Syngas Fermentation to Acetate and Ethanol with Adaptative Electroactive Carboxydotrophs in Single Chambered Microbial Electrochemical System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of MES
2.2. Microbiome, Electrolyte and Feed Stock
2.3. System Operation
2.4. Performance Analysis
2.5. Electrochemical Characteristics
2.6. Substrate Fixation and Conversion
2.7. Metagenomic Analysis
3. Results
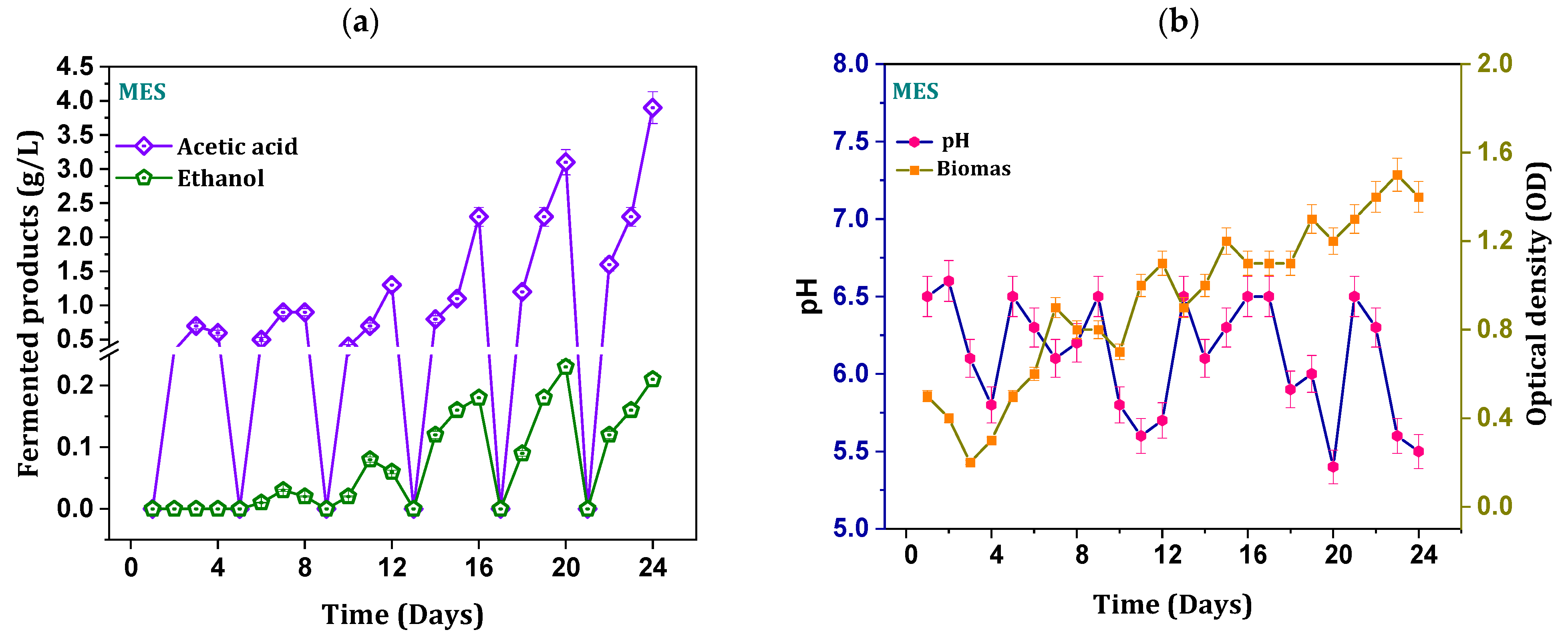
3.1. Productivity and Composition of Fermentation Products
3.2. Biomass and pH
3.3. Fixation and Conversion Efficiencies
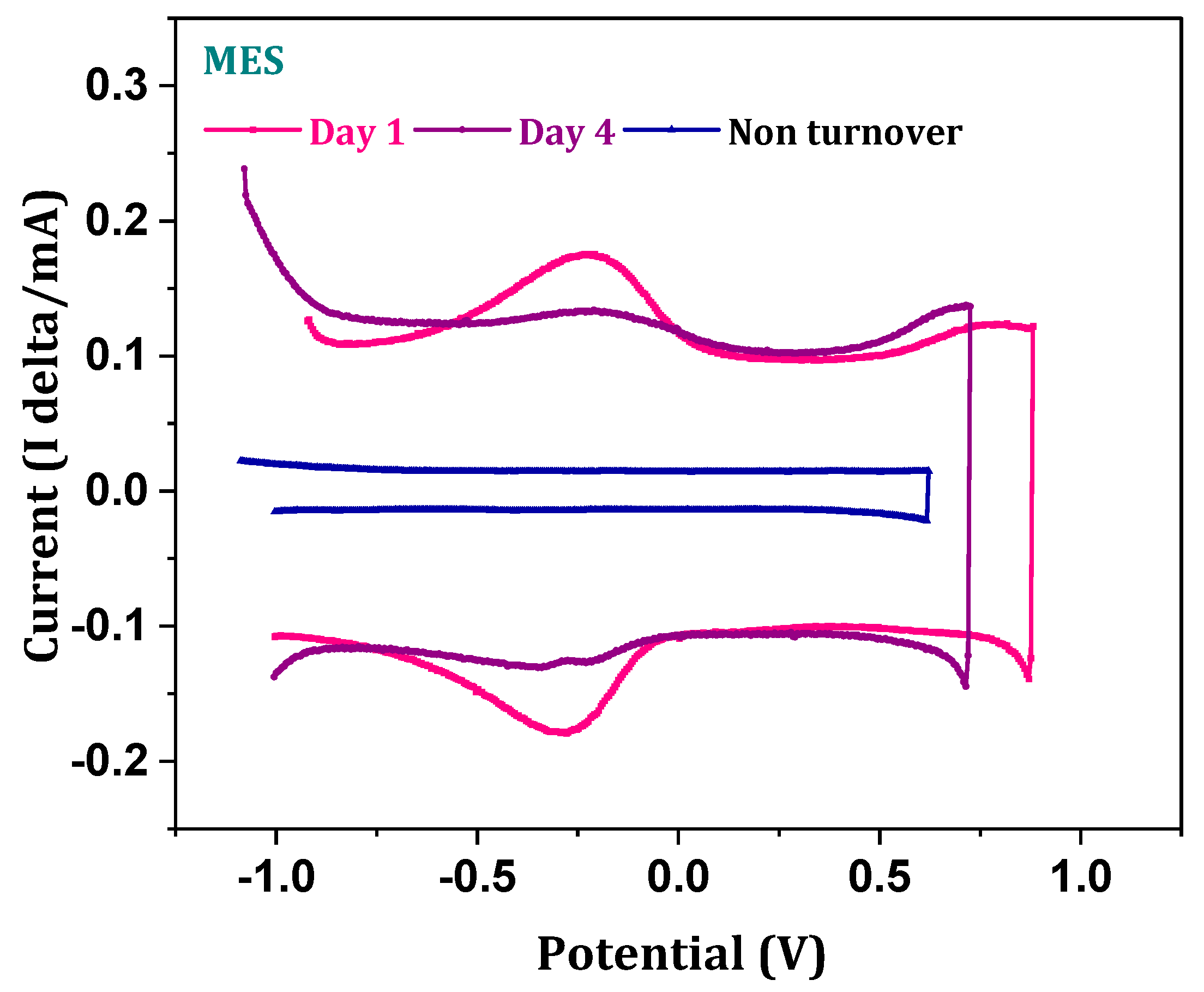
3.4. Electrotrophy of Biocathode
3.5. Kinetics of Charge Transfer-Biocathode Response
3.6. Electrochemically Active Substrate Profile
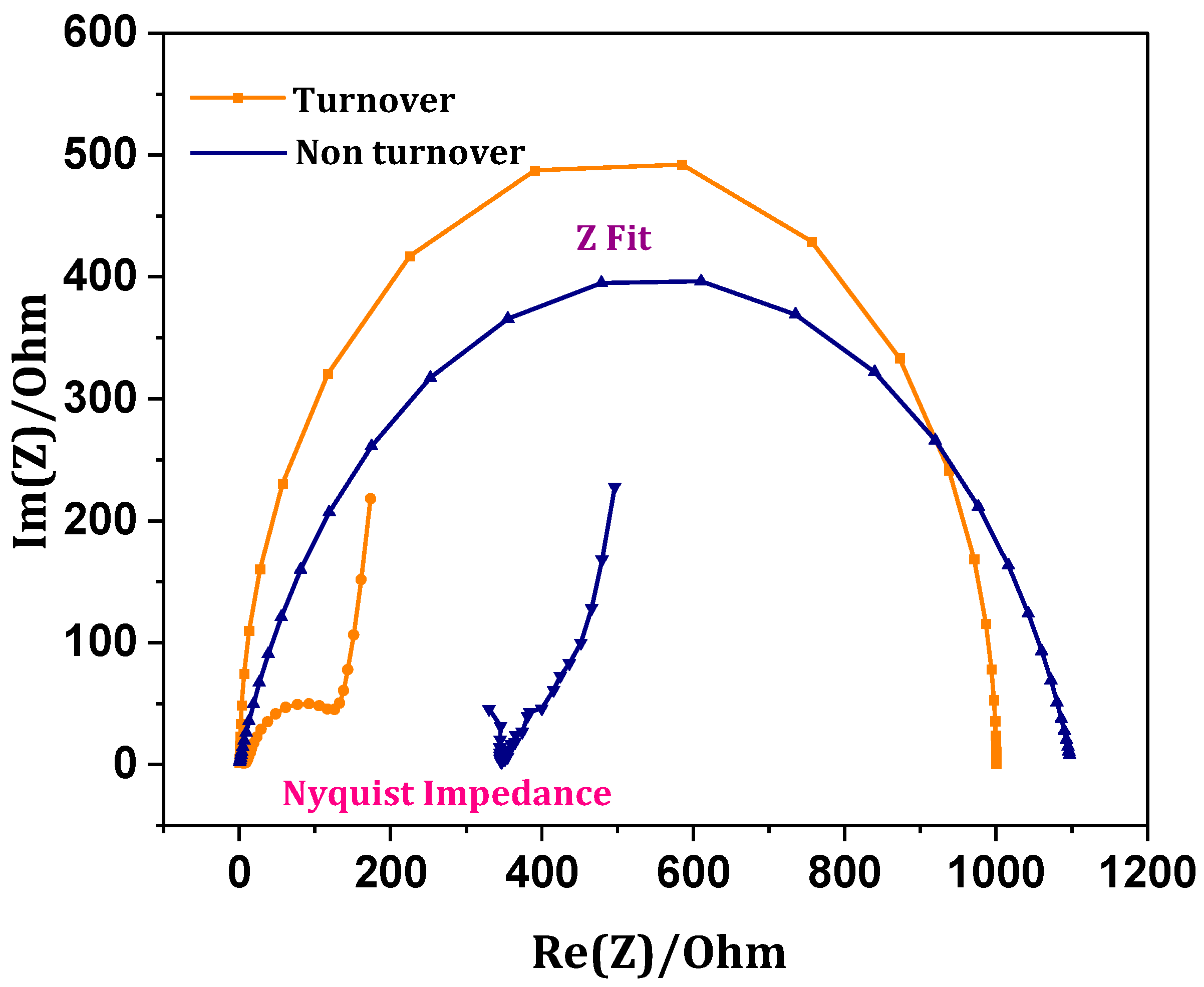
3.7. Internal Resistance-Charge Transfer Mechanics
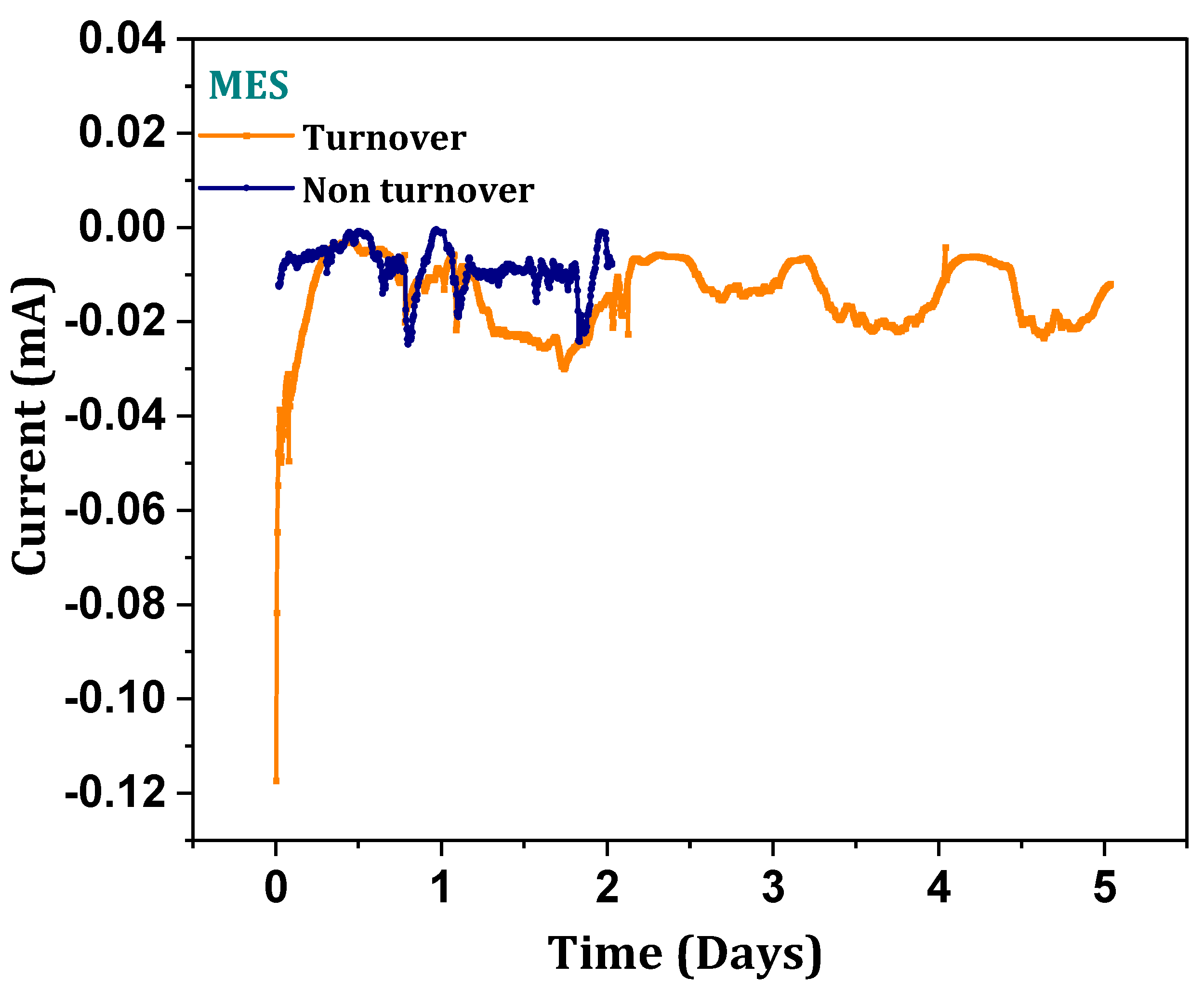
3.8. Cathodic Reduction Responses
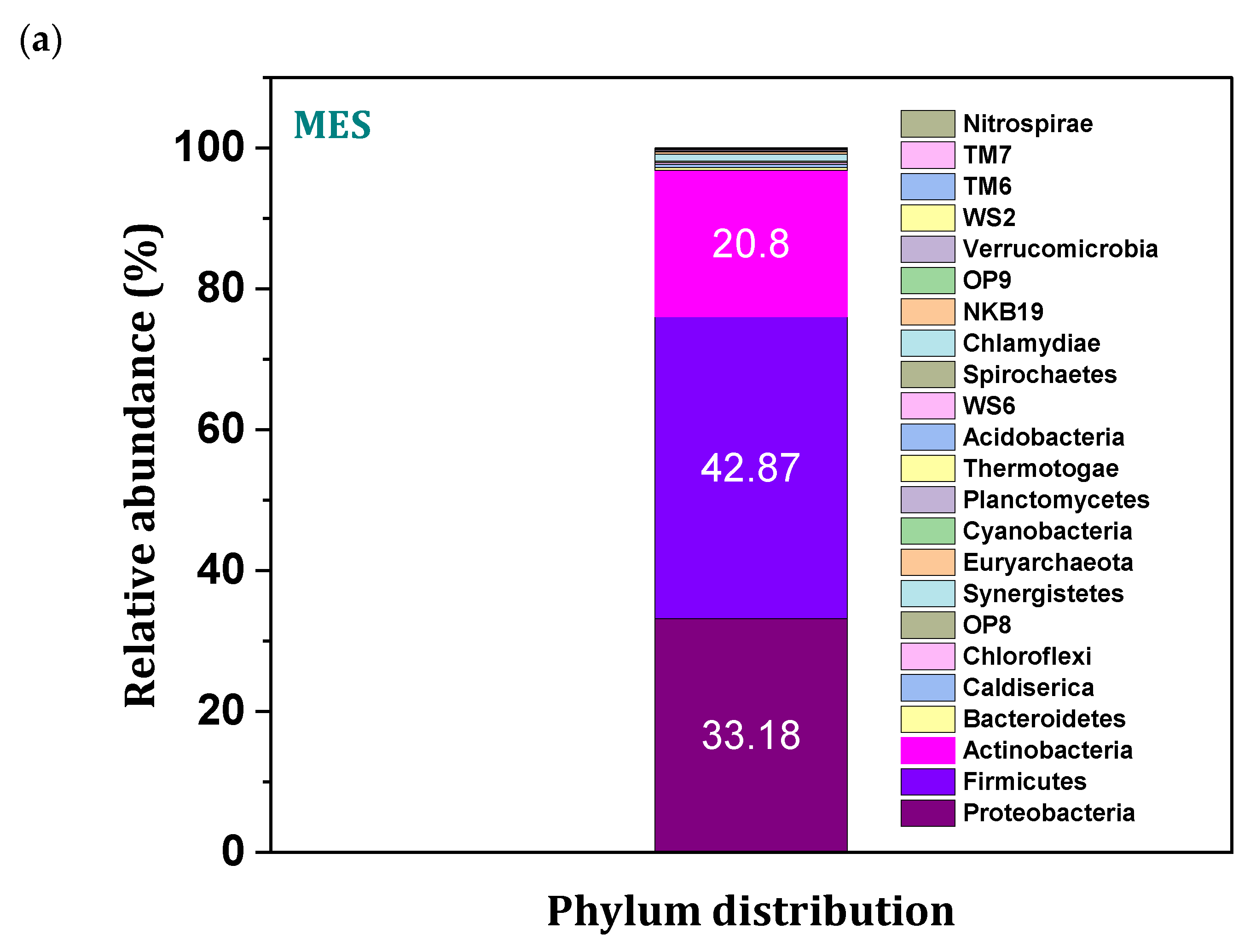
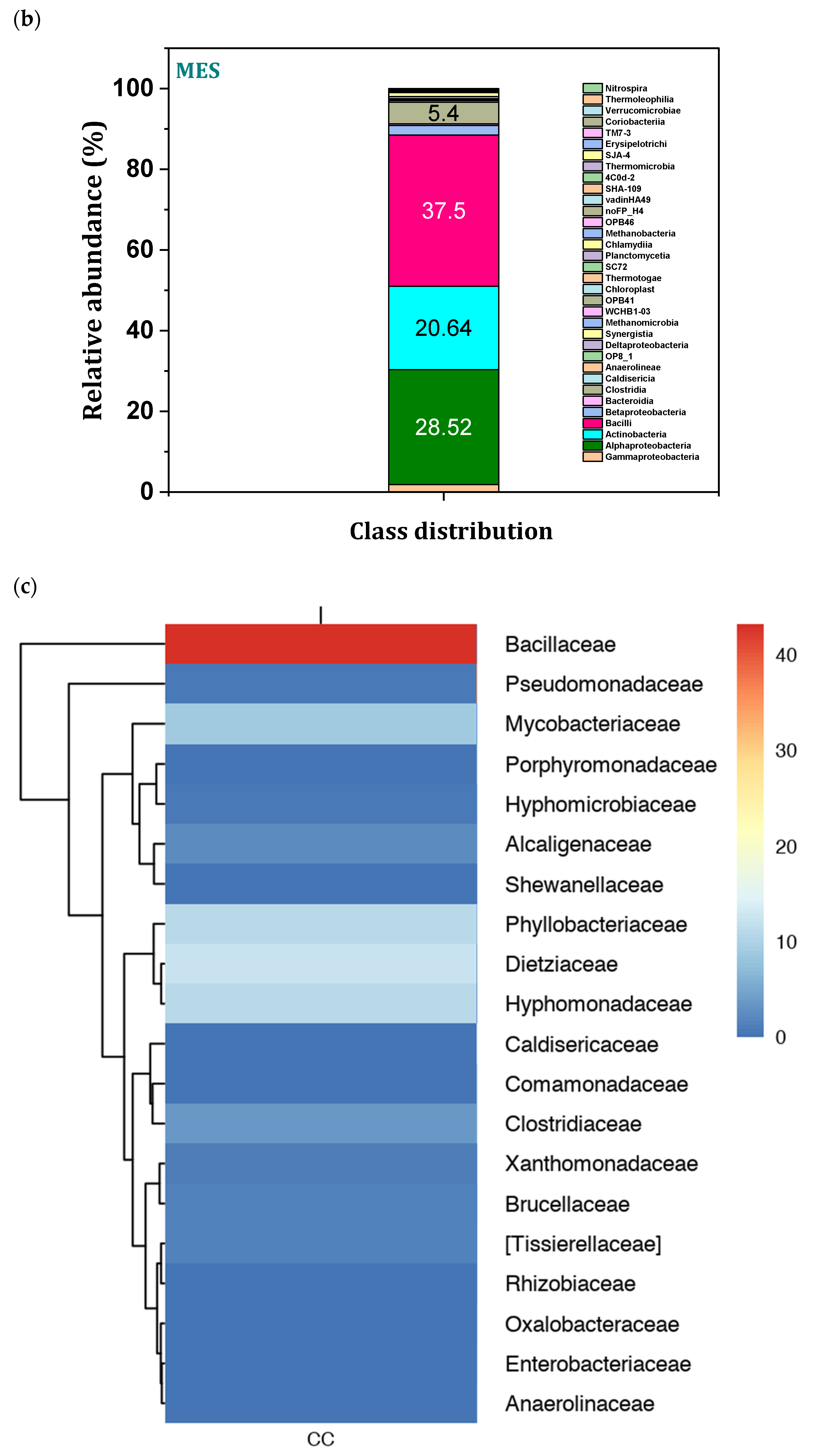
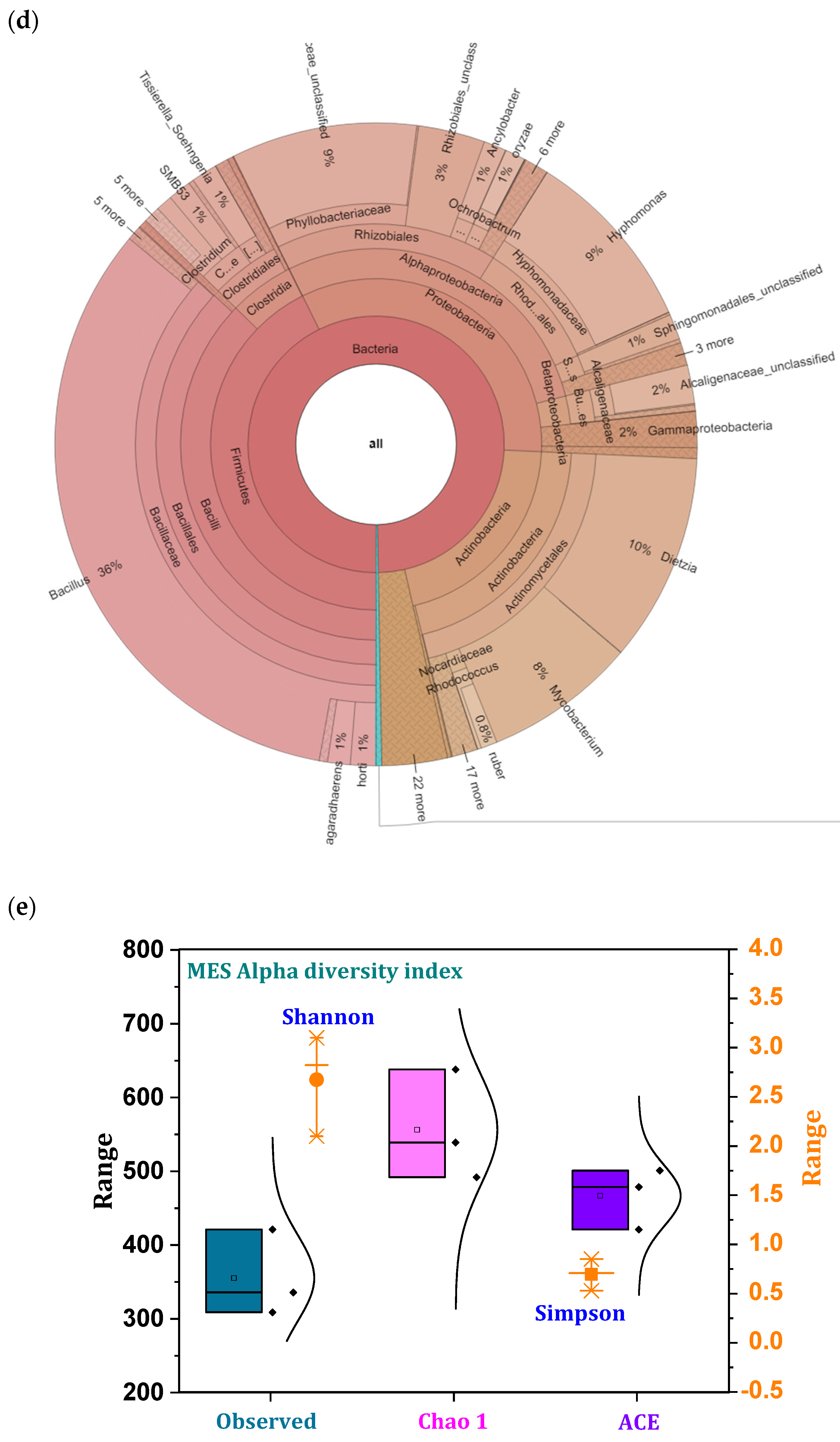
3.9. Microbial Community Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivalingam, V.; Ahmadi, V.; Babafemi, O.; Dinamarca, C. Integrating syngas fermentation into a single-cell microbial electrosynthesis (MES) reactor. Catalysts 2020, 11, 40. [Google Scholar] [CrossRef]
