Supercritical Fluids and Nanoparticles in Cancer Therapy
Abstract
:1. Introduction
2. Production of Nanoparticles
2.1. Carrier-Free Nanoparticles
2.2. Coprecipitated Carrier and API Nanoparticles
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fages, J.; Lochard, H.; Letourneau, J.J.; Sauceau, M.; Rodier, E. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol. 2004, 141, 219–226. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, A.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; Vaiano, V.; De Marco, I. Supercritical Carbon Dioxide-Based Processes in Photocatalytic Applications. Molecules 2021, 26, 2640. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Subramaniam, B.; Rajewski, R.A.; Snavely, K. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 1997, 86, 885–890. [Google Scholar] [CrossRef]
- Cooper, A.I. Polymer synthesis and processing using supercritical carbon dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Extraction of rotenoids from Derris elliptica using supercritical CO2. J. Chem. Technol. Biotechnol. 2018, 93, 3656–3660. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical extraction of volatile and fixed oils from Petroselinum crispum L. seeds: Chemical composition and biological activity. Nat. Prod. Res. 2022, 36, 1883–1888. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Zahran, F.; Martín, D.; Cabañas, A.; Pando, C. Preparation of 5-fluorouracil microparticles and 5-fluorouracil/poly(L-lactide) composites by a supercritical CO2 antisolvent process. J. Supercrit. Fluids 2019, 143, 64–71. [Google Scholar] [CrossRef]
- Palazzo, I.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Zein/luteolin microparticles formation using a supercritical fluids assisted technique. Powder Technol. 2019, 356, 899–908. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 133, 239–252. [Google Scholar] [CrossRef]
- Ajiboye, A.L.; Trivedi, V.; Mitchell, J.C. Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv. Transl. Res. 2018, 8, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Schiavo Rappo, E.; Cardea, S. Flexible supercritical CO2-assisted process for poly(methyl methacrylate) structure formation. Polym. Eng. Sci. 2006, 46, 188–197. [Google Scholar] [CrossRef]
- Cardea, S.; Sessa, M.; Reverchon, E. Supercritical phase inversion to form drug-loaded poly(vinylidene fluoride-co-hexafluoropropylene) membranes. Ind. Eng. Chem. Res. 2010, 49, 2783–2789. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A versatile supercritical assisted process for the one-shot production of liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Sharifi, F.; Zhou, R.; Lim, C.; Jash, A.; Abbaspourrad, A.; Rizvi, S.S.H. Generation of liposomes using a supercritical carbon dioxide eductor vacuum system: Optimization of process variables. J. CO2 Util. 2019, 29, 163–171. [Google Scholar] [CrossRef]
- García-Casas, I.; Crampon, C.; Montes, A.; Pereyra, C.; Martínez de la Ossa, E.J.; Badens, E. Supercritical CO2 impregnation of silica microparticles with quercetin. J. Supercrit. Fluids 2019, 143, 157–161. [Google Scholar] [CrossRef]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; De Marco, I. Supercritical impregnation of mesoglycan into calcium alginate aerogel for wound healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Tsai, W.C.; Wang, Y. Progress of supercritical fluid technology in polymerization and its applications in biomedical engineering. Prog. Polym. Sci. 2019, 98, 101161. [Google Scholar] [CrossRef]
