Facile Electrochemical Sensor for Sensitive and Selective Determination of Guaifenesin, Phenylephrine and Paracetamol on Electrochemically Pretreated Pencil Graphite Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Electrochemical Pretreatment of PGE
2.3. Sample Analysis
3. Results and Discussion
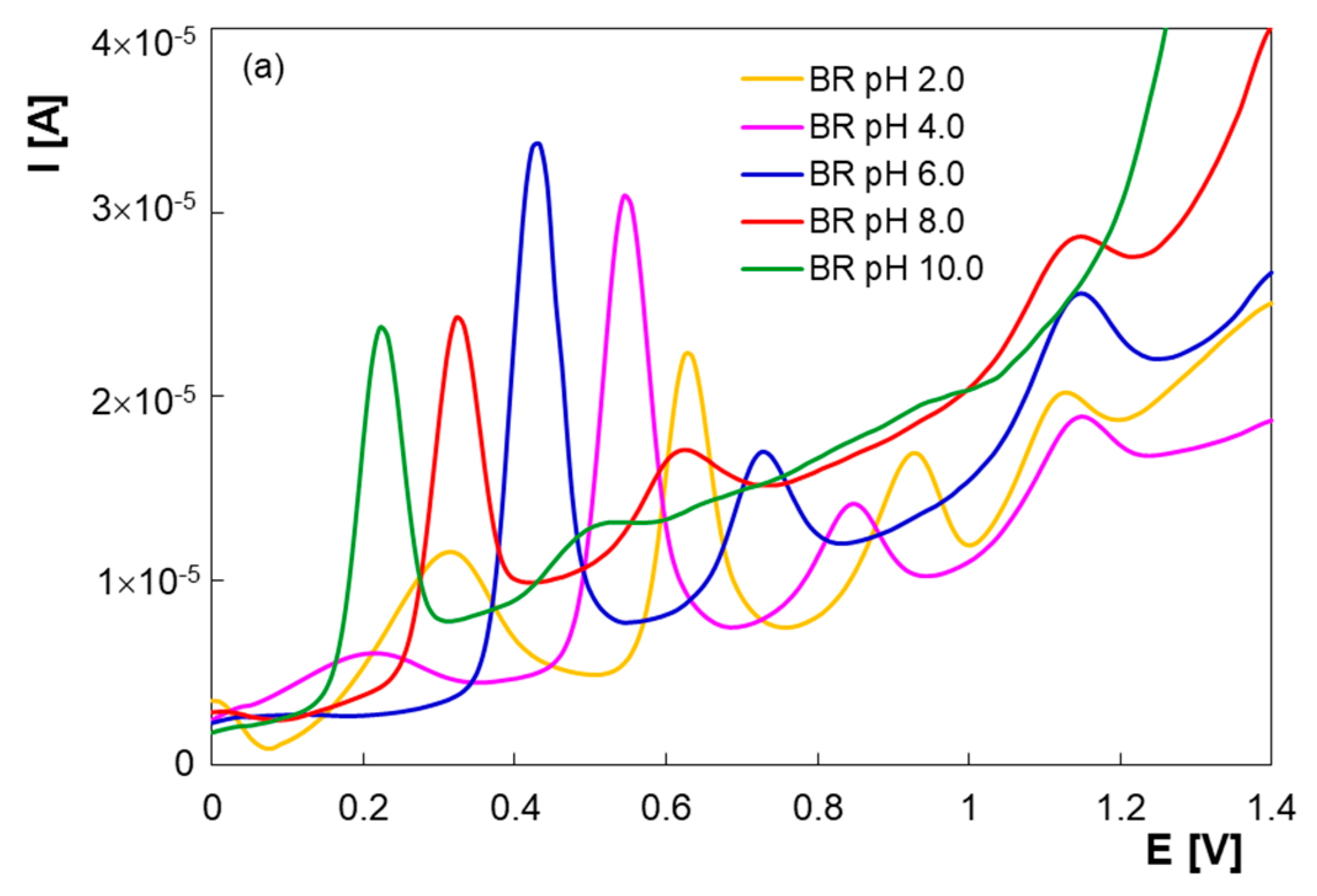
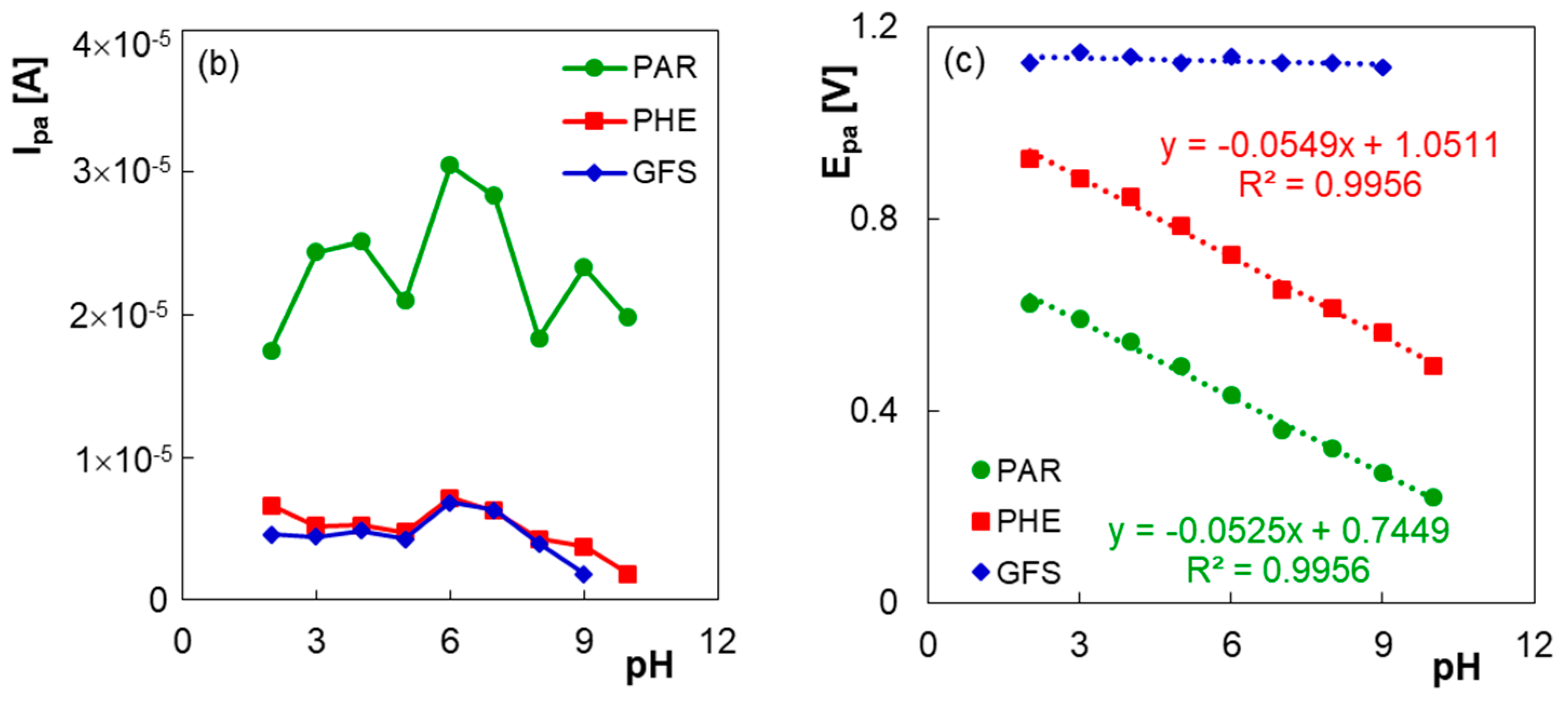
3.1. Electrochemical Behavior of PAR, PHE and GFS at PGE*
3.2. Electrochemical Simultaneous Determination of PAR, PHE and GFS at PGE*
3.3. Analytical Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janin, A.; Monnet, J. Bioavailability of paracetamol, phenylephrine hydrochloride and guaifenesin in a fixed-combination syrup versus an oral reference product. Int. J. Med. Res. 2014, 42, 347–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, H.H.; Dicpinigaitis, P.V.; Guenin, E.P. Role of guaifenesin in the management of chronic bronchitis and upper respiratory tract infections. Multidiscip. Respir. Med. 2017, 12, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatton, R.C.; Winterstein, A.G.; McKelvey, R.P.; Shuster, J.; Hendeles, L. Efficacy and safety of oral phenylephrine: Systematic review and meta-analysis. Ann. Pharmacother. 2007, 41, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Marzilawati, A.R.; Ngau, Y.Y.; Mahadeva, S. Low rates of hepatotoxicity among Asian patients with paracetamol overdose: A review of 1024 cases. BMC Pharmacol. Toxicol. 2012, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okic, M.; Johnson, T.; Crifasi, J.A.; Long, C.; Mitchell, E.K. Swift onset of central nervous system depression and asystole following an overdose of guaifenesin. J. Anal. Toxicol. 2013, 37, 318–319. [Google Scholar] [CrossRef] [Green Version]
- Enohumah, K.O.; Immanuel, H.; Whitty, R.; Connolly, J. The adverse effects of inadvertent intraoperative intravenous phenylephrine administration: A case report. S. Afr. J. Anaesth. Analg. 2012, 18, 218–220. [Google Scholar] [CrossRef]
- Bosch, M.E.; Ruiz Sanchez, A.J.; Sanchez Rojas, F.; Bosch Ojeda, C. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 2006, 42, 291–321. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B.C. Analytical techniques for the determination of acetaminophen: A review. TrAC-Trends Anal. Chem. 2018, 108, 122–134. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Barka, N. Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples. J. Pharm. Anal. 2021, 11, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Yehia, A.M.; Essam, H.M. Development and validation of a generic high-performance liquid chromatography for the simultaneous separation and determination of six cough ingredients: Robustness study on core-shell particles. J. Sep. Sci. 2016, 39, 3357–3367. [Google Scholar] [CrossRef] [PubMed]
