Mechanical Characterization and Analysis of Different-Type Polyimide Feedthroughs Based on Tensile Test and FEM Simulation for an Implantable Package
Abstract
:1. Introduction
2. Concept of Implantable Package
3. Tensile Test of PSPI and FPCB
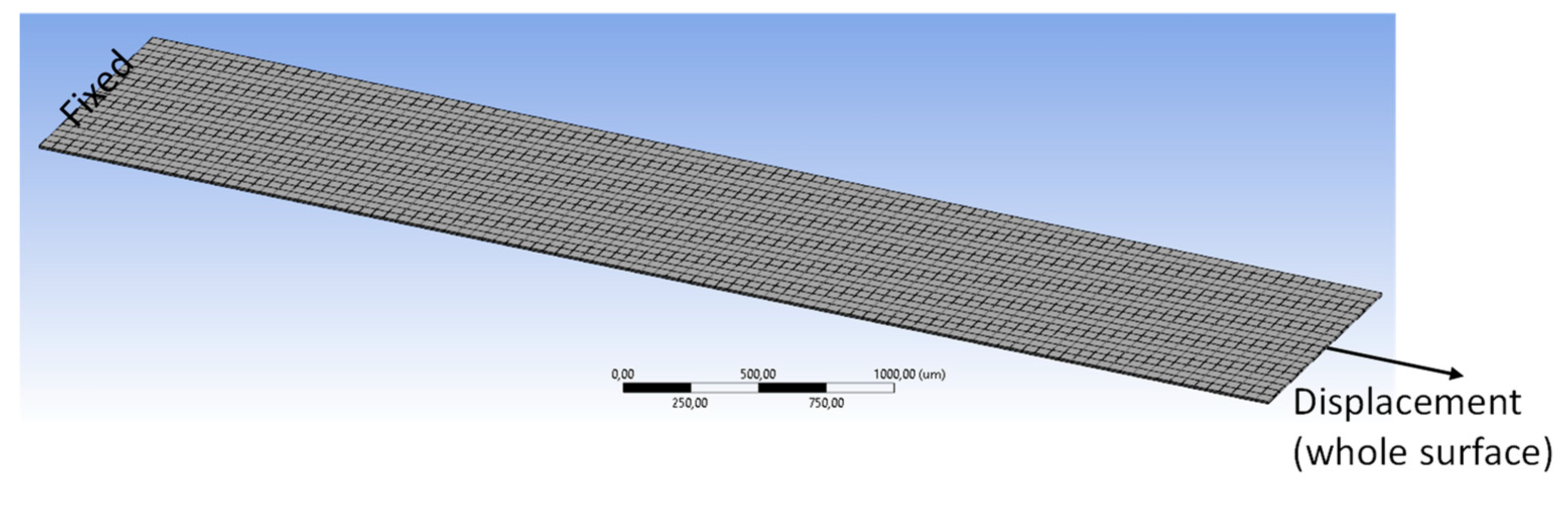
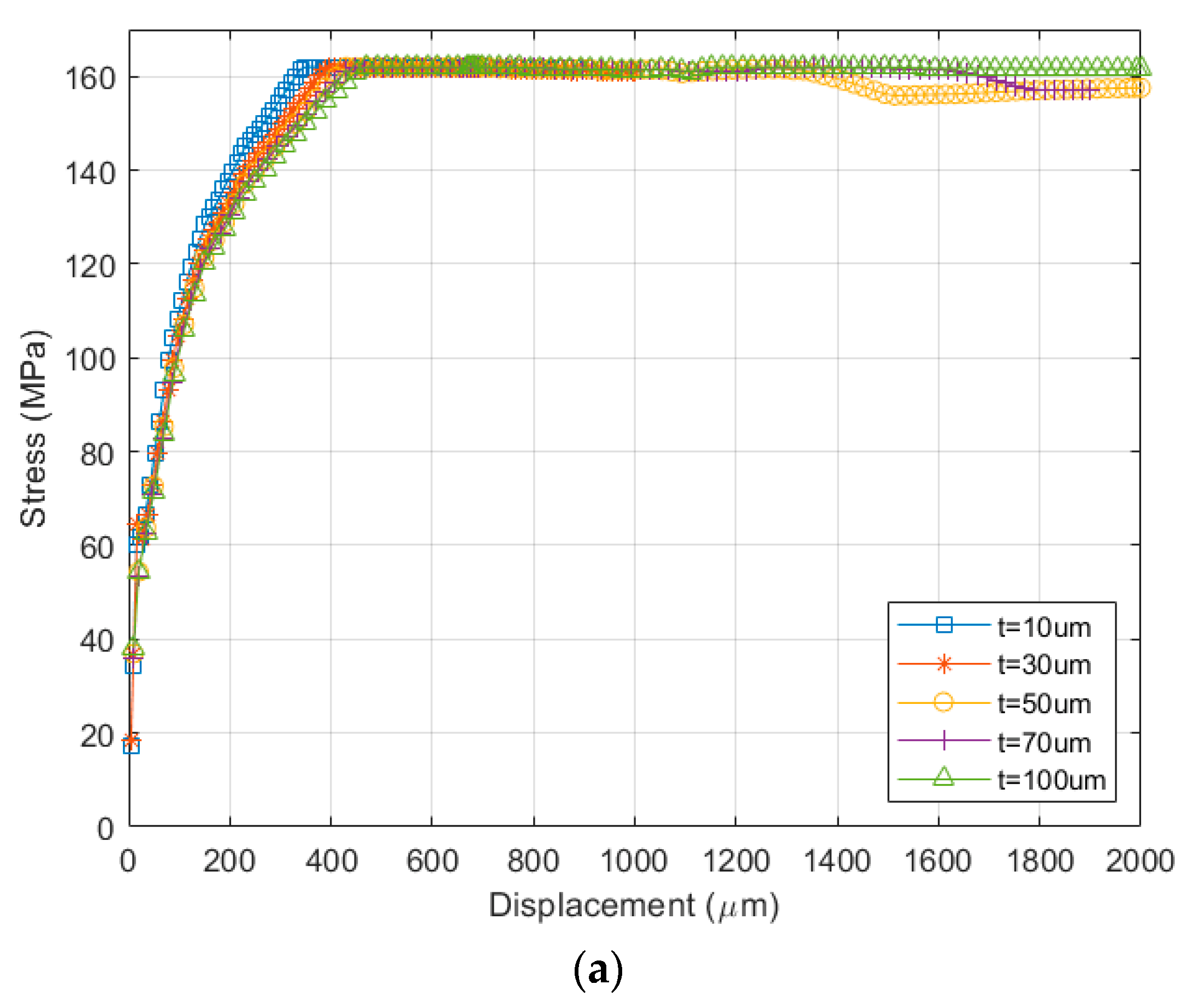
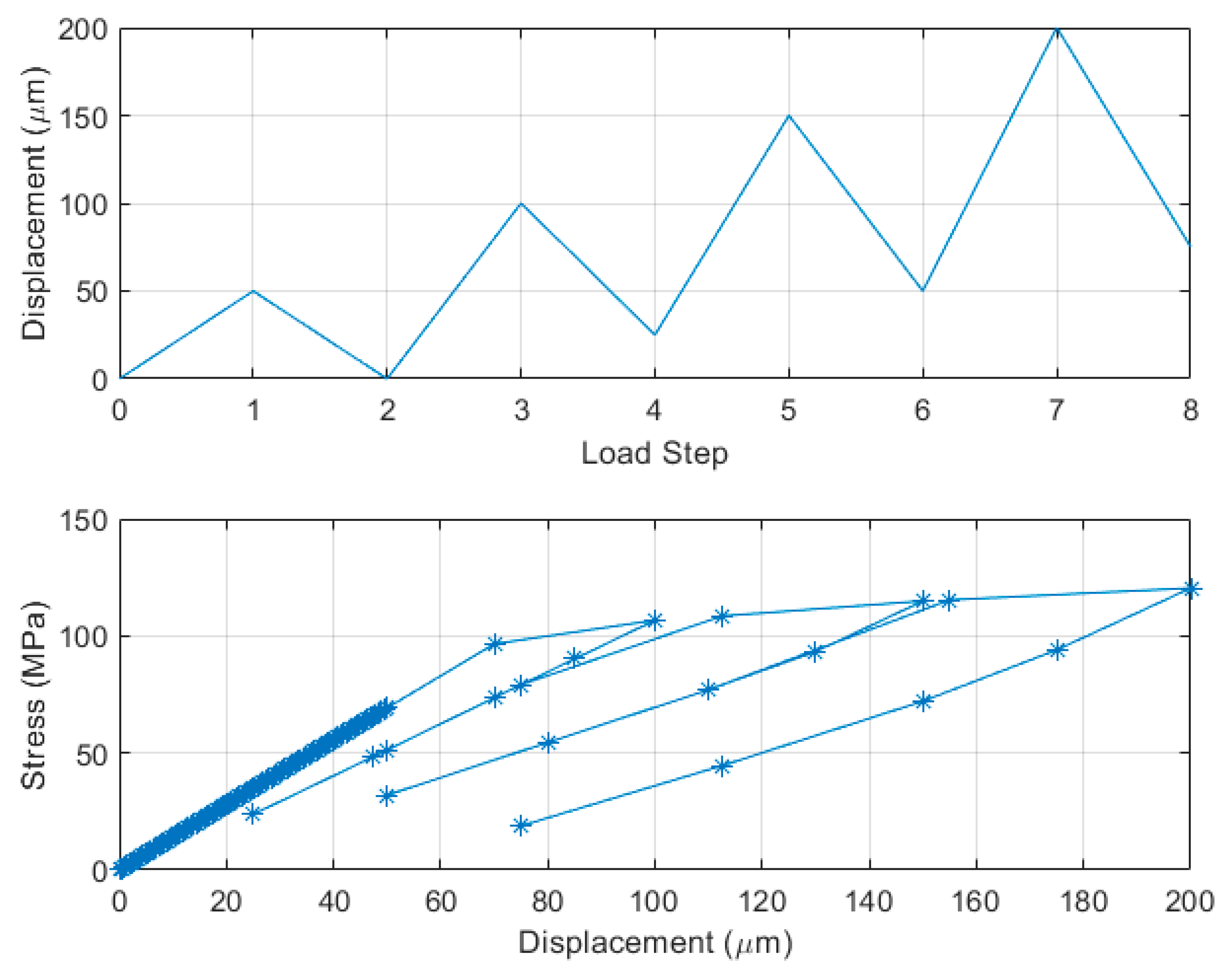
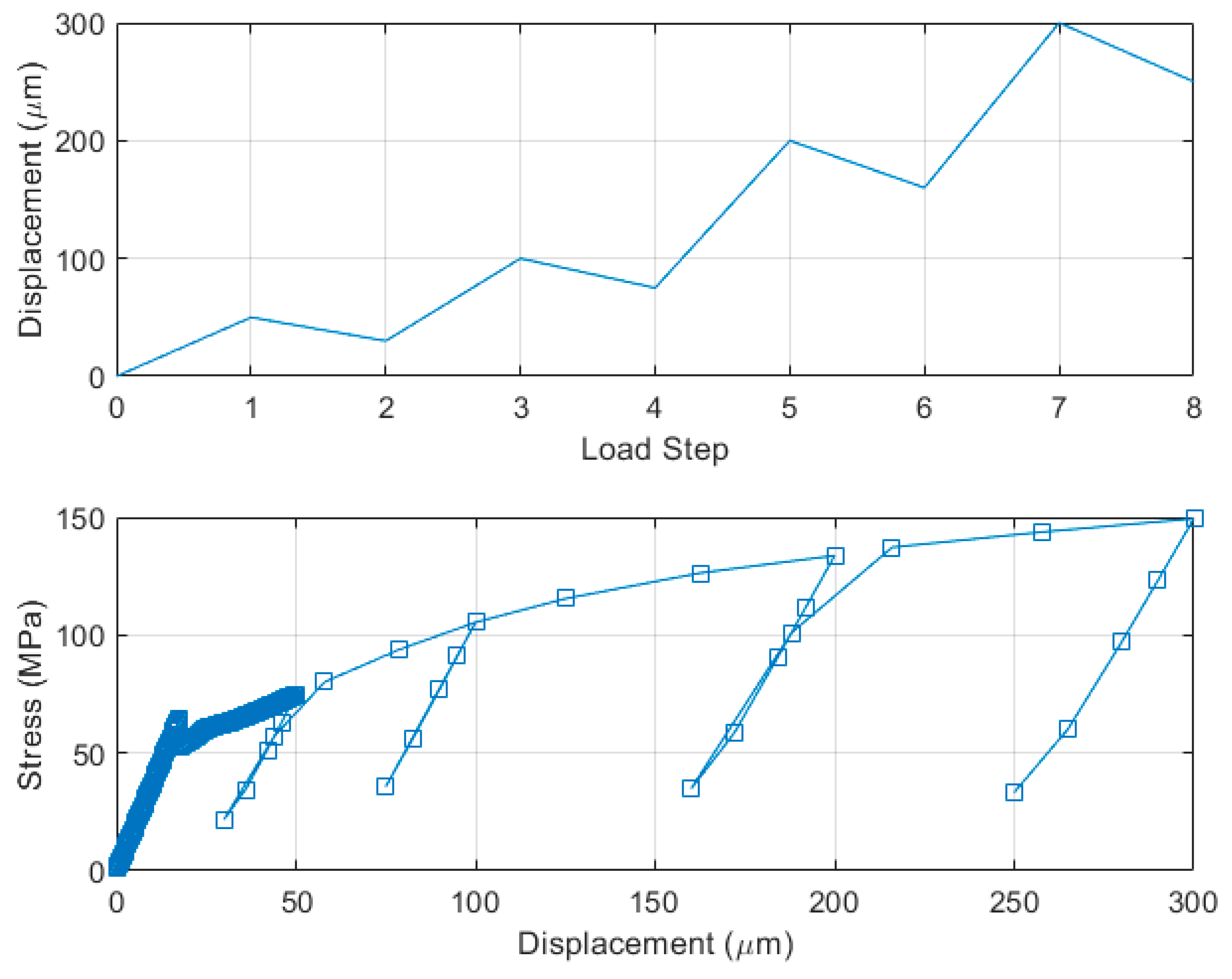
4. FEM Modeling and Simulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Velten, T.; Ruf, H.H.; Barrow, D.; Aspragathos, N.; Lazarou, P.; Jung, E.; Malek, C.K.; Richter, M.; Kruckow, J.; Wackerle, M. Packaging of bio-MEMS: Strategies, technologies, and applications. IEEE Trans. Adv. Packag. 2005, 28, 533–546. [Google Scholar] [CrossRef]
- Seok, S. Polymer-Based Biocompatible Packaging for Implantable Devices: Packaging Method, Materials, and Reliability Simulation. Micromachines 2021, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, D.S.; Kim, I.G.; Sohn, D.W.; Park, J.Y.; Choi, B.K.; Kim, S.W. Evaluation