Hermetic Packaging Based on Cu–Sn and Au–Au Dual Bonding for High-Temperature Graphene Pressure Sensor
Abstract
1. Introduction
2. Materials and Methods
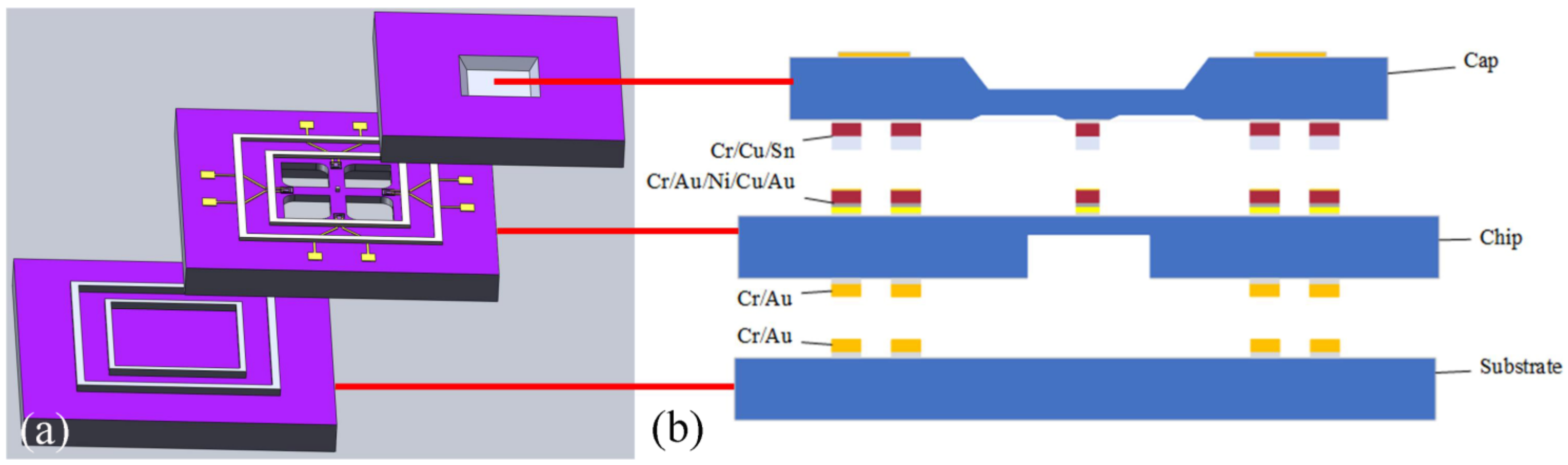
2.1. Test Vehicle Design
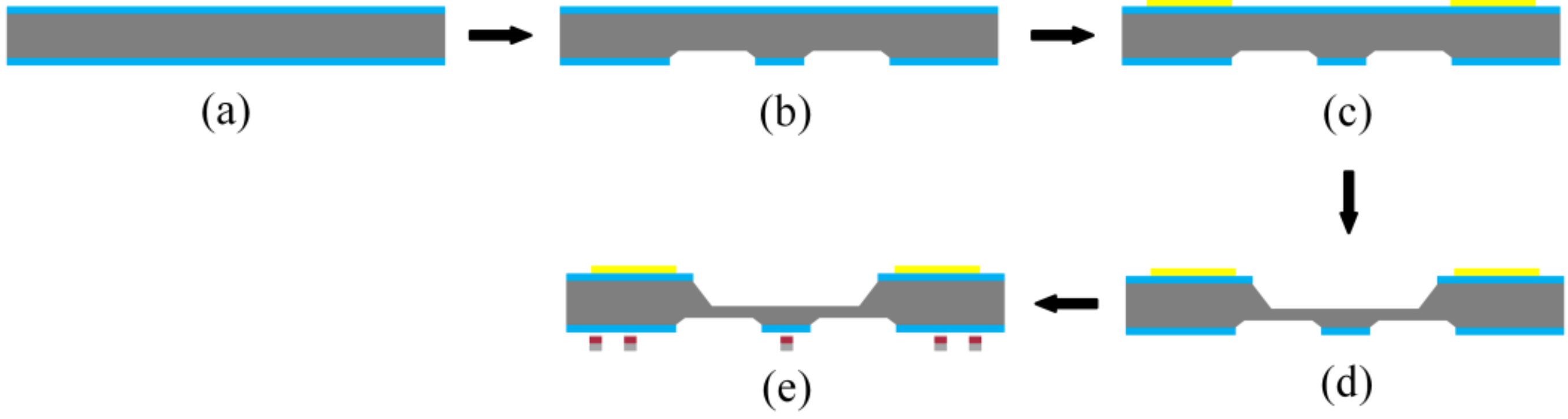
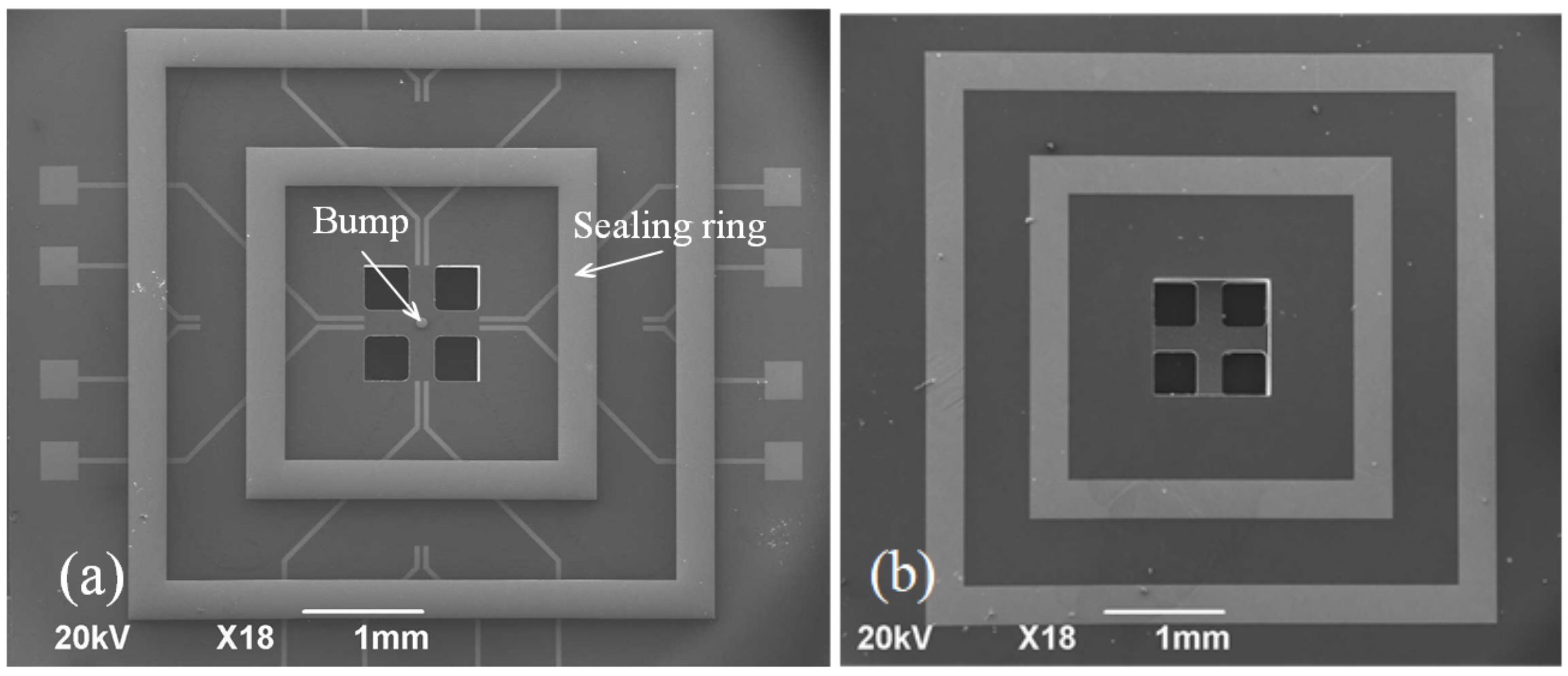
2.2. Vehicle Fabrication
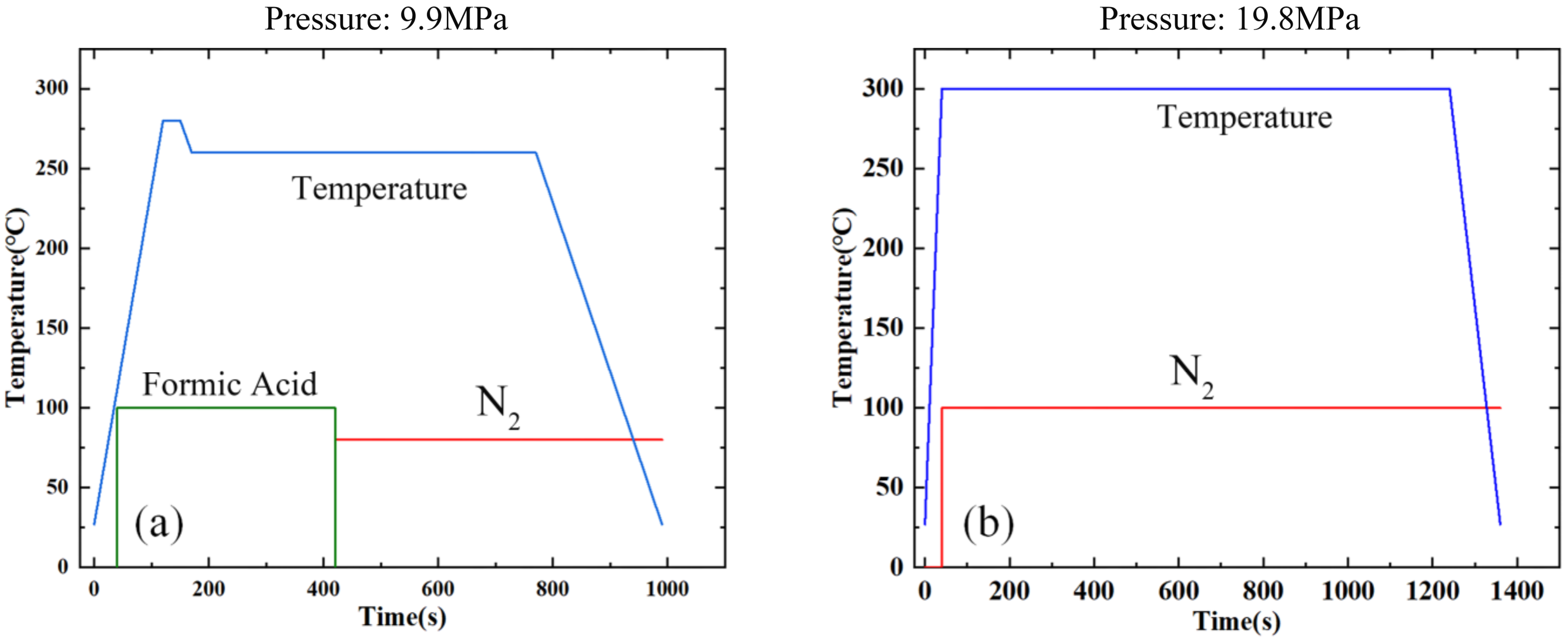
2.3. Cu–Sn and Au–Au Bonding
3. Results and Discussion
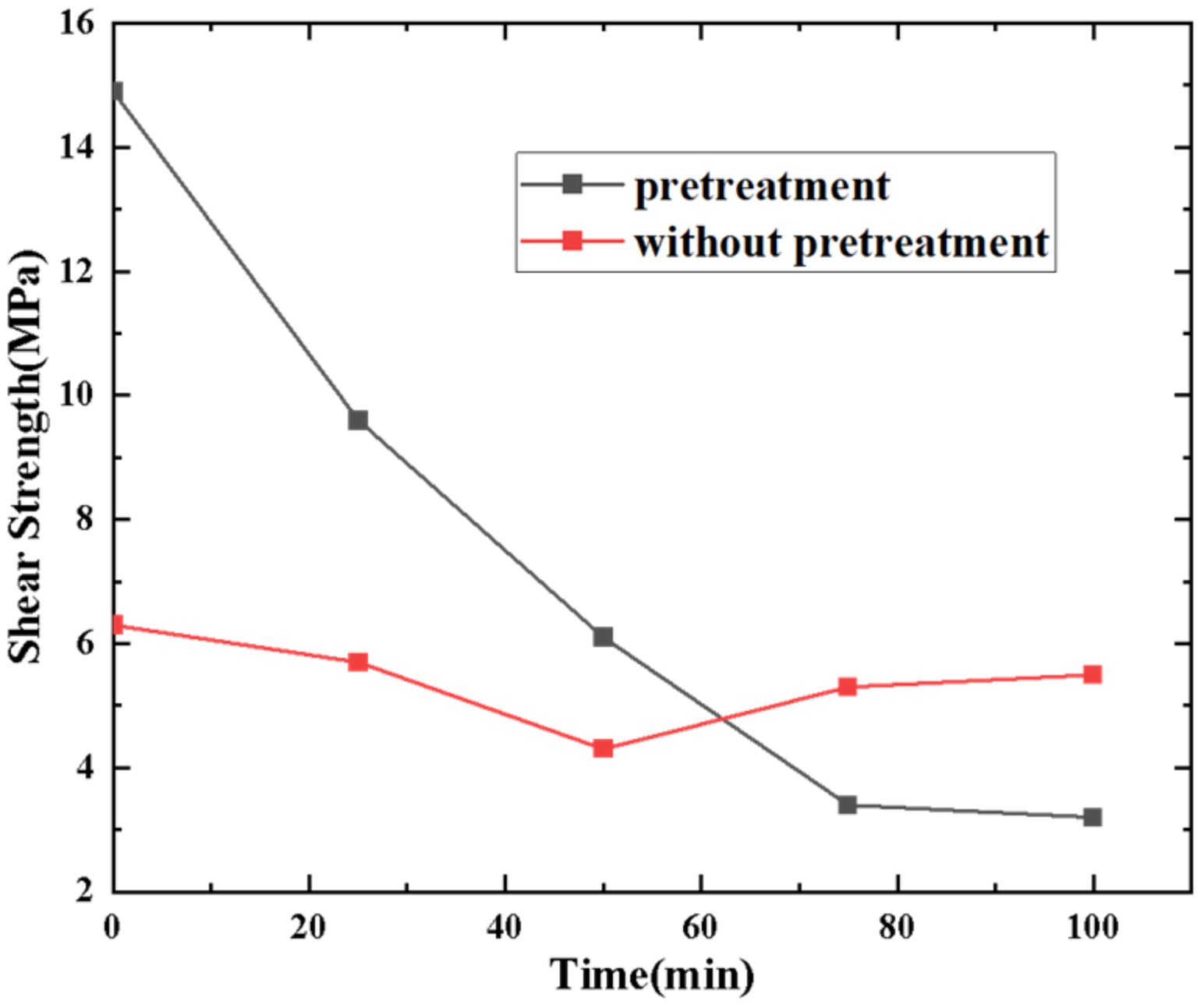
3.1. Au Pretreatment Optimization
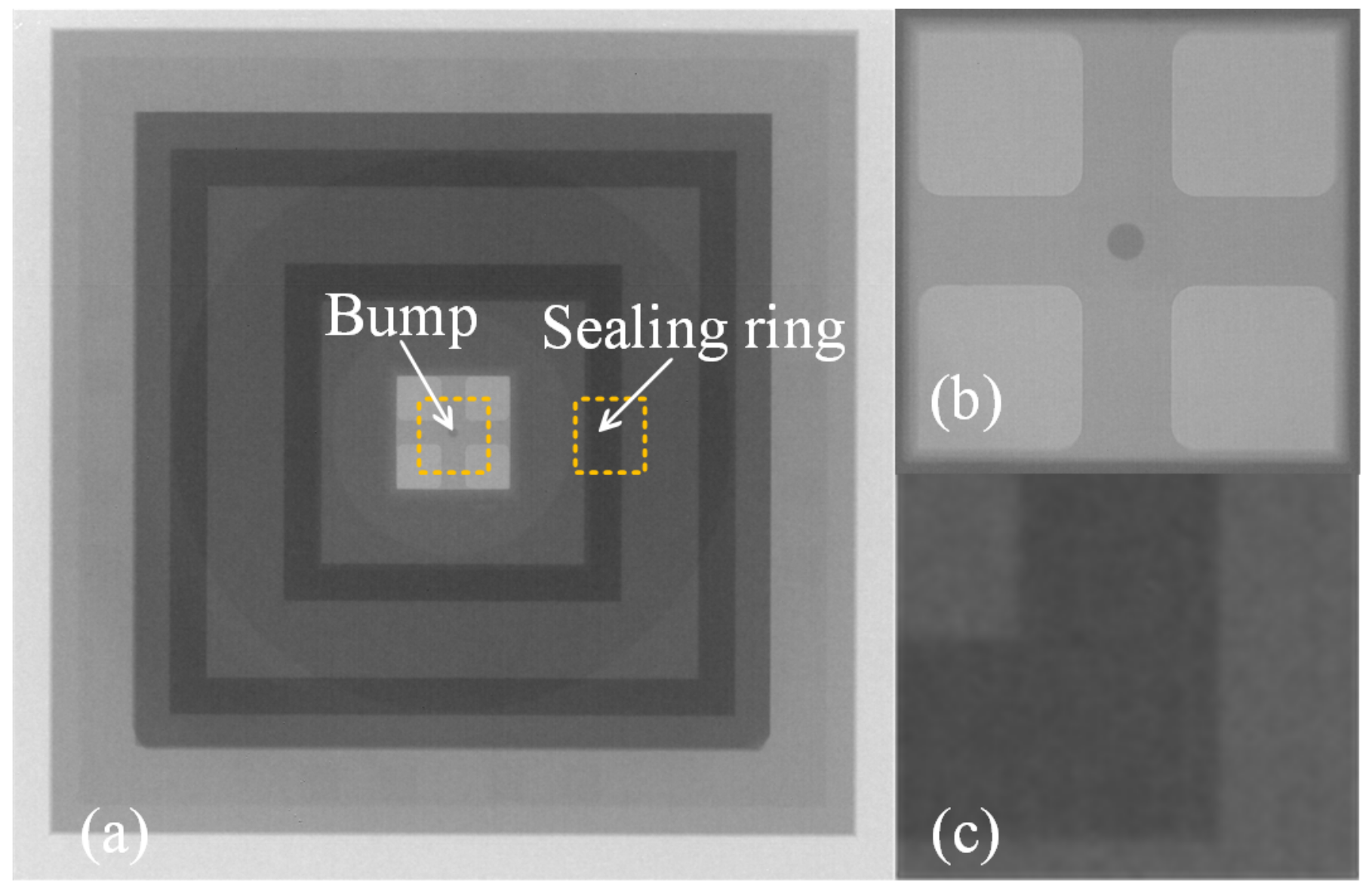
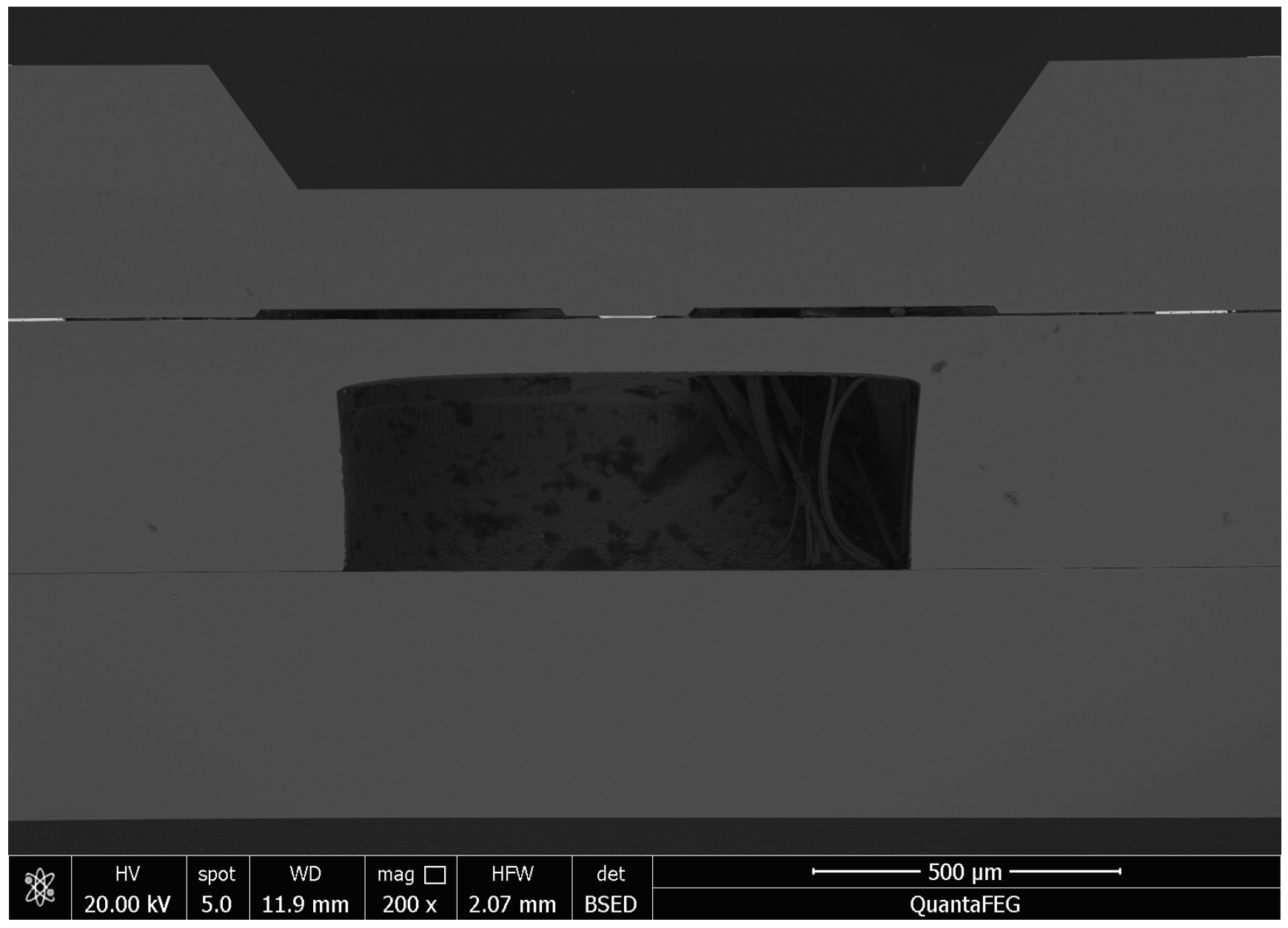
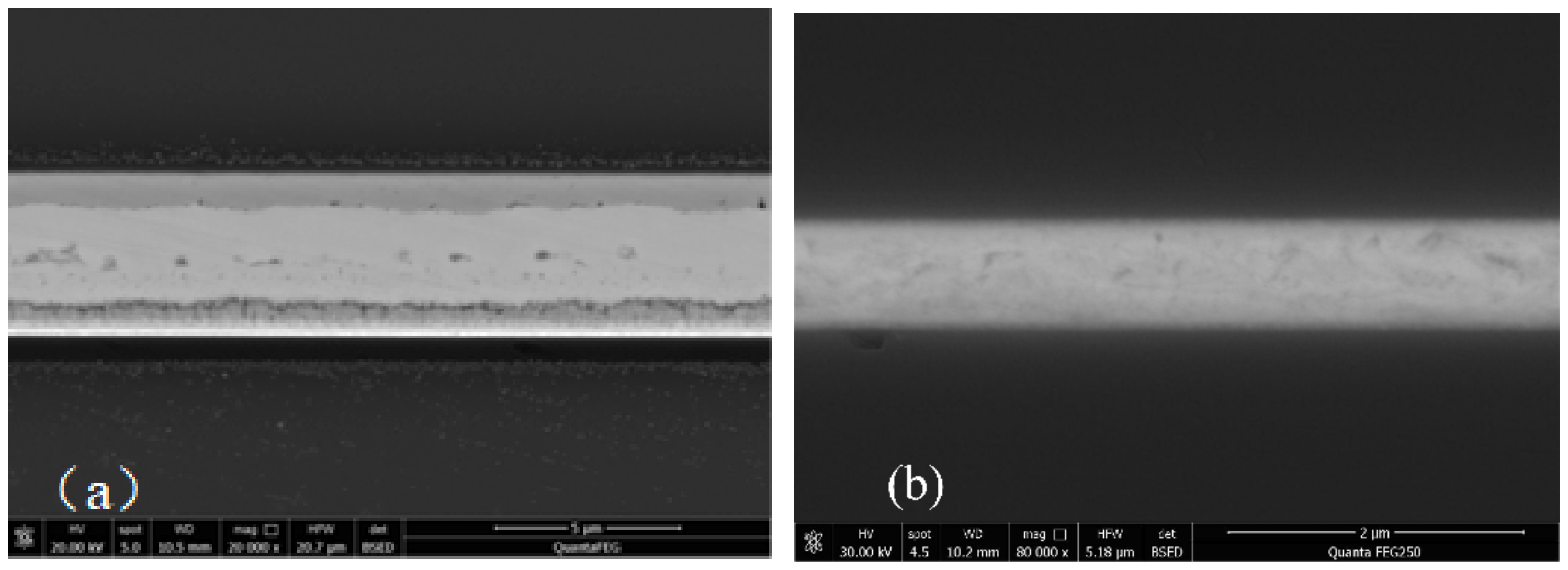
3.2. Interfacial Analysis
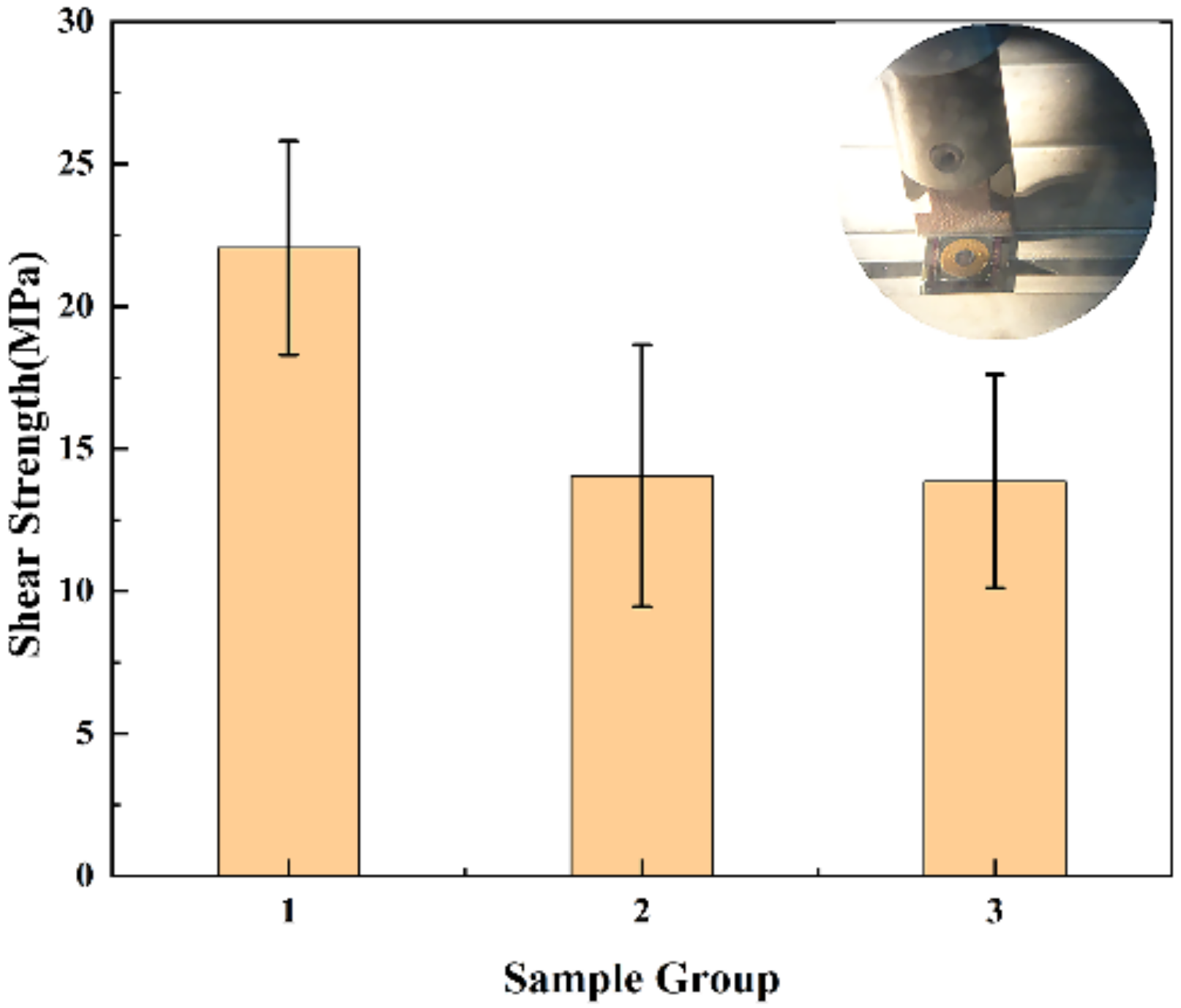
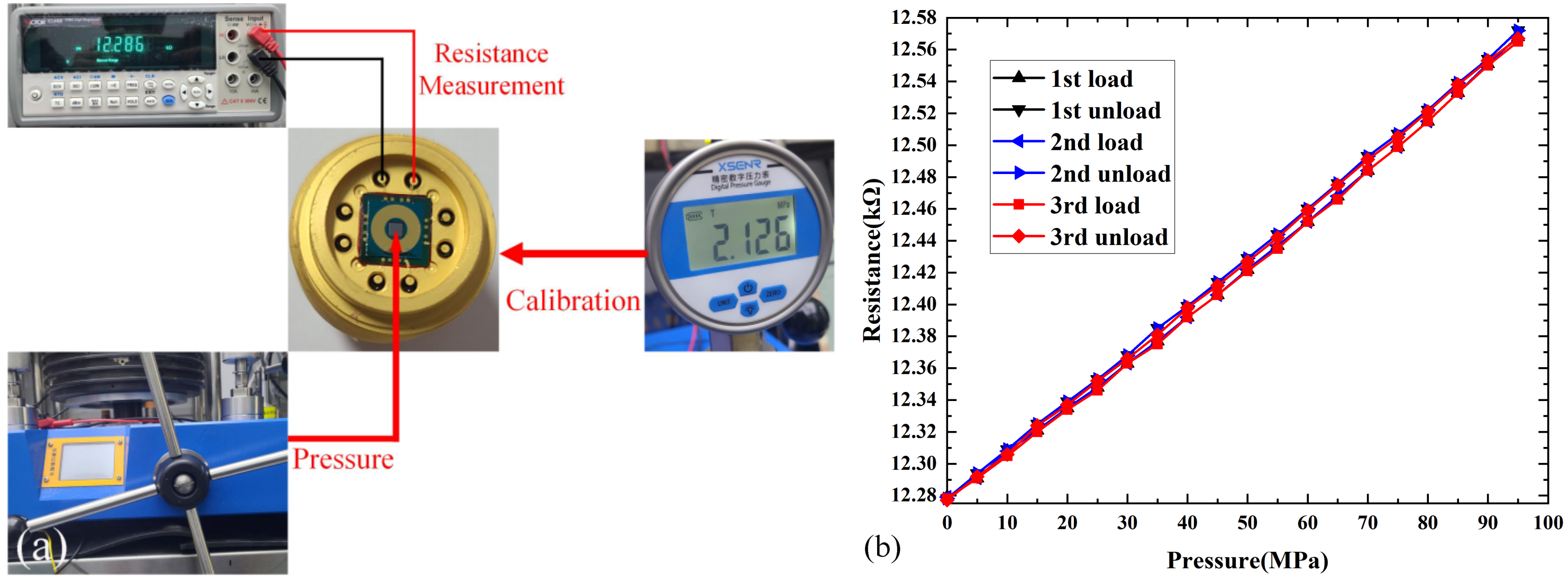
3.3. Shear Strength and Hermeticity Detection
3.4. High-Temperature Reliability
3.5. Static Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chun, S.; Kim, Y.; Jung, H.; Park, W. A flexible graphene touch sensor in the general human touch range. Appl. Phys. Lett. 2014, 105, 041907. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Chen, Y.; Zhang, H.; Zhang, J. Substrate Engineering by Hexagonal Boron Nitride/SiO2for Hysteresis-Free Graphene FETs and Large-Scale Graphene p-n Junctions. Chem. Asian J. 2013, 8, 2446–2452. [Google Scholar] [CrossRef] [PubMed]