- Polloni, A.E.; Veneral, J.G.; Rebelatto, E.A.; de Oliveira, D.; Oliveira, J.V.; Araújo, P.H.H.; Sayer, C. Enzymatic ring opening polymerization of ω-pentadecalactone using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 119, 221–228. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Barani, M.; Hosseinikhah, S.M.; Rahdar, A.; Farhoudi, L.; Arshad, R.; Cucchiarini, M.; Pandey, S. Nanotechnology in bladder cancer: Diagnosis and treatment. Cancers 2021, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Yezhelyev, M.V.; Gao, X.; Xing, Y.; Al-Hajj, A.; Nie, S.; O’Regan, R.M. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006, 7, 657–667. [Google Scholar] [CrossRef]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef]
- Ghaferi, M.; Amari, S.; Vivek Mohrir, B.; Raza, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Preparation, characterization, and evaluation of cisplatin-loaded polybutylcyanoacrylate nanoparticles with improved in vitro and in vivo anticancer activities. Pharmaceuticals 2020, 13, 44. [Google Scholar] [CrossRef]
- Sukumar, U.K.; Bhushan, B.; Dubey, P.; Matai, I.; Sachdev, A.; Packirisamy, G. Emerging applications of nanoparticles for lung cancer diagnosis and therapy. Int. Nano Lett. 2013, 3, 45. [Google Scholar] [CrossRef]
- Vinhas, R.; Mendes, R.; Fernandes, A.R.; Baptista, P.V. Nanoparticles—Emerging potential for managing leukemia and lymphoma. Front. Bioeng. Biotechnol. 2017, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bathula, S.R.; Yang, Q.; Huang, L. Targeted Nanoparticles Deliver siRNA to Melanoma. J. Investig. Dermatol. 2010, 130, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Zimta, A.A.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 12, 4593. [Google Scholar] [CrossRef]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010, 62, 346–361. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, J.Z.; Chen, J.; Ko, L.; Amanie, J.; Gulavita, S.; Pervez, N.; Yee, D.; Moore, R.; Roa, W. Enhanced radiation sensitivity in prostate cancer by gold-nanoparticles. Clin. Investig. Med. 2008, 31, E160–E167. [Google Scholar] [CrossRef]
- Koppolu, B.; Bhavsar, Z.; Wadajkar, A.S.; Nattama, S.; Rahimi, M.; Nwariaku, F.; Nguyen, K.T. Temperature-sensitive polymer-coated magnetic nanoparticles as a potential drug delivery system for targeted therapy of thyroid cancer. J. Biomed. Nanotechnol. 2012, 8, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Ricci, G.; Severini, G.M.; Romano, F.; Biffi, S. Imaging and therapy of ovarian cancer: Clinical application of nanoparticles and future perspectives. Theranostics 2018, 8, 4279–4294. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory Overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm. Res. 2013, 30, 307–324. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef]
- Türk, M. Particle synthesis by rapid expansion of supercritical solutions (RESS): Current state, further perspectives and needs. J. Aerosol Sci. 2022, 161, 105950. [Google Scholar] [CrossRef]
- Matson, D.W.; Petersen, R.C.; Smith, R.D. Formation of Silica Powders from the Rapid Expansion of Supercritical Solutions. Adv. Ceram. Mater. 1986, 1, 242–246. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, R.B. Rapid expansion of supercritical solution with solid cosolvent (RESS-SC) process: Formation of griseofulvin nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 7380–7387. [Google Scholar] [CrossRef]
- Sane, A.; Thies, M.C. The Formation of Fluorinated Tetraphenylporphyrin Nanoparticles via Rapid Expansion Processes: RESS vs RESOLV. J. Phys. Chem. B 2005, 109, 19688–19695. [Google Scholar] [CrossRef]
- Türk, M.; Lietzow, R. Stabilized nanoparticles of phytosterol by rapid expansion from supercritical solution into aqueous solution. AAPS PharmSciTech 2004, 5, 36–45. [Google Scholar] [CrossRef]
