Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thiolation of Chitosan
2.2. Development of Dissolvable Microneedle Patches
2.3. Simvastatin Loading in dMNPs
2.4. Optical Microscopic Evaluation of dMNPs
2.5. Microneedle Patch Thickness
2.6. Tensile Strength and Percentage Elongation
2.7. Moisture Contents (%)
2.8. Scanning Electron Microscopy
2.9. In Vitro Penetration Study
2.10. Fourier Transform Infrared Spectroscopy
2.11. Differential Scanning Calorimetry
2.12. Thermogravimetric Analysis
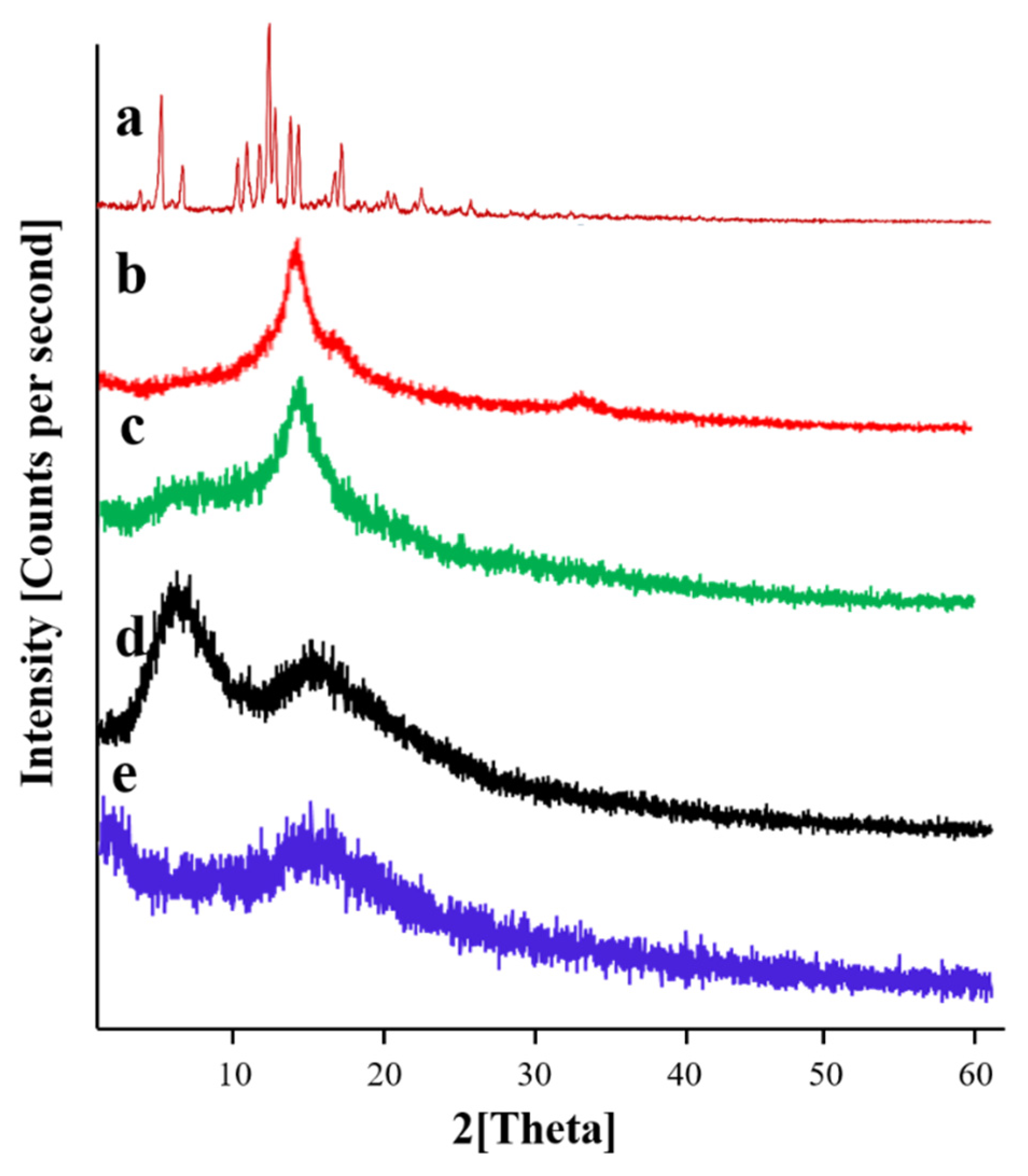
2.13. Powder X-ray Diffraction Studies
2.14. Skin Irritation Studies of dMNPs
2.15. Simvastatin Loading Efficiency (%)
2.16. Histopathological Examination
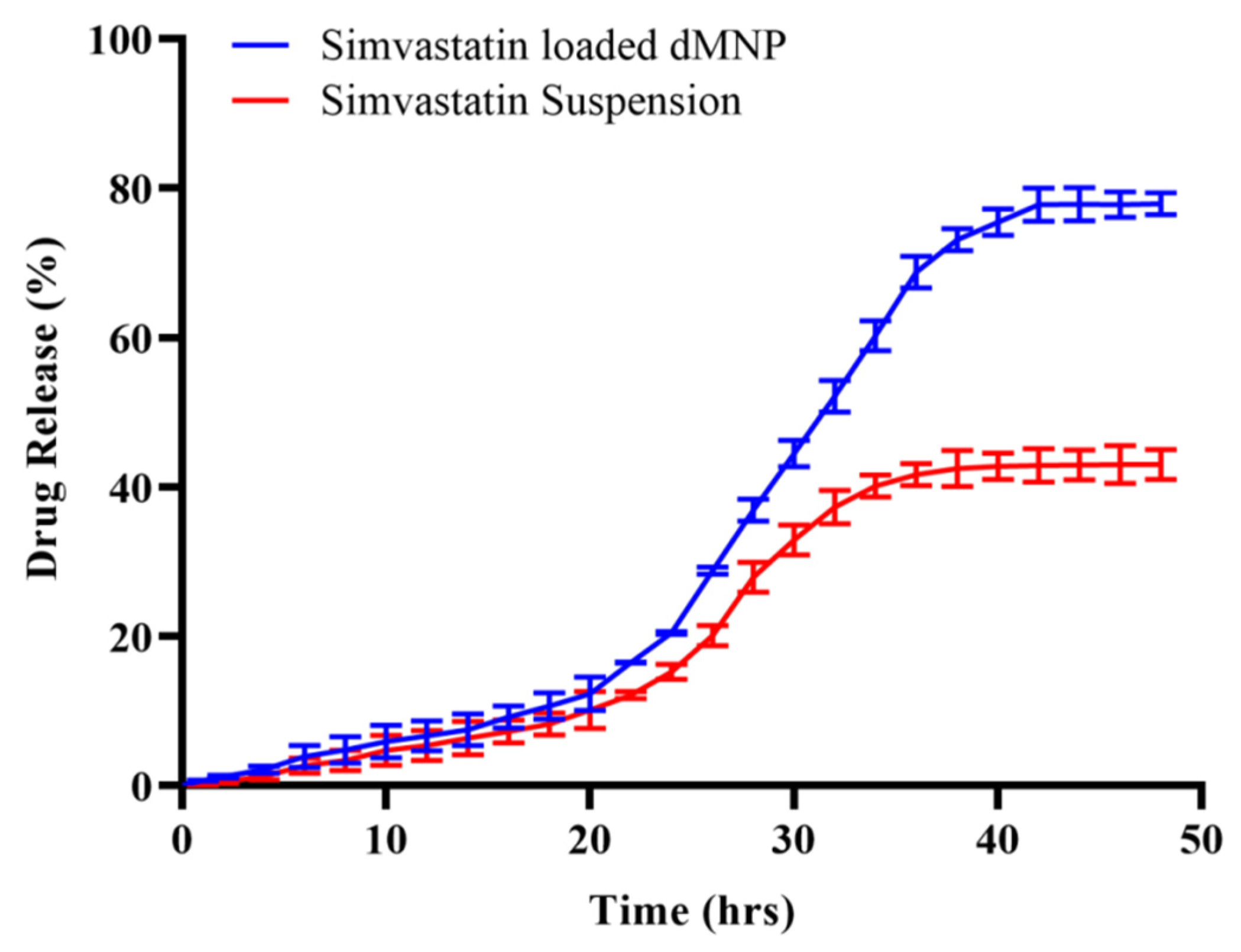
2.17. In Vitro Release Study
2.18. Pharmacokinetic Evaluation
2.19. Statistics
3. Results and Discussion
3.1. Thiolation of Chitosan and Development of Dissolvable Microneedle Patches
3.2. Optical Microscopic Evaluation of dMNPs
3.3. Microneedle Patch Thickness
3.4. Tensile Strength and Percentage Elongation
3.5. Moisture Contents (%)
3.6. Scanning Electron Microscopy
3.7. In Vitro Penetration Study
3.8. Fourier Transform Infrared Spectroscopy
3.9. Differential Scanning Calorimetry
3.10. Thermogravimetric Analysis
3.11. Powder X-ray Diffraction Studies
3.12. Skin Irritation Studies of dMNPs
3.13. Simvastatin Loading Efficiency (%)
3.14. Histopathological Examination
3.15. In Vitro Release Study
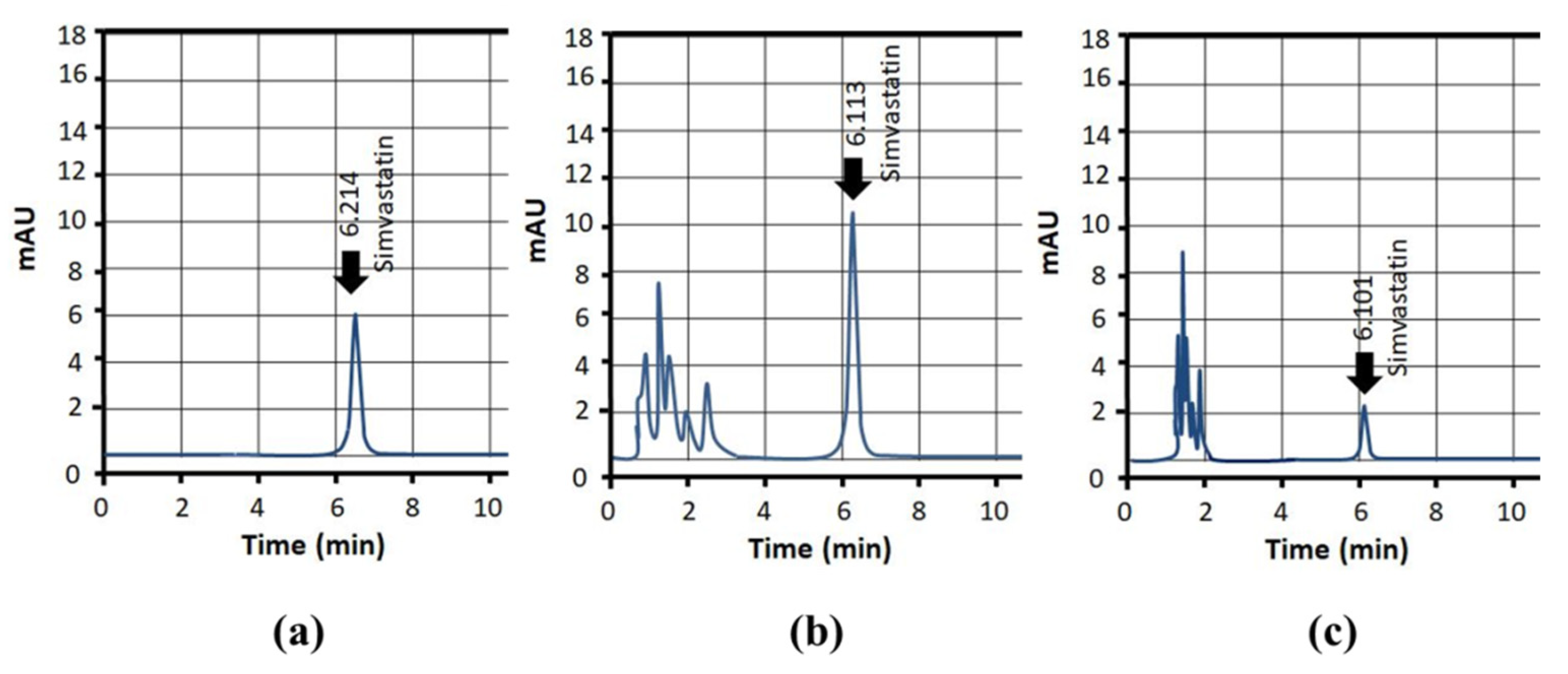
3.16. Pharmacokinetic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Häfeli, U.O. Magnetically modulated therapeutic systems. Int. J. Pharm. 2004, 277, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic liquid transdermal delivery system: Progress, prospects, and challenges. J. Mol. Liq. 2022, 351, 118643. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Administration strategies for proteins and peptides. Int. J. Pharm. 2014, 477, 578–589. [Google Scholar] [CrossRef]