- Kühne, M.; Paolucci, F.; Popovic, J.; Ostrovsky, P.; Maier, J.; Smet, J.H. Ultrafast lithium diffusion in bilayer graphene. Nat. Nanotechnol. 2017, 12, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Li, C.; Wang, S.; He, T.; Zhou, Z.; Wei, D.; Guo, H.; Yang, H.; Liu, Y. Highly responsive and broadband photodetectors based on WS2–graphene van der Waals epitaxial heterostructures. J. Mater. Chem. C 2017, 5, 1494–1500. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Wu, D.; Ye, C.; Li, X.; Tra, V.T.; Wei, Q.; Fu, L.; Yu, A.; Li, L.-J.; Lin, C.-T. Hall effect biosensors with ultraclean graphene film for improved sensitivity of label-free DNA detection. Biosens. Bioelectron. 2018, 99, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; Elashery, S.E.; Zakria, A.M.; Eltaweil, A.S.; Oh, H. Recent advances in graphene sheets as new generation of flame retardant materials. Mater. Sci. Eng. B 2021, 274, 115460. [Google Scholar] [CrossRef]
- Elashery, S.E.A.; Attia, N.F.; Mohamed, G.G.; Omar, M.M.; Tayea, H.M.I. Hybrid Nanocomposite Based Graphene Sensor for Ultrasensitive Clomipramine HCl Detection. Electroanalysis 2021, 33, 2361–2371. [Google Scholar] [CrossRef]
- Karimov, K.S.; Sulaiman, K.; Ahmad, Z.; Akhmedov, K.M.; Mateen, A. Novel pressure and displacement sensors based on carbon nanotubes. Chin. Phys. B 2015, 24, 018801. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Q.; Gan, X.; Hu, M.; Zhang, T.; Li, C.; Kang, F.; Terrones, M.; Lv, R. Ultrasensitive Pressure Detection of Few-Layer MoS2. Adv. Mater. 2017, 29, 1603266. [Google Scholar] [CrossRef]
- Bolotin, K.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Wang, L.; Meric, I.; Huang, P.Y.; Gao, Q.; Gao, Y.; Tran, H.; Taniguchi, T.; Watanabe, K.; Campos, L.M.; Muller, D.A.; et al. One-Dimensional Electrical Contact to a Two-Dimensional Material. Science 2013, 342, 614–617. [Google Scholar] [CrossRef]
- Yin, Y.; Cheng, Z.; Wang, L.; Jin, K.; Wang, W. Graphene, a material for high temperature devices–intrinsic carrier density, carrier drift velocity and lattice energy. Electroanalysis 2014, 4, 5758. [Google Scholar] [CrossRef]