- Sane, A.; Thies, M.C. Effect of material properties and processing conditions on RESS of poly(l-lactide). J. Supercrit. Fluids 2007, 40, 134–143. [Google Scholar] [CrossRef]
- Antonov, E.N.; Krotova, L.I.; Mishakov, G.V.; Popov, V.K. Micronization of Levofloxacin Using the RESS Method. Russ. J. Phys. Chem. B 2020, 14, 1225–1228. [Google Scholar] [CrossRef]
- De Melo, S.A.B.V.; Danh, L.T.; Mammucari, R.; Foster, N.R. Dense CO2 antisolvent precipitation of levothyroxine sodium: A comparative study of GAS and ARISE techniques based on morphology and particle size distributions. J. Supercrit. Fluids 2014, 93, 112–120. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; De Marco, I.; Laudani, C.; Spada, A. Pigment Red 60 micronization using supercritical fluids based techniques. J. Supercrit. Fluids 2005, 35, 76–82. [Google Scholar] [CrossRef]
- Kang, Y.; Yin, G.; Ouyang, P.; Huang, Z.; Yao, Y.; Liao, X.; Chen, A.; Pu, X. Preparation of PLLA/PLGA microparticles using solution enhanced dispersion by supercritical fluids (SEDS). J. Colloid Interface Sci. 2008, 322, 87–94. [Google Scholar] [CrossRef]
- Campardelli, R.; Della Porta, G.; Gomez, V.; Irusta, S.; Reverchon, E.; Santamaria, J. Encapsulation of titanium dioxide nanoparticles in PLA microspheres using supercritical emulsion extraction to produce bactericidal nanocomposites. J. Nanopartic. Res. 2013, 15, 1987. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Supercritical carbon dioxide+ethanol mixtures for the antisolvent micronization of hydrosoluble materials. Chem. Eng. J. 2012, 187, 401–409. [Google Scholar] [CrossRef]
- De Marco, I. Production of carrier/antioxidant particles by Supercritical Assisted Atomization as an adjuvant treatment of the COVID-19 pathology. J. Supercrit. Fluids 2022, 186, 105604. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Di Capua, A.; Scognamiglio, M.; Reverchon, E. Production of PEA composite microparticles with polyvinylpyrrolidone and luteolin using Supercritical Assisted Atomization. J. Supercrit. Fluids 2019, 143, 82–89. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Xiang, S.T.; Chen, B.Q.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Solubility measurement and RESOLV-assisted nanonization of gambogic acid in supercritical carbon dioxide for cancer therapy. J. Supercrit. Fluids 2019, 150, 147–155. [Google Scholar] [CrossRef]
- Pathak, P.; Prasad, G.L.; Meziani, M.J.; Joudeh, A.A.; Sun, Y.P. Nanosized paclitaxel particles from supercritical carbon dioxide processing and their biological evaluation. Langmuir 2007, 23, 2674–2679. [Google Scholar] [CrossRef]
- Sharma, S.K.; Al Hosani, S.; Kalmouni, M.; Nair, A.R.; Palanikumar, L.; Pasricha, R.; Sadler, K.C.; Magzoub, M.; Jagannathan, R. Supercritical CO2 Processing Generates Aqueous Cisplatin Solutions with Enhanced Cancer Specificity. ACS Omega 2020, 5, 4558–4567. [Google Scholar] [CrossRef]
- Pessi, J.; Lassila, I.; Meriläinen, A.; Räikkönen, H.; Hæggström, E.; Yliruusi, J. Controlled Expansion of Supercritical Solution: A Robust Method to Produce Pure Drug Nanoparticles with Narrow Size-Distribution. J. Pharm. Sci. 2016, 105, 2293–2297. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 140, 72–84. [Google Scholar] [CrossRef]
- Amani, M.; Saadati Ardestani, N.; Majd, N.Y. Utilization of supercritical CO2 gas antisolvent (GAS) for production of Capecitabine nanoparticles as anti-cancer drug: Analysis and optimization of the process conditions. J. CO2 Util. 2021, 46, 101465. [Google Scholar] [CrossRef]