- Sayiner, H.S.; Kandemirli, F.; Dalgic, S.S.; Monajjemi, M.; Mollaamin, F. Carbazochrome carbon nanotube as drug delivery nanocarrier for anti-bleeding drug: Quantum chemical study. J. Mol. Model. 2021, 28, 11. [Google Scholar] [CrossRef]
- Sala, M.; Elaissari, A.; Fessi, H. Advances in psoriasis physiopathology and treatments: Up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J. Control. Release Off. J. Control. Release Soc. 2016, 239, 182–202. [Google Scholar] [CrossRef]
- Margetts, L.; Sawyer, R. Transdermal drug delivery: Principles and opioid therapy. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 171–176. [Google Scholar] [CrossRef]
- Badri, W.; Eddabra, R.; Fessi, H.; Elaissari, A. Biodegradable Polymer Based Nanoparticles: Dermal and Transdermal Drug Delivery. J. Colloid Sci. Biotechnol. 2014, 3, 141–149. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Ripolin, A.; Quinn, J.; Larrañeta, E.; Vicente-Perez, E.M.; Barry, J.; Donnelly, R.F. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 2017, 521, 92–101. [Google Scholar] [CrossRef]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Badnikar, K.; Jayadevi, S.N.; Pahal, S.; Vemula, P.K.; Nayak, M.M.; Subramanyam, D.N. Microscale engineering of hollow microneedle tips: Design, manufacturing, optimization and validation. Drug Deliv. Transl. Res. 2022, 12, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Moffatt, K.; Donnelly, R.F. 9—Long-lasting drug delivery systems based on microneedles. In Long-Acting Drug Delivery Systems; Larrañeta, E., Raghu Raj Singh, T., Donnelly, R.F., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 249–287. [Google Scholar]