of the biocompatibility of a coating mate-rial for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, T.; Zhao, S.; Xiong, S.; Peng, B.; Humayun, M.S. Chronically implantable package based on alumina ceramics and titanium with high-density feedthroughs for medical implants. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3382–3385. [Google Scholar]
- Kramar, T.; Michalec, I.; Kovacocy, P. The laser beam welding of titanium grade 2 alloy. GRANT J. 2012, 1, 77–79. [Google Scholar]
- Op de Beeck, M.; O’Callaghan, J.; Qian, K.; Malachowski, K.; Vanfleteren, J.; Van Hoof, C. Biocompatible packaging solutions for implantable electronic systems for medical applications. In Proceedings of the IEEE Circuits and Systems Society Forum on Emerging and Selected Topics, Seoul, Korea, 20 May 2012. [Google Scholar]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P., Jr.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.R.; Miller, J.A.; Reichert, W.M. Polyimides as biomaterials: Preliminary biocompatibility testing. Biomaterials 1993, 14, 627–635. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Jin, Q. Preparation and Characteristics of a Novel Silicone Rubber Nanocomposite Based on Organophilic Montmorillonite. High Perform. Polym. 2006, 18, 325–340. [Google Scholar] [CrossRef]
- Brancato, L.; Weydts, T.; Oosterlinck, W.; Herijgers, P.; Puers, R. Biocompatible packaging of an epicardial accel-erometer for real-time assessment of cardiac motion. Procedia Eng. 2016, 168, 80–83. [Google Scholar] [CrossRef]
- Brancato, L.; Weydts, T.; De Clercq, H.; Dimiaux, T.; Herijgers, P.; Puers, R. Biocompatible Packaging and Testing of an Endocardial Accelerometer for Heart Wall Motion Analysis. Procedia Eng. 2015, 120, 840–844. [Google Scholar] [CrossRef]
- Kirsten, S.; Schubert, M.; Braunschweig, M.; Woldt, G.; Voitsekhivska, T.; Wolter, K.