- Timmis, K.; de Vos, W.M.; Ramos, J.L.; Vlaeminck, S.E.; Prieto, A.; Danchin, A.; Verstraete, W.; de Lorenzo, V.; Lee, S.Y.; Brüssow, H.; et al. The contribution of microbial biotechnology to sustainable development goals. Microb. Biotechnol. 2017, 10, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Katakojwala, R.; Tharak, A.; Sarkar, O.; Venkata Mohan, S. Design and evaluation of Gas fermentation system for CO2 reduction to C2 and C4 fatty acids: Metabolic regulation with pressure, pH and reaction time. Bioresour. Technol. 2022, 351, 126–937. [Google Scholar] [CrossRef] [PubMed]
- Arantes, A.L.; Moreira, J.P.; Diender, M.; Parshina, S.N.; Stams, A.J.; Alves, M.M.; Alves, J.I.; Sousa, D.Z. Enrichment of anaerobic syngas-converting communities and isolation of a novel carboxydotrophic Acetobacterium wieringae strain JM. Front. Microbiol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surywanshi, G.D.; Pillai, B.B.K.; Patnaikuni, V.S.; Vooradi, R.; Anne, S.B. 4-E analyses of chemical looping combustion based subcritical, supercritical and ultra-supercritical coal-fired power plants. Energy Convers. Manag. 2019, 200, 112050. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Alves, J.I.; Alves, M.M. Bioelectrochemical systems (BESs) towards conversion of carbon monoxide/syngas: A mini-review. Renew. Sustain. Energy Rev. 2021, 135, 110–358. [Google Scholar] [CrossRef]
- Sinharoy, A.; Pakshirajan, K.; Lens, P.N. Syngas Fermentation for Bioenergy Production: Advances in Bioreactor Systems. Renew. Energy Technol. Energy Effic. Sustain. Dev. 2022, 325–358. [Google Scholar]