- Li, W.X.; Zhang, X.P.; Chen, B.Z.; Fei, W.M.; Cui, Y.; Zhang, C.Y.; Guo, X.D. An update on microneedle-based systems for diabetes. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Meng, F.; Hasan, A.; Mahdi Nejadi Babadaei, M.; Hashemi Kani, P.; Jouya Talaei, A.; Sharifi, M.; Cai, T.; Falahati, M.; Cai, Y. Polymeric-based microneedle arrays as potential platforms in the development of drugs delivery systems. J. Adv. Res. 2020, 26, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, E.; Ozer, O.; Prausnitz, M.R. Fabrication of pure-drug microneedles for delivery of montelukast sodium. Drug Deliv. Transl. Res. 2022, 12, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Howells, O.; Blayney, G.J.; Gualeni, B.; Birchall, J.C.; Eng, P.F.; Ashraf, H.; Sharma, S.; Guy, O.J. Design, fabrication, and characterisation of a silicon microneedle array for transdermal therapeutic delivery using a single step wet etch process. Eur. J. Pharm. Biopharm. 2022, 171, 19–28. [Google Scholar] [CrossRef]
- Sadeqi, A.; Kiaee, G.; Zeng, W.; Rezaei Nejad, H.; Sonkusale, S. Hard polymeric porous microneedles on stretchable substrate for transdermal drug delivery. Sci. Rep. 2022, 12, 1853. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Lee, I.C.; He, J.-S.; Tsai, M.-T.; Lin, K.-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J. Mater. Chem. B 2015, 3, 276–285. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhang, S.; Zhao, X.; Gao, Y. Dissolving Microneedles Loaded With Etonogestrel Microcrystal Particles for Intradermal Sustained Delivery. J. Pharm. Sci. 2018, 107, 1037–1045. [Google Scholar] [CrossRef]