- Dongala, T.; Katari, N.K.; Palakurthi, A.K.; Jonnalagadda, S.B. Development and validation of a generic RP-HPLC PDA method for the simultaneous separation and quantification of active ingredients in cold and cough medicines. Biomed. Chromatogr. 2019, 33, e4641. [Google Scholar] [CrossRef] [PubMed]
- Yehia, A.M.; Mohamed, H.M. Chemometrics resolution and quantification power evaluation: Application on pharmaceutical quaternary mixture of paracetamol, guaifenesin, phenylephrine and p-aminophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Sohrabi, M.R.; Motiee, F.; Davallo, M. Rapid simultaneous spectrophotometric determination of acetaminophen, phenylephrine, and guaifenesin in a cold syrup formulation based on continuous wavelet transform and first derivative transform methods. Optik 2021, 230, 166323. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Mirzabeygi, V.; Davallo, M. Use of continuous wavelet transform approach for simultaneous quantitative determination of multicomponent mixture by UV–Vis spectrophotometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 201, 306–314. [Google Scholar] [CrossRef]
- Hendawy, H.A.M.; Ibrahim, A.M.; Hassan, W.S.; Shalaby, A.; El-Sayed, H.M. Voltammetric method for simultaneous determination of ascorbic acid, paracetamol and guaifenesin using a sequential experimentation strategy. Microchem. J. 2019, 145, 428–434. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Attia, A.K.; Elwy, H.M. Electrochemical sensor based on multiwalled carbon nanotube, alizarine red S and chitosan for simultaneous determination of oxomemazine hydrochloride, paracetamol and guaifenesin. Electroanalysis 2017, 29, 506–513. [Google Scholar] [CrossRef]
- Habibi, B.; Ayazi, Z.; Zalvand, F. Electrochemical behavior and determination of phenylephrine at the multi-walled carbon nanotubes/ionic liquid nanocomposite modified electrode in the presence of acetaminophen. Int. J. Nanosci. Nanotechnol. 2017, 13, 203–218. [Google Scholar]
- Lotfi, S.; Veisi, H. Pd nanoparticles decorated poly-methyldopa@GO/Fe3O4 nanocomposite modified glassy carbon electrode as a new electrochemical sensor for simultaneous determination of acetaminophen and phenylephrine. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110112. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Nejad-Darzi, S.K.H.; Ilkhani, H. A new sensor for determination of paracetamol, phenylephrine hydrochloride and chlorpheniramine maleate in pharmaceutical samples using nickel phosphate nanoparticles modified carbon paste electrode. Anal. Bioanal. Electrochem. 2011, 3, 134–145. [Google Scholar]