- Kalantarian, P.; Najafabadi, A.R.; Haririan, I.; Vatanara, A.; Yamini, Y.; Darabi, M.; Gilani, K. Preparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary delivery. Int. J. Nanomed. 2010, 5, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, J.; Liu, G.; Chen, B.; Jiang, Y.; Xie, Q. In vitro and in vivo anti-tumor efficacy of 10-hydroxycamptothecin polymorphic nanoparticle dispersions: Shape- and polymorph-dependent cytotoxicity and delivery of 10-hydroxycamptothecin to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zu, Y.; Li, Q.; Wang, M.; Zu, B.; Zhang, X.; Jiang, R.; Zu, C. Preparation and characterization of camptothecin powder micronized by a supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 51, 412–419. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, X.; Li, Q.; Zu, Y.; Fu, Y.; Zu, B.; Meng, X.; Liu, C. In vitro and in vivo evaluation of camptothecin nanosuspension: A novel formulation with high antitumor efficacy and low toxicity. Int. J. Pharm. 2012, 423, 586–588. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Jiang, Y. Recrystallization and micronization of camptothecin by the supercritical antisolvent process: Influence of solvents. Ind. Eng. Chem. Res. 2013, 52, 15049–15056. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Zhao, Z.; Li, Z.; Li, G.; Chen, Y.; He, X.; Chen, A.; Li, J.; Lin, X.; et al. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int. J. Pharm. 2015, 496, 732–740. [Google Scholar] [CrossRef]
- Xu, J.; Luo, K.Q. Enhancing the solubility and bioavailability of isoflavone by particle size reduction using a supercritical carbon dioxide-based precipitation process. Chem. Eng. Res. Des. 2014, 92, 2542–2549. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zu, Y.; Jiang, R.; Zhao, D. Recrystallization and micronization of taxol using the supercritical antisolvent (SAS) process. Ind. Eng. Chem. Res. 2012, 51, 9591–9597. [Google Scholar] [CrossRef]
- Margulis, K.; Neofytou, E.A.; Beygui, R.E.; Zare, R.N. Celecoxib Nanoparticles for Therapeutic Angiogenesis. ACS Nano 2015, 9, 9416–9426. [Google Scholar] [CrossRef]
- Tay, K.J.; Schulman, A.A.; Sze, C.; Tsivian, E.; Polascik, T.J. New advances in focal therapy for early stage prostate cancer. Expert Rev. Anticancer. Ther. 2017, 17, 737–743. [Google Scholar] [CrossRef]
- Hua, M.; Hua, X. Polymer Nanoparticles Prepared by Supercritical Carbon Dioxide for In Vivo Anti-cancer Drug Delivery. Nano-Micro Lett. 2014, 6, 20–23. [Google Scholar] [CrossRef]
- Shanmugam, S.; Park, J.-H.; Chi, S.-C.; Yong, C.S.; Choi, H.-G.; Woo, J.S. Antitumor efficacy of solid dispersion of paclitaxel prepared by supercritical antisolvent process in human mammary tumor xenografts. Int. J. Pharm. 2011, 403, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Gonzalez-Valdivieso, J.; Santos, M.; Rodriguez-Rojo, S.; Arias, F.J. Production of elastin-like recombinamer-based nanoparticles for docetaxel encapsulation and use as smart drug-delivery systems using a supercritical anti-solvent process. J. Ind. Eng. Chem. 2021, 93, 361–374. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, R.; Zu, Y.; Wang, Y.; Zhao, Q.; Zu, B.; Zhao, D.; Wang, M.; Sun, Z. Process optimization studies of 10-Hydroxycamptothecin (HCPT)-loaded folate-conjugated chitosan nanoparticles by SAS-ionic crosslink combination using response surface methodology (RSM). Appl. Surf. Sci. 2012, 258, 2000–2005. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, D.; Zhao, X.; Jiang, R.; Zhang, Q.; Zhao, D.; Li, Y.; Zu, B.; Sun, Z. A novel preparation method for camptothecin (CPT) loaded folic acid conjugated dextran tumor-targeted nanoparticles. Int. J. Mol. Sci. 2011, 12, 4237–4249. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Zhao, X.; Zu, Y.; Wang, Y.; Zhang, B.; Zhao, D.; Zhao, Q.; Su, L.; Gao, Y.; et al. Preparation, characterization and targeting of micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles to cancer cells. Int. J. Nanomed. 2011, 6, 397–405. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Cetin-Uyanikgil, E.O. In vitro release kinetics of polycaprolactone encapsulated plant extract fabricated by supercritical antisolvent process and solvent evaporation method. J. Supercrit. Fluids 2012, 62, 219–225. [Google Scholar] [CrossRef]