- Kathuria, H.; Lim, D.; Cai, J.; Chung, B.G.; Kang, L. Microneedles with Tunable Dissolution Rate. ACS Biomater. Sci. Eng. 2020, 6, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, M.-R.; Park, J.-H. Polymer microneedles for transdermal drug delivery. J. Drug Target. 2013, 21, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 30, 391–396. [Google Scholar] [CrossRef]
- Kast, C.E.; Bernkop-Schnürch, A. Thiolated polymers—Thiomers: Development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Kramadhati, S.; Thyagarajan, K. Optical properties of pure and doped (KnO3 & MgCl2) polyvinyl alcohol polymer thin films. Int. J. Eng. Res. Dev. 2013, 6, 15–18. [Google Scholar]
- Noushini, A.; Samali, B.; Vessalas, K. Effect of polyvinyl alcohol (PVA) fibre on dynamic and material properties of fibre reinforced concrete. Constr. Build. Mater. 2013, 49, 374–383. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.; Chu, G.S.; Kim, K.S.; Sung, J.H.; Kim, B. Transdermal delivery of cosmetic ingredients using dissolving polymer microneedle arrays. Biotechnol. Bioprocess Eng. 2015, 20, 543–549. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Koutsonanos, D.G.; Del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pani, A.; Di Rocco, A.; Menichelli, D.; Gazzaniga, G.; Farcomeni, A.; D’Erasmo, L.; Angelico, F.; Del Ben, M.; Baratta, F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 2022, 88, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Khan, M.I.; Siddique, M.I.; Sarwar, H.S.; Shahnaz, G.; Hussain, S.Z.; Bukhari, N.I.; Hussain, I.; Sohail, M.F. Fabrication and Characterization of Thiolated Chitosan Microneedle Patch for Transdermal Delivery of Tacrolimus. AAPS PharmSciTech 2020, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Nanjappa, S.H.; Honnavar, S.; Salwa, M.; Murthy, S.N. Rapidly Dissolving Microneedle Patches for Transdermal Iron Replenishment Therapy. J. Pharm. Sci. 2018, 107, 1642–1647. [Google Scholar] [CrossRef]