- Amiri, M.; Rezapour, F.; Bezaatpour, A. Hydrophilic carbon nanoparticulates at the surface of carbon paste electrode improve determination of paracetamol, phenylephrine and dextromethorphan. J. Electroanal. Chem. 2014, 735, 10–18. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Srivastava, A.K. Simultaneous voltammetric determination of paracetamol, cetirizine and phenylephrine using a multiwalled carbon nanotube-platinum nanoparticles nanocomposite modified carbon paste electrode. Sens. Actuators B Chem. 2016, 233, 237–248. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. Simultaneous determination of ascorbic acid, paracetamol and phenylephrine: Carbon nanotubes ceramic electrode as a renewable electrode. Anal. Bioanal. Electrochem. 2015, 7, 45–58. [Google Scholar]
- Salamanca-Neto, C.A.R.; Marcheafave, G.G.; Mattos, G.J.; Moraes, J.T.; Schwarzova-Peckova, K.; Sartori, E.R. Boron-doped diamond film and multiple linear regression-based calibration applied to the simultaneous electrochemical determination of paracetamol, phenylephrine hydrochloride, and loratadine in fixed-dose combinations. Microchem. J. 2021, 162, 105831. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandra, M. Pencil graphite electrodes: A versatile tool in electroanalysis. J. Anal. Meth. Chem. 2017, 2017, 1905968. [Google Scholar] [CrossRef] [Green Version]
- David, I.G.; Buleandra, M.; Popa, D.E.; Cheregi, M.C.; David, V.; Iorgulescu, E.E.; Tartareanu, G.O. Recent developments in voltammetric analysis of pharmaceuticals using disposable pencil graphite electrodes. Processes 2022, 10, 472. [Google Scholar] [CrossRef]
- Tasic, Z.Z.; Petrovic Mihajlovic, M.B.; Simonovic, A.T.; Radovanovic, M.B.; Antonijevic, M.M. Review of applied surface modifications of pencil graphite electrodes for paracetamol sensing. Results Phys. 2021, 22, 103911. [Google Scholar] [CrossRef]
- Dehnavi, A.; Soleynanpour, A. Silver nanoparticules/poly (L-cysteine) nanocomposite modified pencil grafite for selective electrochemical measurement of guaifenesin in real samples. Measurement 2021, 175, 109103. [Google Scholar] [CrossRef]
- David, I.G.; Florea, M.A.; Cracea, O.G.; Popa, D.E.; Buleandra, M.; Iorgulescu, E.E.; David, V.; Badea, I.A.; Ciucu, A.A. Voltammetric determination of vitamin B1 and vitamin B6 on a disposable electrode. Chem. Pap. 2015, 69, 901–910. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Badea, I.A.; Balan, A.; Stamatin, I.; Mihailciuc, C.; Ciucu, A.A. Voltammetric determination of dihydroxybenzene isomers using a disposable pencil graphite electrode modified with cobalt-phthalocyanine. Microchim. Acta 2017, 184, 1481–1488. [Google Scholar] [CrossRef]