- Bunch, J.S.; van der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Electromechanical Resonators from Graphene Sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, V.; Zhang, Y.W. Graphene-based pressure nano-sensors. J. Mol. Model. 2011, 17, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Niklaus, F.; Paussa, A.; Vaziri, S.; Fischer, A.C.; Sterner, M.; Forsberg, F.; Delin, A.; Esseni, D.; Palestri, P.; et al. Electromechanical Piezoresistive Sensing in Suspended Graphene Membranes. Nano Lett. 2013, 13, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Vaziri, S.; Niklaus, F.; Fischer, A.; Sterner, M.; Delin, A.; Östling, M.; Lemme, M. Pressure sensors based on suspended graphene membranes. Solid-State Electron. 2013, 88, 89–94. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, W.; Dong, L. Graphene “microdrums” on a freestanding perforated thin membrane for high sensitivity MEMS pressure sensors. Nanoscale 2016, 8, 7663–7671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.; Li, M.; Wang, P.; Li, M. Solid–liquid interdiffusion bonding of Cu–Sn–Cu interconnection and sealing for high-temperature pressure sensor based on graphene. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 10, 65–71. [Google Scholar] [CrossRef]
- Choi, W.-B.; Ju, B.-K.; Jeong, S.-J.; Lee, N.-Y.; Koh, K.-H.; Haskard, M.; Sung, M.-Y.; Oh, M.-H. Anodic bonding technique under low-temperature and low-voltage using evaporated glass. J. Vac. Sci. Technol. B 2002, 15, 477–481. [Google Scholar] [CrossRef]
- Noguchi, K.; Ikeda, M.; Shimizu, I.; Kawamura, Y.; Ohno, Y. Effect of Cooling Rate on Microstructure and Strength Properties of Tin-Silver-Copper Solder Ball Bonding. Mater. Trans. 2001, 42, 761–768. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Ning, W.; Le, L. Wafer-level package with simultaneous TSV connection and cavity hermetic sealing by solder bonding for MEMS device. IEEE Trans. Electron. Packag. Manuf. 2009, 32, 125–132. [Google Scholar]
- Zhang, S.; Yang, M.; Wu, Y.; Du, J.; Lin, T.; He, P.; Huang, M.; Paik, K.W. A study on the optimization of anisotropic conductive films for Sn-3Ag-0.5Cu-based flex-on-board application at a 250 degrees C bonding temperature. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 383–391. [Google Scholar] [CrossRef]