- Habib, R.; Azad, A.K.; Akhlaq, M.; Al-Joufi, F.A.; Shahnaz, G.; Mohamed, H.R.H.; Naeem, M.; Almalki, A.S.A.; Asghar, J.; Jalil, A.; et al. Thiolated Chitosan Microneedle Patch of Levosulpiride from Fabrication, Characterization to Bioavailability Enhancement Approach. Polymers 2022, 14, 415. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Ye, Y.; Yang, Z.; Ruan, Z.; Jin, N. Fabrication of sponge-forming microneedle patch for rapidly sampling interstitial fluid for analysis. Biomed. Microdevices 2019, 21, 63. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Ghareeb, M.M.; Hamzah, Z.O.; Kadhim, Z.M. Formulation and characterization of Flurbiprofen nanoparticles loaded microneedles. Kerbala J. Pharm. Sci. 2021, 1, 90–107. [Google Scholar]
- Obaidat, R.; BaniAmer, F.; Assaf, S.M.; Yassin, A. Fabrication and Evaluation of Transdermal Delivery of Carbamazepine Dissolving Microneedles. AAPS PharmSciTech 2021, 22, 253. [Google Scholar] [CrossRef]
- Arshad, M.S.; Hassan, S.; Hussain, A.; Abbas, N.; Kucuk, I.; Nazari, K.; Ali, R.; Ramzan, S.; Alqahtani, A.; Andriotis, E.G.; et al. Improved transdermal delivery of cetirizine hydrochloride using polymeric microneedles. DARU J. Pharm. Sci. 2019, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Azukizawa, H.; Matsuo, K.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Suzuki, H.; Katayama, I.; Okada, N.; Nakagawa, S. Development and Clinical Study of a Self-Dissolving Microneedle Patch for Transcutaneous Immunization Device. Pharm. Res. 2013, 30, 2664–2674. [Google Scholar] [CrossRef]
- Dey, S.; De, A.; Mandal, S.K.; Pradhan, P.K.; Patel, C.; Shah, S.; Patel, B. Development and Validation of Rp-Hplc Method for the Estimation of Simvastatin in Bulk and Pharmaceutical Dosage Form. Indo Am. J. Pharm. Res. 2013, 3, 7376–7384. [Google Scholar]