- Liu, C.; Wang, W.; Yang, Z.; Zhang, S.; Liu, G.; Luo, G. Microbial insights of enhanced anaerobic conversion of syngas into volatile fatty acids by co-fermentation with carbohydrate-rich synthetic wastewater. Biotechnol. Biofuels 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Liu, C.; Luo, G.; Liu, H.; Yang, Z.; Angelidaki, I.; Sompong, O.; Liu, G.; Zhang, S.; Wang, W. CO as electron donor for efficient medium chain carboxylate production by chain elongation: Microbial and thermodynamic insights. Chem. Eng. J. 2020, 390, 124–577. [Google Scholar] [CrossRef]
- Modestra, J.A.; Katakojwala, R.; Venkata Mohan, S. CO2 fermentation to short chain fatty acids using selectively enriched chemolithoautotrophic acetogenic bacteria. Chem. Eng. J. 2020, 394, 124759. [Google Scholar] [CrossRef]
- Dang, J.; Wang, N.; Atiyeh, H.K. Review of dissolved CO and H2 measurement methods for syngas fermentation. Sensors 2021, 21, 2165. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rene, E.R.; Lens, P.N.; Veiga, M.C.; Kennes, C. Enrichment of a solventogenic anaerobic sludge converting carbon monoxide and syngas into acids and alcohols. Bioresour. Technol. 2019, 272, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Gunes, B. A critical review on biofilm-based reactor systems for enhanced syngas fermentation processes. Renew. Sustain. Energy Rev. 2021, 143, 110–950. [Google Scholar] [CrossRef]
- Ayol, A.; Peixoto, L.; Keskin, T.; Abubackar, H.N. Reactor designs and configurations for biological and bioelectrochemical C1 gas conversion: A review. Int. J. Environ. Res. Public Health 2021, 18, 11683. [Google Scholar] [CrossRef] [PubMed]
