Lan, J.; Sui, G.; Zhang, H.; Yang, X. Flexible Reduced Graphene Oxide/Polyacrylonitrile Dielectric Nanocomposite Films for High-Temperature Electronics Applications. ACS Appl. Nano Mater. 2020, 3, 7005–7015. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, S.; Zakhidov, A.A.; Lee, S.B.; Aliev, A.E.; Williams, C.D.; Atkinson, K.R.; Baughman, R.H. Strong, transparent, multifunctional, carbon nanotube sheets. Science 2005, 309, 1215–1219. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.J.; Hong, W.G.; Kim, W.J.; Jun, Y.; Kim, B.H. A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef]
- Shathi, M.A.; Chen, M.; Khoso, N.A.; Rahman, M.T.; Bhattacharjee, B. Graphene coated textile based highly flexible and washable sports bra for human health monitoring. Mater. Des. 2020, 193, 108792. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.N.; Zhang, Y.; Tao, L.Q.; Li, Y.-X.; Wang, D.-Y.; Yang, Y. Tian-Ling, R. Flexible, wearable, and functional graphene-textile composites. Appl. Phys. Lett. 2017, 110, 261903. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, Y.J.; Lee, K.E.; Oh, Y.; Um, M.K.; Seong, D.G.; Lee, J.U. Textile-Based Organic Transistors Using Graphene/Ag Nanoparticle Electrode. Nanomaterials 2016, 6, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Lee, K.; Cha, H.J. Highly Conductive Graphene/Ag Hybrid Fibers for Flexible Fiber-Type Transistors. Sci. Rep. 2015, 5, 16366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Nair, N.M.; Nagasarvari, G.; Ray, D.; Swaminathan, P. A review of silver nanowire-based composites for flexible electronic applications. Flex. Print. Electron. 2022, 7, 014009. [Google Scholar] [CrossRef]
- Rodriguez, R.D. Ultra-robust flexible electronics by laser driven polymer nanomaterials integration. Adv. Funct. Mater. 2021, 31, 2008818. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M.; Rahdar, A. Graphene-Based Polymer Composites for Flexible Electronic Applications. Micromachines 2022, 13, 1123. https://doi.org/10.3390/mi13071123
Díez-Pascual AM, Rahdar A. Graphene-Based Polymer Composites for Flexible Electronic Applications. Micromachines. 2022; 13(7):1123. https://doi.org/10.3390/mi13071123
Chicago/Turabian StyleDíez-Pascual, Ana M., and Abbas Rahdar. 2022. "Graphene-Based Polymer Composites for Flexible Electronic Applications" Micromachines 13, no. 7: 1123. https://doi.org/10.3390/mi13071123
APA StyleDíez-Pascual, A. M., & Rahdar, A. (2022). Graphene-Based Polymer Composites for Flexible Electronic Applications. Micromachines, 13(7), 1123. https://doi.org/10.3390/mi13071123