- Thomas, J.R. Effects of age and diet on rat skin histology. Laryngoscope 2005, 115, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Yu, X.; Huang, K.; Zheng, T.; Cheng, X.; Zeng, S.; Liu, X. Hematoxylin and eosin staining of intact tissues via delipidation and ultrasound. Sci. Rep. 2018, 8, 12259. [Google Scholar] [CrossRef]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Dathathri, E.; Lal, S.; Mittal, M.; Thakur, G.; De, S. Fabrication of low-cost composite polymer-based micro needle patch for transdermal drug delivery. Appl. Nanosci. 2020, 10, 371–377. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating Formulations for Microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef]
- Mao, J.; Wang, H.; Xie, Y.; Fu, Y.; Li, Y.; Liu, P.; Du, H.; Zhu, J.; Dong, L.; Hussain, M.; et al. Transdermal delivery of rapamycin with poor water-solubility by dissolving polymeric microneedles for anti-angiogenesis. J. Mater. Chem. B 2020, 8, 928–934. [Google Scholar] [CrossRef]
- Yavuz, B.; Chambre, L.; Harrington, K.; Kluge, J.; Valenti, L.; Kaplan, D.L. Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. ACS Appl. Bio Mater. 2020, 3, 5375–5382. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Ling, M.-H.; Lai, K.-Y.; Pramudityo, E. Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Amodwala, S.; Kumar, P.; Thakkar, H.P. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: A patient friendly approach to manage arthritis. Eur. J. Pharm. Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef] [PubMed]

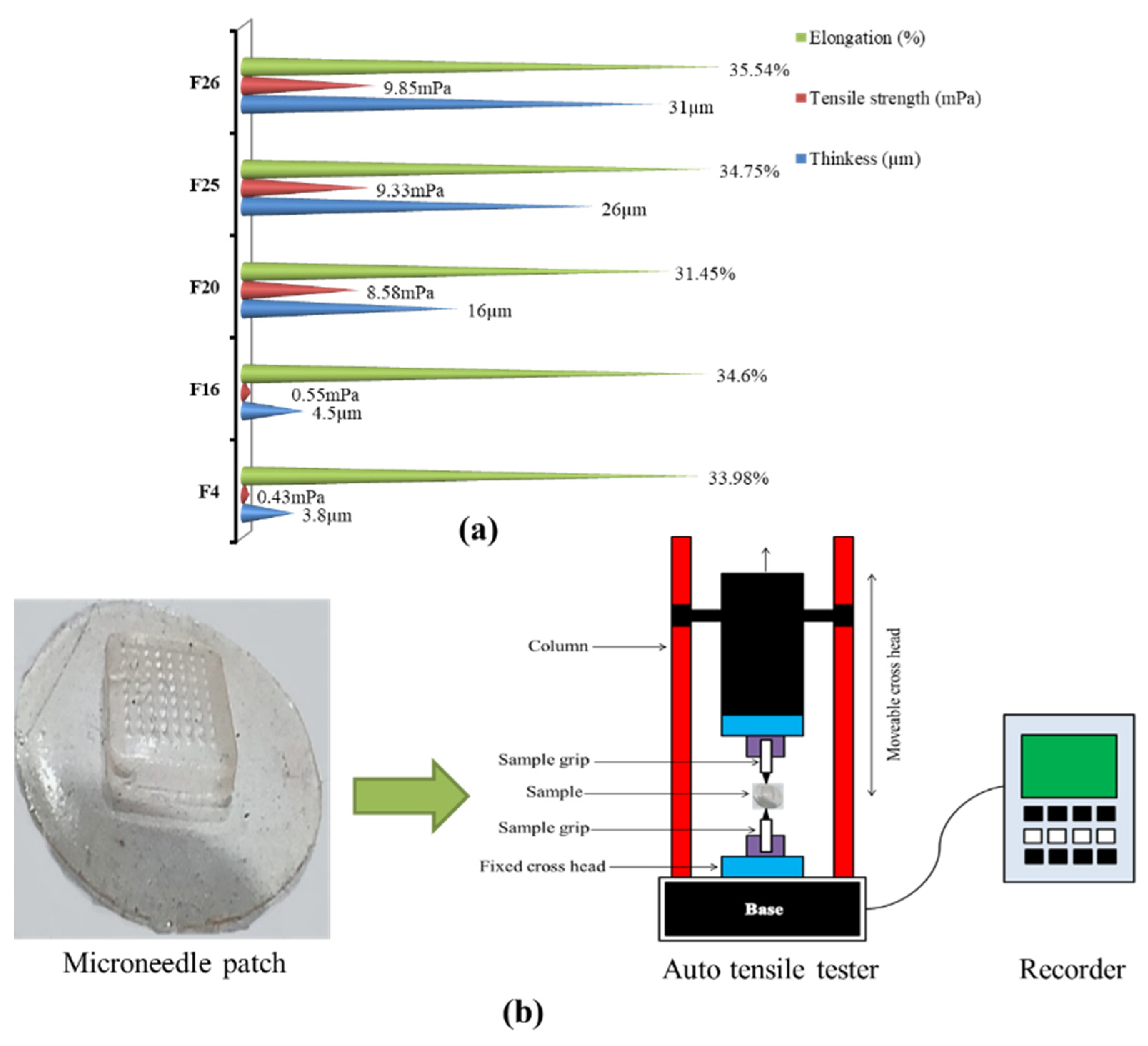

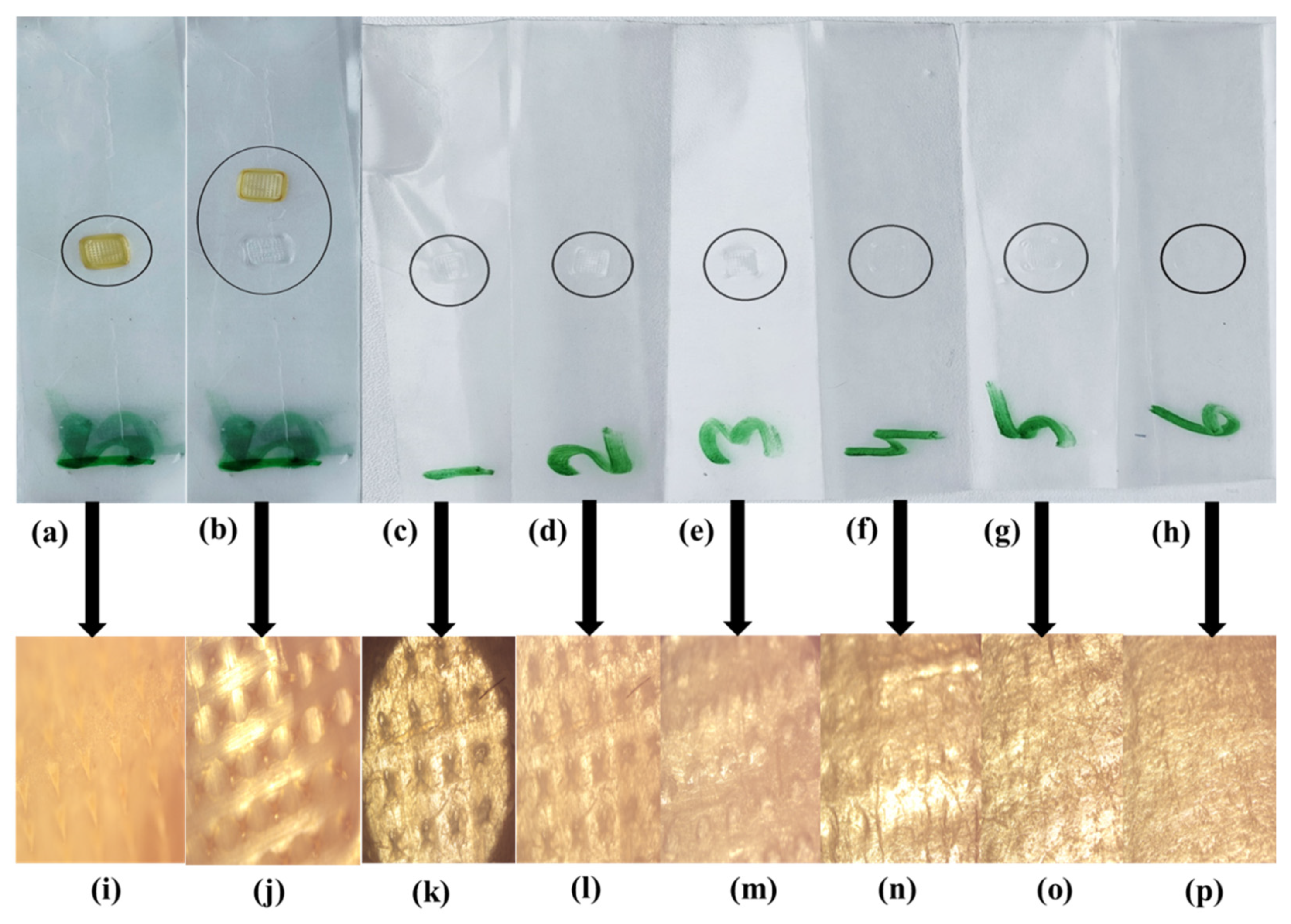






| Polymers (%) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | F19 | F20 | F21 | F22 | F23 | F24 | F25 | F26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | 0.5 | 1 | 1.5 | 2 | - | - | - | - | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 |