- Parera Olm, I.; Sousa, D.Z. Conversion of Carbon Monoxide to Chemicals Using Microbial Consortia. In One-Carbon Feedstocks for Sustainable Bioproduction; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Amulya, K.; Venkata Mohan, S. Augmenting succinic acid production by bioelectrochemical synthesis: Influence of applied potential and CO2 availability. Chem. Eng. J. 2021, 411, 128377. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Mohanakrishna, G.; Mohan, S.V. Rhizosphere mediated electrogenesis with the function of anode placement for harnessing bioenergy through CO2 sequestration. Bioresour. Technol. 2012, 124, 364–370. [Google Scholar] [CrossRef]
- Kim, D.; Chang, I.S. Electricity generation from synthesis gas by microbial processes: CO fermentation and microbial fuel cell technology. Bioresour. Technol. 2009, 100, 4527–4530. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Łężyk, M.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Enrichment of syngas-converting mixed microbial consortia for ethanol production and thermodynamics-based design of enrichment strategies. Biotechnol. Biofuels 2018, 11, 198. [Google Scholar] [CrossRef]
- He, Y.; Cassarini, C.; Marciano, F.; Lens, P.N. Homoacetogenesis and solventogenesis from H2/CO2 by granular sludge at 25, 37 and 55 C. Chemosphere 2021, 265, 128649. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Y.; Gan, W.; Xia, A.; Liao, Q.; Zhu, X. Ethanol production from gas fermentation: Rapid enrichment and domestication of bacterial community with continuous CO/CO2 gas. Renew. Energy 2021, 175, 337–344. [Google Scholar] [CrossRef]