- Kalantarian, P.; Haririan, I.; Najafabadi, A.R.; Shokrgozar, M.A.; Vatanara, A. Entrapment of 5-fluorouracil into PLGA matrices using supercritical antisolvent processes. J. Pharm. Pharmacol. 2011, 63, 500–506. [Google Scholar] [CrossRef]
- Xu, P.Y.; Kankala, R.K.; Pan, Y.J.; Yuan, H.; Wang, S.B.; Chen, A.Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018, 13, 4685–4698. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Kang, Y.-Q.; Wang, S.-B.; Tang, N.; Su, X.-Q. Preparation and antitumor effect evaluation of composite microparticles co-loaded with siRNA and paclitaxel by a supercritical process. J. Mater. Chem. B 2015, 3, 6439–6447. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, J.; Yin, G.; Huang, Z.; Liao, X.; Yao, Y.; Ouyang, P.; Wang, H.; Yang, Q. Characterization and Biological Evaluation of Paclitaxel-Loaded Poly(l-lactic acid) Microparticles Prepared by Supercritical CO2. Langmuir 2008, 24, 7432–7441. [Google Scholar] [CrossRef] [PubMed]
- Alias, D.; Yunus, R.; Chong, G.H.; Che Abdullah, C.A. Single step encapsulation process of tamoxifen in biodegradable polymer using supercritical anti-solvent (SAS) process. Powder Technol. 2017, 309, 89–94. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Coprecipitation of Polyvinylpyrrolidone/β-Carotene by Supercritical Antisolvent Processing. Ind. Eng. Chem. Res. 2015, 54, 11568–11575. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic acid–PVP nanostructured composite microparticles by supercritical antisolvent precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/flavonoid coprecipitation by supercritical antisolvent process. Chem. Eng. Process. 2019, 146, 107689. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Kankala, R.K.; He, G.-Y.; Yang, D.-Y.; Li, G.-P.; Wang, P.; Wang, S.-B.; Zhang, Y.S.; Chen, A.-Z. Supercritical Fluid-Assisted Fabrication of Indocyanine Green-Encapsulated Silk Fibroin Nanoparticles for Dual-Triggered Cancer Therapy. ACS Biomater.-Sci. Eng. 2018, 4, 3487–3497. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Li, Y.; He, X.; Chen, X.; Chen, Y.; Zhu, J.; Xu, G.; Wu, X.; Lan, P. Supercritical carbon dioxide-developed silk fibroin nanoplatform for smart colon cancer therapy. Int. J. Nanomed. 2017, 12, 7751–7761. [Google Scholar] [CrossRef]
- Peng, H.H.; Hong, D.X.; Guan, Y.X.; Yao, S.J. Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer. Int. J. Pharm. 2019, 558, 82–90. [Google Scholar] [CrossRef]
- Silva, A.S.; Shopsowitz, K.E.; Correa, S.; Morton, S.W.; Dreaden, E.C.; Casimiro, T.; Aguiar-Ricardo, A.; Hammond, P.T. Rational design of multistage drug delivery vehicles for pulmonary RNA interference therapy. Int. J. Pharm. 2020, 591, 119989. [Google Scholar] [CrossRef]