| PVP | - | - | 5 | - | 10 | - | 10 | - | 5 | - | 10 | - | 5 | - | 10 | - | 5 | 5 | 10 | 10 | 5 | 10 | 10 | 5 | ||
| PVA | - | - | - | - | - | 5 | - | 10 | - | 10 | - | 5 | - | 10 | - | 5 | - | 10 | 5 | 5 | 10 | 10 | 10 | 5 | 5 | 10 |
| Sr. No. | Description | Thickness (mm) | Thickness (mm) | Thickness (mm) | Average Thickness (mm) |
|---|---|---|---|---|---|
| 1 | Thickness of two glass slides | 2.20 | 2.21 | 2.19 | 2.20 |
| 2 | Thickness of slides with 9 layers of Paraffin M films | 3.48 | 3.47 | 3.46 | 3.47 |
| 3 | Thickness of one Paraffin film M layer | 0.142 | 0.140 | 0.141 | 0.141 |
| Polymer | Functional Groups | Peaks |
|---|---|---|
| PVP | C=O | 1642.13 cm−1 |
| CH2 | 2981.67 cm−1 | |
| CH | 1290.14 cm−1 | |
| CH | 1425.13 cm−1 | |
| C-N | 1313.11 cm−1 | |
| PVA | OH | 3296 cm−1 |
| CH | 2921 cm−1 | |
| OH | 1411 cm−1 | |
| C-O- | 1090 cm−1 | |
| Thiolated Chitosan | OH | 3352.5 cm−1 |
| NH | 3201 cm−1 | |
| Acylamino | 1631 cm−1 | |
| Amino group with thioglycolic acid | 1605 cm−1 | |
| Simvastatin | –OH | 3550 cm−1 |
| –CH | 3747 cm−1 | |
| –C-O- | 2950 cm−1 | |
| –C=O | 1740 cm−1 | |
| Simvastatin loaded dMNP (F26) | –C-O- | 1644.35 cm−1 |
| –CH | 2950 cm−1 |
| Sr. No. | Drug Release Behaviour | Reference |
|---|---|---|
| 1 | In vitro drug release was found to be 65% in 48 h. | Habib R. et al. (2022) [37] |
| 2 | dMNPs made of PVP matrix resulted in drug release of 80%. | Mao J. et al. (2020) [51] |
| 3 | Drug release reported in this study is 82.5%. | Ahmad Z. et al. (2020) [35] |
| 4 | Drug release was found to be 82.7%. | Yavuz B. et al. (2020) [52] |
| 5 | Bovine serum albumin release was up to 95% from MNPs. | Chen M-C. et al. (2012) [53] |