- Tharak, A.; Venkata Mohan, S. Electrotrophy of biocathodes regulates microbial-electro-catalyzation of CO2 to fatty acids in single chambered system. Bioresour. Technol. 2021, 320, 124–272. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Mohan, S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017, 20, 190–199. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Seelam, J.S.; Vanbroekhoven, K.; Pant, D. An enriched electroactive homoacetogenic biocathode for the microbial electrosynthesis of acetate through carbon dioxide reduction. Faraday Discuss. 2015, 183, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Mohan, S.V. Influence of terminal electron acceptor availability to the anodic oxidation on the electrogenic activity of microbial fuel cell (MFC). Bioresour. Technol. 2012, 123, 480–487. [Google Scholar] [CrossRef]
- Sravan, J.S.; Mohan, S.V. Bioelectrocatalytic Reduction of Tellurium Oxyanions toward Their Cathodic Recovery: Concentration Dependence and Anodic Electrogenic Activity. ACS EST Water 2021, 2, 40–51. [Google Scholar] [CrossRef]
- Pasupuleti, S.B.; Srikanth, S.; Mohan, S.V.; Pant, D. Continuous mode operation of microbial fuel cell (MFC) stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. Int. J. Hydrog. Energy 2015, 40, 12424–12435. [Google Scholar] [CrossRef]
- Velvizhi, G.; Mohan, S.V. Biocatalyst behavior under self-induced electrogenic microenvironment in comparison with anaerobic treatment: Evaluation with pharmaceutical wastewater for multi-pollutant removal. Bioresour. Technol. 2011, 102, 10784–10793. [Google Scholar] [CrossRef]