- Hang, C.; Liu, J.; Wang, J.; Fu, X.; Chen, H.; Li, M. A low-temperature Cu-to-Cu interconnection method by using nanoporous Cu fabricated by dealloying electroplated Cu–Zn. J. Mater. Sci. Mater. Electron. 2020, 31, 18381–18388. [Google Scholar] [CrossRef]
- Jiang, H.; Robertson, S.; Zhou, Z.; Liu, C. Cu–Cu bonding with Cu nanowire arrays for electronics integration. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference (ESTC), Tønsberg, Norway, 15–18 September 2020. [Google Scholar]
- Hao, F.; Wang, J.; Li, M. Low-temperature anodic bonding for wafer-level Al–Al interconnection in MEMS grating gyroscope. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 11, 19–24. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Wu, Z.; Wang, D.; Cai, J. Solid-state-diffusion bonding for wafer-level fine-pitch Cu/Sn/Cu interconnect in 3-D integration. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 7, 19–26. [Google Scholar] [CrossRef]
- Kurashima, Y.; Matsumae, T.; Takagi, H. Room-temperature Au–Au bonding in atmospheric air using direct transferred atomically smooth Au film on electroplated patterns. Microelectron. Eng. 2018, 189, 33–38. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Z.; Qi, Y.; Li, M. A Novel Crossbeam Structure with Graphene Sensing Element for N/MEMS Mechanical Sensors. Nanomaterials 2022, 12, 2101. [Google Scholar] [CrossRef] [PubMed]
- Matsumae, T.; Kurashima, Y.; Umezawa, H.; Mokuno, Y.; Takagi, H. Room-temperature bonding of single-crystal diamond and Si using Au/Au atomic diffusion bonding in atmospheric air. Microelectron. Eng. 2018, 195, 68–73. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsumae, T.; Kurashima, Y.; Takagi, H.; Suga, T.; Takamatsu, S.; Itoh, T.; Higurashi, E. Effect of Au Film Thickness and Surface Roughness on Room-Temperature Wafer Bonding and Wafer-Scale Vacuum Sealing by Au-Au Surface Activated Bonding. Micromachines 2020, 11, 454. [Google Scholar] [CrossRef]
- Yamamoto, M.; Higurashi, E.; Suga, T.; Sawada, R.; Itoh, T. Properties of various plasma surface treatments for low-temperature Au–Au bonding. Jpn. J. Appl. Phys. 2018, 57, 04FC12. [Google Scholar] [CrossRef]
- Wu, D.; Tian, W.; Wang, C.; Huo, R.; Wang, Y. Research of Wafer Level Bonding Process Based on Cu–Sn Eutectic. Micromachines 2020, 11, 789. [Google Scholar] [CrossRef] [PubMed]