| Models | Parameters | F26 | Simvastatin Solution |
|---|---|---|---|
| Zero Order | R2 | 0.989 | 0.91 |
| T25 | 14.842 | 24.728 | |
| T50 | 29.683 | 49.457 | |
| T75 | 44.525 | 74.185 | |
| First Order | R2 | 0.8916 | 0.8732 |
| T25 | 11.171 | 22.500 | |
| T50 | 26.915 | 41.213 | |
| T75 | 53.830 | 62.425 | |
| Higuchi Model | R2 | 0.89 | 0.93 |
| T25 | 6.041 | 22.500 | |
| T50 | 24.163 | 54.213 | |
| T75 | 54.367 | 72.425 | |
| Korsemeyer Peppas | R2 | 0.977 | 0.93 |
| N | 1.436 | 0.987 |
| Parameters | F26 (Mean ± S.D) | Table Simva Solution (Mean ± S.D.) |
|---|---|---|
| Cmax (µg/mL) | 1.97 ± 0.002 | 2.55 ± 0.089 |
| tmax (h) | 9 ± 0.453 | 3 ± 0.786 |
| t1/2 (h) | 10.11 ± 0.342 | 2.31 ± 1.23 |
| AUC 0-t (µg/mL·h) | 46.243 ± 0.123 | 14.20 ± 0.871 |
| AUMC 0-∞ (µg/mL·h2) | 48.941 ± 0.223 | 15.19 ± 0.342 |
| MRT (h) | 19.91 ± 0.334 | 5.91 ± 0.233 |
| Clearance (mg)/(μg/mL)/h | 0.408 ± 0.033 | 1.314 ± 0.223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulcaif; Zafar, N.; Mahmood, A.; Sarfraz, R.M.; Elaissari, A. Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance. Micromachines 2022, 13, 1304. https://doi.org/10.3390/mi13081304
Zulcaif, Zafar N, Mahmood A, Sarfraz RM, Elaissari A. Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance. Micromachines. 2022; 13(8):1304. https://doi.org/10.3390/mi13081304
Chicago/Turabian StyleZulcaif, Nadiah Zafar, Asif Mahmood, Rai Muhammad Sarfraz, and Abdelhamid Elaissari. 2022. "Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance" Micromachines 13, no. 8: 1304. https://doi.org/10.3390/mi13081304
APA StyleZulcaif, Zafar, N., Mahmood, A., Sarfraz, R. M., & Elaissari, A. (2022). Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance. Micromachines, 13(8), 1304. https://doi.org/10.3390/mi13081304