- Buleandra, M.; Popa, D.E.; David, I.G.; Bacalum, E.; David, V.; Ciucu, A.A. Electrochemical behavior study of some selected phenylurea herbicides at activated pencil graphite electrode. Electrooxidation of linuron and monolinuron. Microchem. J. 2019, 147, 1109–1116. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Koyun, O.; Gorduk, S.; Arvas, M.B.; Sahin, Y. Electrochemically treated pencil graphite electrodes prepared in one step for the electrochemical determination of paracetamol. Russ. J. Electrochem. 2018, 54, 796–808. [Google Scholar] [CrossRef]
- Calam, T.T. A modified pencil graphite electrode with 2-thiobarbituric acid for the efficient and cheap voltammetric sensing of 4-aminophenol in water samples and child syrup sample. J. Food Compos. Anal. 2021, 98, 103809. [Google Scholar] [CrossRef]
- Nematollahi, D.; Shayani-Jam, H.; Alimoradi, M.; Niroomand, S. Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochim. Acta 2009, 54, 7407–7415. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F. Voltammetric determination of phenylephrine hydrochloride using a multi-walled carbon nanotube-modified carbon paste electrode. R. Soc. Open Sci. 2018, 5, 181264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, Z.; Zhao, W.; Pang, D. Voltammetric behavior and determination of phenylephrine at a glassy carbon electrode modified with multi-wall carbon nanotubes. Sens. Actuators B Chem. 2016, 119, 308–314. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rawoola, C.R.; Srivastava, A.K. Fabrication of graphene nanosheets-multiwalled carbon nanotubes polyaniline modified carbon paste electrode for simultaneous electrochemical determination of terbutaline sulphate and guaifenesin. New J. Chem. 2017, 41, 7061–7072. [Google Scholar] [CrossRef]
- Huang, S.J.; Kannaiyan, S.; Venkatesh, K.; Cheemalapati, S.; Haidyrah, A.S.; Ramaraj, S.K.; Yang, C.C.; Karuppiah, C. Synthesis and fabrication of Ni-SiO2 nanosphere-decorated multilayer graphene nanosheets composite electrode for highly sensitive amperometric determination of guaifenesin drug. Microchem. J. 2021, 167, 106325. [Google Scholar] [CrossRef]
- Mecozzi, F.; Dong, J.J.; Saisaha, P.; Browne, W.R. Oxidation of vicinal diols to α-hydroxy ketones with H2O2 and a simple manganese catalyst. Eur. J. Org. Chem. 2017, 46, 6919–6925. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, X.; Zhang, J.; Zhang, H.; Li, Y. Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode. Biosens. Bioelectron. 2019, 130, 315–321. [Google Scholar] [CrossRef]



| C [M] | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| Mean Concentration ± SD [M] | r.e.% | RSD% | Mean Concentration ± SD [M] | r.e.% | RSD% | |