| As bonded | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
| 1.53 | 1.48 | 1.66 | 2.47 | 1.46 |
| Stored | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
| 1.65 | 1.87 | 2.39 | 2.86 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, H.; Chen, X.; Li, M. Hermetic Packaging Based on Cu–Sn and Au–Au Dual Bonding for High-Temperature Graphene Pressure Sensor. Micromachines 2022, 13, 1191. https://doi.org/10.3390/mi13081191
Wang J, Zhang H, Chen X, Li M. Hermetic Packaging Based on Cu–Sn and Au–Au Dual Bonding for High-Temperature Graphene Pressure Sensor. Micromachines. 2022; 13(8):1191. https://doi.org/10.3390/mi13081191
Chicago/Turabian StyleWang, Junqiang, Haikun Zhang, Xuwen Chen, and Mengwei Li. 2022. "Hermetic Packaging Based on Cu–Sn and Au–Au Dual Bonding for High-Temperature Graphene Pressure Sensor" Micromachines 13, no. 8: 1191. https://doi.org/10.3390/mi13081191
APA StyleWang, J., Zhang, H., Chen, X., & Li, M. (2022). Hermetic Packaging Based on Cu–Sn and Au–Au Dual Bonding for High-Temperature Graphene Pressure Sensor. Micromachines, 13(8), 1191. https://doi.org/10.3390/mi13081191