-J. Biocompatible packaging for implantable miniaturized pressure sensor device used for stent grafts: Concept and choice of materials. In Proceedings of the 2014 IEEE 16th Electronics Packaging Technology Conference (EPTC), Singapore, 3–5 December 2014; pp. 719–724. [Google Scholar]
- De Beeck, M.O.; Verplancke, R.; Schaubroeck, D.; Cuypers, D.; Cauwe, M.; Vandecasteele, B.; O’Callaghan, J.; Braeken, D.; Andrei, A.; Firrincieli, A.; et al. Ultra-thin biocompatible implantable chip for bidirectional communication with peripheral nerves. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference, Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Lee, C.D.; Meng, E. Mechanical properties of thin-film Parylene-Metal-Parylene devices. Front. Mech. Eng. 2015, 1, 10. [Google Scholar] [CrossRef]
- Bowman, L.; Meindl, J.D. The packaging of implantable integrated sensors. IEEE Trans. Biomed. Eng. 1986, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, P.E.K. The encapsulation of microelectronic devices for long-term surgical implantation. IEEE Trans. Biomed. Eng. 1976, 23, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, P.E.K. Experimental visual prosthesis. Proc. Inst. Electr. Eng. 1973, 120, 281–298. [Google Scholar] [CrossRef]
- Donaldson, P.E.K. The life of neurological protheses. J. Biomed. Eng. 1981, 3, 294–296. [Google Scholar] [CrossRef]
- Seok, S.; Park, H.; Kim, J. Characterization and Analysis of Metal Adhesion to Parylene Polymer Substrate Using Scotch Tape Test for Peripheral Neural Probe. Micromachines 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Boeser, F.; Ordonez, J.S.; Schuettler, M.; Stieglitz, T.; Plachta, D.T.T. Non-hermetic encapsulation for implantable electronic devices based on epoxy. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 809–812. [Google Scholar] [CrossRef]
- Shamma-Donoghue, S.A.; May, G.A.; Cotter, N.E.; White, R.L.; Simmons, F.B. Thin film microelectrode arrays for a cochlear prosthesis. IEEE Trans. Electron Devices 1982, 29, 136–144. [Google Scholar] [CrossRef]