| PAR | ||||||
| 2.50 × 10−6 | 2.59 × 10−6 ± 4.49 × 10−8 | 3.45 | 1.74 | 2.57 × 10−6 ± 6.78 × 10−8 | 2.89 | 2.64 |
| 1.00 × 10−5 | 1.02 × 10−5 ± 2.11 × 10−7 | 2.07 | 2.06 | 1.02 × 10−5 ± 2.46 × 10−7 | 2.43 | 2.40 |
| 1.00 × 10−4 | 9.85 × 10−5 ± 2.08 × 10−6 | −1.22 | 2.11 | 9.97 × 10−5 ± 2.18 × 10−6 | −0.34 | 2.19 |
| PHE | ||||||
| 5.00 × 10−6 | 5.15 × 10−6 ± 2.55 × 10−7 | 2.96 | 4.95 | 5.13 × 10−6 ± 2.70 × 10−7 | 5.27 | 2.66 |
| 2.00 × 10−5 | 2.07 × 10−5 ± 8.75 × 10−7 | 3.50 | 4.23 | 2.08 × 10−5 ± 9.14 × 10−7 | 4.40 | 3.89 |
| 2.00 × 10−4 | 2.02 × 10−4 ± 7.92 × 10−6 | 0.95 | 3.92 | 2.06 × 10−4 ± 8.60 × 10−6 | 4.18 | 2.78 |
| GFS | ||||||
| 2.50 × 10−6 | 2.51 × 10−6 ± 1.09 × 10−7 | 0.34 | 4.35 | 2.51 × 10−6 ± 1.30 × 10−7 | 0.44 | 5.17 |
| 2.00 × 10−5 | 2.03 × 10−5 ± 6.92 × 10−7 | 1.61 | 3.40 | 2.07 × 10−5 ± 7.35 × 10−7 | 3.66 | 3.56 |
| 2.00 × 10−4 | 1.96 × 10−4 ± 4.71 × 10−6 | −1.99 | 2.40 | 1.96 × 10−4 ± 4.92 × 10−6 | −1.86 | 2.50 |
| PAR | GFS | PHE | |
|---|---|---|---|
| Declared content (mg) | 500 | 200 | 10 |
| Determined content (mg) | 509.12 ± 0.78 | 197.10 ± 1.21 | 10.60 ± 0.15 |
| Recovery ± SD (%) | 101.82 ± 3.92 | 98.55 ± 0.60 | 100.56 ± 1.50 |
| RSD% | 0.77 | 0.61 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buleandră, M.; Pătrașcu, A.A.; Popa, D.E.; David, I.G.; Badea, I.A.; Ciucu, A.A. Facile Electrochemical Sensor for Sensitive and Selective Determination of Guaifenesin, Phenylephrine and Paracetamol on Electrochemically Pretreated Pencil Graphite Electrode. Micromachines 2022, 13, 1213. https://doi.org/10.3390/mi13081213
Buleandră M, Pătrașcu AA, Popa DE, David IG, Badea IA, Ciucu AA. Facile Electrochemical Sensor for Sensitive and Selective Determination of Guaifenesin, Phenylephrine and Paracetamol on Electrochemically Pretreated Pencil Graphite Electrode. Micromachines. 2022; 13(8):1213. https://doi.org/10.3390/mi13081213
Chicago/Turabian StyleBuleandră, Mihaela, Anca Aurelia Pătrașcu, Dana Elena Popa, Iulia Gabriela David, Irinel Adriana Badea, and Anton Alexandru Ciucu. 2022. "Facile Electrochemical Sensor for Sensitive and Selective Determination of Guaifenesin, Phenylephrine and Paracetamol on Electrochemically Pretreated Pencil Graphite Electrode" Micromachines 13, no. 8: 1213. https://doi.org/10.3390/mi13081213
APA StyleBuleandră, M., Pătrașcu, A. A., Popa, D. E., David, I. G., Badea, I. A., & Ciucu, A. A. (2022). Facile Electrochemical Sensor for Sensitive and Selective Determination of Guaifenesin, Phenylephrine and Paracetamol on Electrochemically Pretreated Pencil Graphite Electrode. Micromachines, 13(8), 1213. https://doi.org/10.3390/mi13081213