- Mukherjee, T.; Mohan, S.V. Magnetite-Bacillus subtilis Synergy on the Metabolic Selection of Products in Electrofermentation System. Bioresour. Technol. 2022, 357, 127267. [Google Scholar] [CrossRef]
- Chu, N.; Liang, Q.; Zhang, W.; Ge, Z.; Hao, W.; Jiang, Y.; Zeng, R.J. Waste C1 gases as alternatives to pure CO2 improved the microbial electrosynthesis of C4 and C6 carboxylates. ACS Sustain. Chem. Eng. 2020, 8, 8773–8782. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Bond, A.M.; Briggs, M.H. Multielement determination in biological materials by differential pulse voltammetry. Anal. Chem. 1985, 57, 1386–1390. [Google Scholar] [CrossRef]
- Hassan, Q.; Ahmadi, S.; Kerman, K. Recent advances in monitoring cell behavior using cell-based impedance spectroscopy. Micromachines 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Venkata Mohan, S. Capacitive biocathodes driving electrotrophy towards enhanced CO2 reduction for microbial electrosynthesis of fatty acids. Bioresour. Technol. 2019, 294, 122–181. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Reesh, I.M.A.; Vanbroekhoven, K.; Pant, D. Microbial electrosynthesis feasibility evaluation at high bicarbonate concentrations with enriched homoacetogenic biocathode. Sci. Total Environ. 2020, 715, 137003. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Srikanth, S.; Raghuvulu, S.V.; Mohanakrishna, G.; Kumar, A.K.; Sarma, P.N. Evaluation of the potential of various aquatic eco-systems in harnessing bioelectricity through benthic fuel cell: Effect of electrode assembly and water characteristics. Bioresour. Technol. 2009, 100, 2240–2246. [Google Scholar]
- Mohan, S.V.; Srikanth, S.; Chiranjeevi, P.; Arora, S.; Chandra, R. Algal biocathode for in situ terminal electron acceptor (TEA) production: Synergetic association of bacteria–microalgae metabolism for the functioning of biofuel cell. Bioresour. Technol. 2014, 166, 566–574. [Google Scholar] [CrossRef]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.; Pant, D.; Strik, D.P. Application of gas diffusion biocathode in microbial electrosynthesis from carbon dioxide. Environ. Sci. Pollut. Res. 2016, 23, 22292–22308. [Google Scholar] [CrossRef]
- Rago, L.; Ruiz, Y.; Baeza, J.A.; Guisasola, A.; Cortés, P. Microbial community analysis in a long-term membrane-less microbial electrolysis cell with hydrogen and methane production. Bioelectrochemistry 2015, 106, 359–368. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.; Heijne, A.T.; Buisman, C.J.; Hamelers, H.V. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2012, 36, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Vavourakis, C.D.; Andrei, A.S.; Mehrshad, M.; Ghai, R.; Sorokin, D.Y.; Muyzer, G. A metagenomics roadmap to the uncultured genome diversity in hypersaline soda lake sediments. Microbiome 2018, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Yadav, R.; Chiranjeevi, P.; Patil, S.A. Direct utilization of industrial carbon dioxide with low impurities for acetate production via microbial electrosynthesis. Bioresour. Technol. 2021, 320, 124289. [Google Scholar] [CrossRef]