- Barth, P.W.; Bernard, S.L.; Angell, J.B. Monolithic silicon fabrication technology for flexible circuit and sensor arrays. In Proceedings of the 1984 International Electron Devices Meeting, San Francisco, CA, USA, 9–12 December 1984. [Google Scholar]
- Milisavljevic-Syed, J.; Petrovic, E.; Ćirić, I.; Mančić, M.; Marković, D.; Djordjević, M.L. Tensile Testing for Different Types of Polymers. In Proceedings of the 29th Danubia—Adria Symposium on Advances in Experimental Mechanics, University of Belgrade, Beograd, Serbia, 26–29 September 2012; pp. 266–269. [Google Scholar]
- Park, H.; Choi, W.; Oh, S.; Kim, Y.; Seok, S.; Kim, J. 2022. A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation. Micromachines 2022, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Pyl, L.; Kalteremidou, K.; van Hemelrijck, D. Exploration of specimen geometry and tab configuration for tensile testing exploiting the potential of 3D printing freeform shape continuous carbon fibre-reinforced nylon matrix composites. Polym. Test. 2018, 71, 318–328. [Google Scholar] [CrossRef]

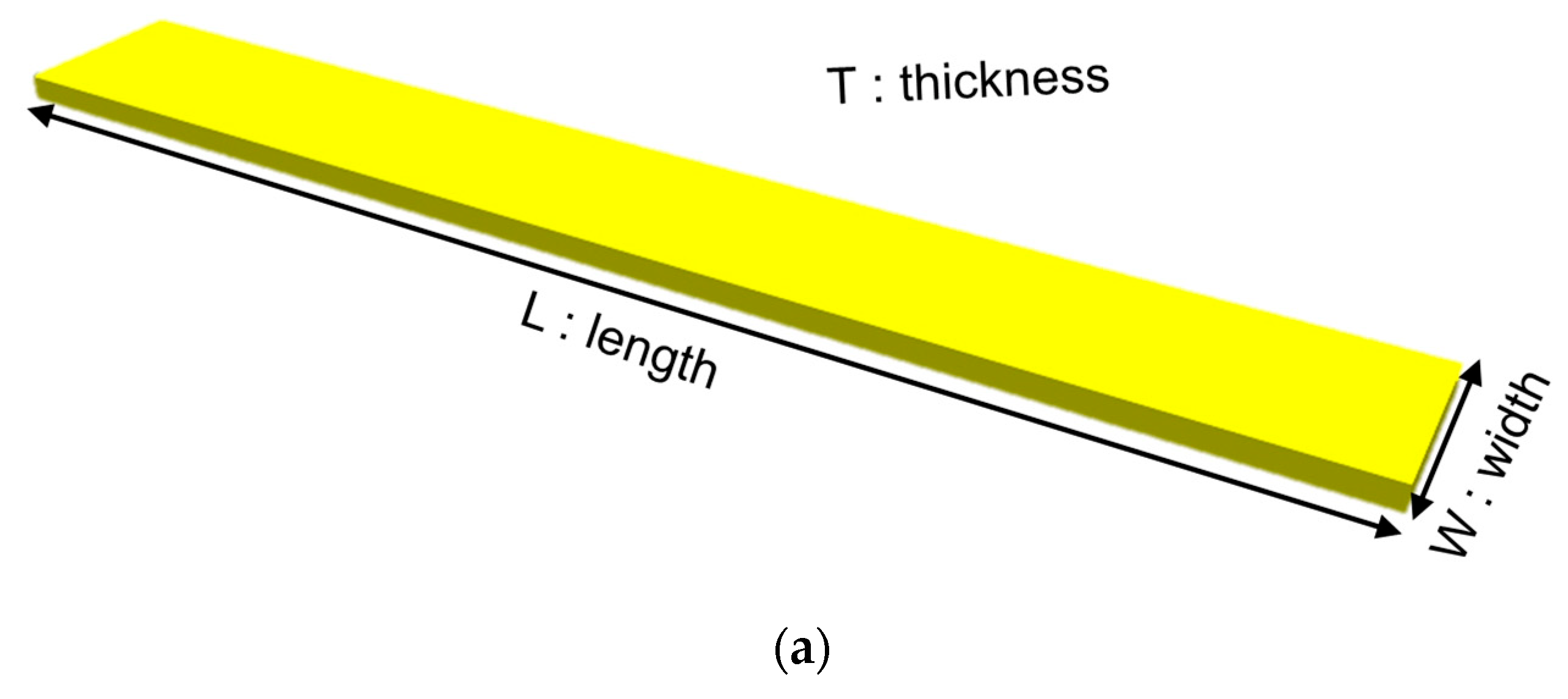


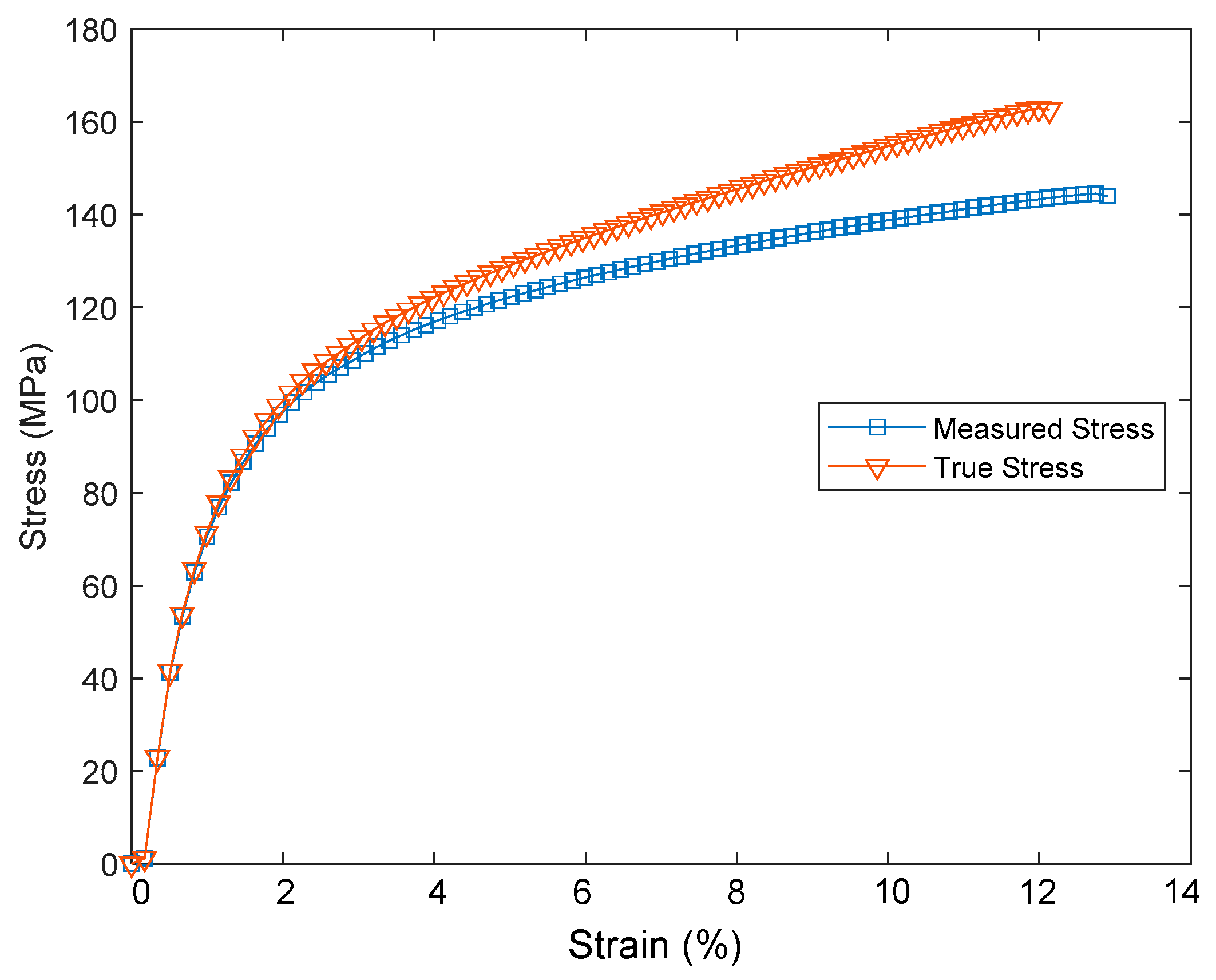
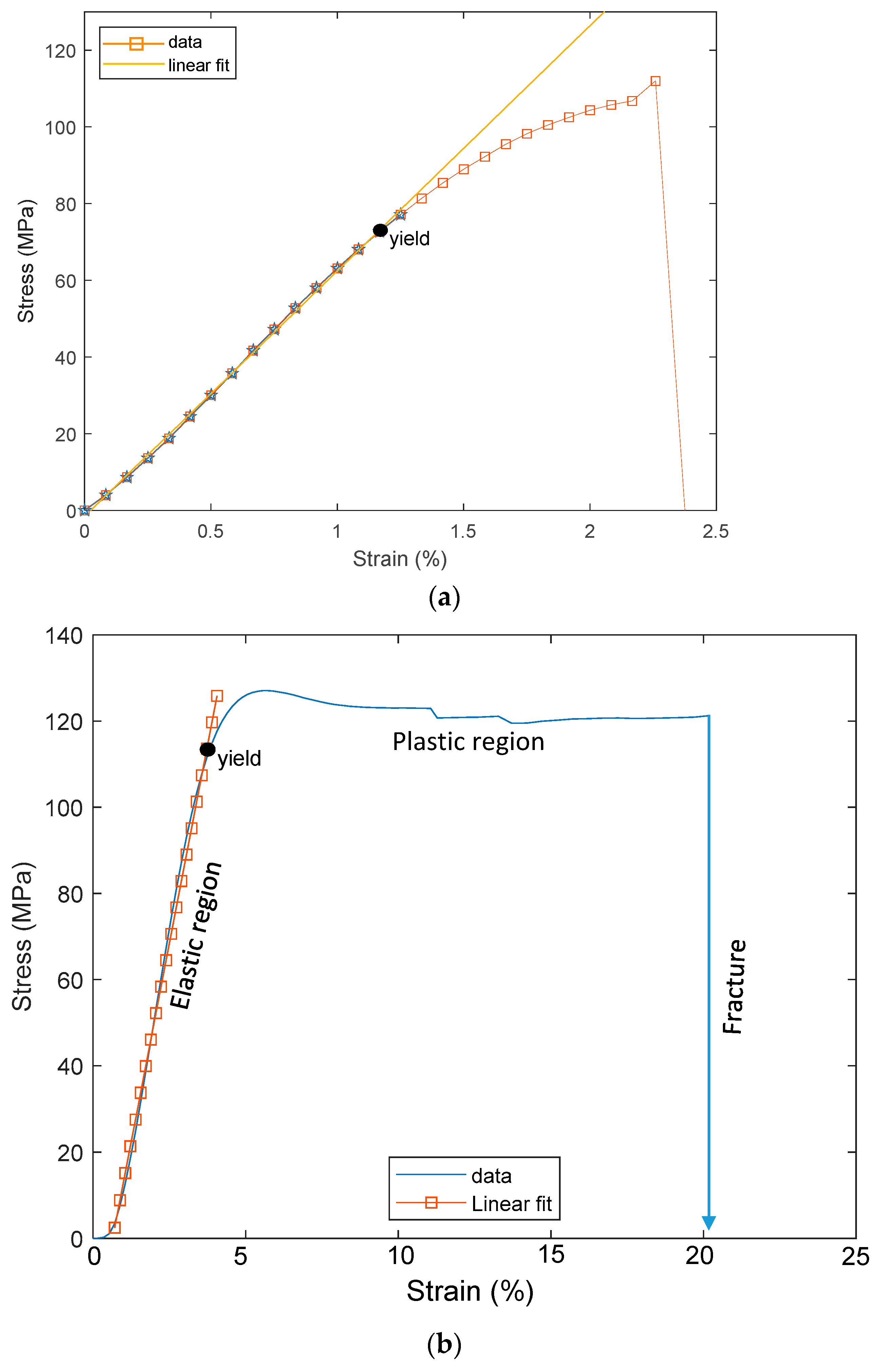





| Samples | Sample Dimension | Elastic Modulus (GPa) | Yield Stress (MPa) | Tensile Strength (MPa) | Tensile Strain (%) |
|---|---|---|---|---|---|
| PSPI I | L = 50 mm W = 5 mm T = 5 µm | 6.3 | 110 | 110 | 2.39 |
| PSPI II | L = 50 mm W = 5 mm T = 25 µm | 3.68 | 125 | 120 | 20 |
| FPCB | L = 103.6 mm W = 4 mm T = 200 µm | 10.5 | 70 | 140 | 12.9 |
| Sample Types | FPCB | PSPI | |
|---|---|---|---|
| Images | |||
| After Tensile Test |  | 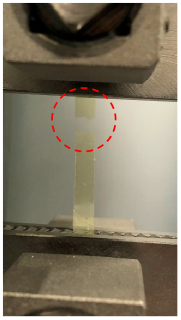 | |
| Microscopic Image of Fractured Region | 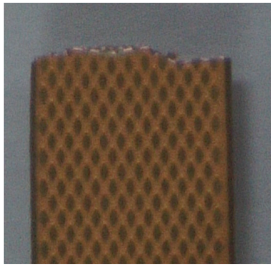 | 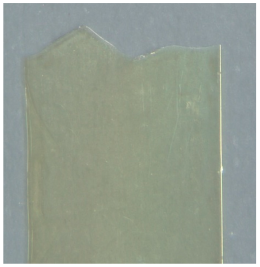 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, S.; Park, H.; Kim, Y.-J.; Kim, J. Mechanical Characterization and Analysis of Different-Type Polyimide Feedthroughs Based on Tensile Test and FEM Simulation for an Implantable Package. Micromachines 2022, 13, 1295. https://doi.org/10.3390/mi13081295
Seok S, Park H, Kim Y-J, Kim J. Mechanical Characterization and Analysis of Different-Type Polyimide Feedthroughs Based on Tensile Test and FEM Simulation for an Implantable Package. Micromachines. 2022; 13(8):1295. https://doi.org/10.3390/mi13081295
Chicago/Turabian StyleSeok, Seonho, HyungDal Park, Yong-Jun Kim, and Jinseok Kim. 2022. "Mechanical Characterization and Analysis of Different-Type Polyimide Feedthroughs Based on Tensile Test and FEM Simulation for an Implantable Package" Micromachines 13, no. 8: 1295. https://doi.org/10.3390/mi13081295
APA StyleSeok, S., Park, H., Kim, Y.-J., & Kim, J. (2022). Mechanical Characterization and Analysis of Different-Type Polyimide Feedthroughs Based on Tensile Test and FEM Simulation for an Implantable Package. Micromachines, 13(8), 1295. https://doi.org/10.3390/mi13081295