- He, P.; Han, W.; Shao, L.; Lü, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Heitkamp, K.; Latorre-Pérez, A.; Nefigmann, S.; Gimeno-Valero, H.; Vilanova, C.; Jahmad, E.; Abendroth, C. Monitoring of seven industrial anaerobic digesters supplied with biochar. Biotechnol. Biofuels 2021, 14, 1–14. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharak, A.; Mohan, S.V. Syngas Fermentation to Acetate and Ethanol with Adaptative Electroactive Carboxydotrophs in Single Chambered Microbial Electrochemical System. Micromachines 2022, 13, 980. https://doi.org/10.3390/mi13070980
Tharak A, Mohan SV. Syngas Fermentation to Acetate and Ethanol with Adaptative Electroactive Carboxydotrophs in Single Chambered Microbial Electrochemical System. Micromachines. 2022; 13(7):980. https://doi.org/10.3390/mi13070980
Chicago/Turabian StyleTharak, Athmakuri, and S. Venkata Mohan. 2022. "Syngas Fermentation to Acetate and Ethanol with Adaptative Electroactive Carboxydotrophs in Single Chambered Microbial Electrochemical System" Micromachines 13, no. 7: 980. https://doi.org/10.3390/mi13070980
APA StyleTharak, A., & Mohan, S. V. (2022). Syngas Fermentation to Acetate and Ethanol with Adaptative Electroactive Carboxydotrophs in Single Chambered Microbial Electrochemical System. Micromachines, 13(7), 980. https://doi.org/10.3390/mi13070980