- De Marco, I.; Franco, P. Effect of the carrier on the coprecipitation of curcumin through supercritical-assisted atomization. ChemEngineering 2021, 5, 59. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Della Porta, G.; Del Gaudio, P.; Reverchon, E. Lincomycin hydrochloride loaded albumin microspheres for controlled drug release, produced by Supercritical Assisted Atomization. J. Supercrit. Fluids 2017, 119, 203–210. [Google Scholar] [CrossRef]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Han, X.; Li, Y.; Wang, H.; Ji, T.; Zhao, Y.; Nie, G. Photothermal Effect Enhanced Cascade-Targeting Strategy for Improved Pancreatic Cancer Therapy by Gold Nanoshell@Mesoporous Silica Nanorod. ACS Nano 2017, 11, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Sousa, A.M.; Cabral, R.P.; Silva, M.C.; Costa, C.; Miguel, S.P.; Bonifácio, V.D.B.; Casimiro, T.; Correia, I.J.; Aguiar-Ricardo, A. Aerosolizable gold nano-in-micro dry powder formulations for theragnosis and lung delivery. Int. J. Pharm. 2017, 519, 240–249. [Google Scholar] [CrossRef]
- Vaz-Ramos, J.; Cordeiro, R.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Costa, B.F.O.; Faneca, H.; Durães, L. Supercritically dried superparamagnetic mesoporous silica nanoparticles for cancer theranostics. Mater. Sci. Eng. C 2020, 115, 111124. [Google Scholar] [CrossRef]
- Silva, M.C.; Silva, A.S.; Fernandez-Lodeiro, J.; Casimiro, T.; Lodeiro, C.; Aguiar-Ricardo, A. Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials 2017, 10, 74. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Wang, Y.; Cui, F.; Zhang, J.; Huang, Q. Preparation and characterization of 5-fluorouracil-loaded PLLA–PEG/PEG nanoparticles by a novel supercritical CO2 technique. Int. J. Pharm. 2012, 436, 272–281. [Google Scholar] [CrossRef]

| Type of Cancer | Outcome | Reference |
|---|---|---|
| Bladder cancer | NPs used in in vitro cancer diagnostics, in vivo imaging enhancement, and drug loading techniques | [24] |
| Breast cancer | Three crucial biomarkers can be detected and accurately quantified in single tumour sections by the use of NPs conjugated with antibodies | [25] |
| Colorectal cancer | Effective release of cytotoxic drugs through targeted NPs; use of nanostructures with surface-bound ligands for the targeted delivery and ablation of the cancer | [26,27] |
| Kidney cancer | Efficacy of the cisplatin encapsulated into polybutylcyanoacrylate NPs | [28] |
| Lung cancer | Therapeutic and diagnostic systems at the nanoscale, which include polymeric NPs-based approaches, metal NPs-based approaches, and bio-NPs-based approaches | [29] |
| Lymphoma | Enhanced sensitivity and selectivity for earlier detection of circulating cancer biomarkers. In vivo, NPs enhance the therapeutic efficacy of anticancer agents, improving vectorization and delivery, and helping to overcome drug resistance | [30] |
| Melanoma | Anisamide-targeted NPs that can systemically deliver siRNA into the cytoplasm of B16F10 murine melanoma cells | [31] |
| Oral and oropharyngeal cancer | Polymeric, metallic, and lipid-based nanosystems that incorporate chemotherapeutics, natural compounds, siRNA, or other molecules | [32] |
| Pancreatic cancer | Gold NPs utilized for targeted drug delivery in pancreatic cancer leading to increased efficacy of traditional chemotherapeutics | [33] |
| Prostate cancer | Use of gold NPs to enhance radiation sensitivity and growth inhibition in radiation-resistant human prostate cancer cells | [34] |
| Thyroid cancer | Temperature-sensitive poly(N-isopropylacrylamide-acrylamide-allylamine)-coated iron oxide magnetic NPs as targeted drug carriers for treatments of advanced thyroid cancer | [35] |
| Uterine and ovarian cancer | Nanostructured probes are a new class of medical tool that can simultaneously provide imaging contrast, target tumor cells, and carry a wide range of medicines resulting in better diagnosis and therapeutic precision | [36] |
| Technique | API | Application | Operating Conditions | Main Results | Reference |
|---|---|---|---|---|---|
| scCO2 used as the solvent with respect to the API | |||||
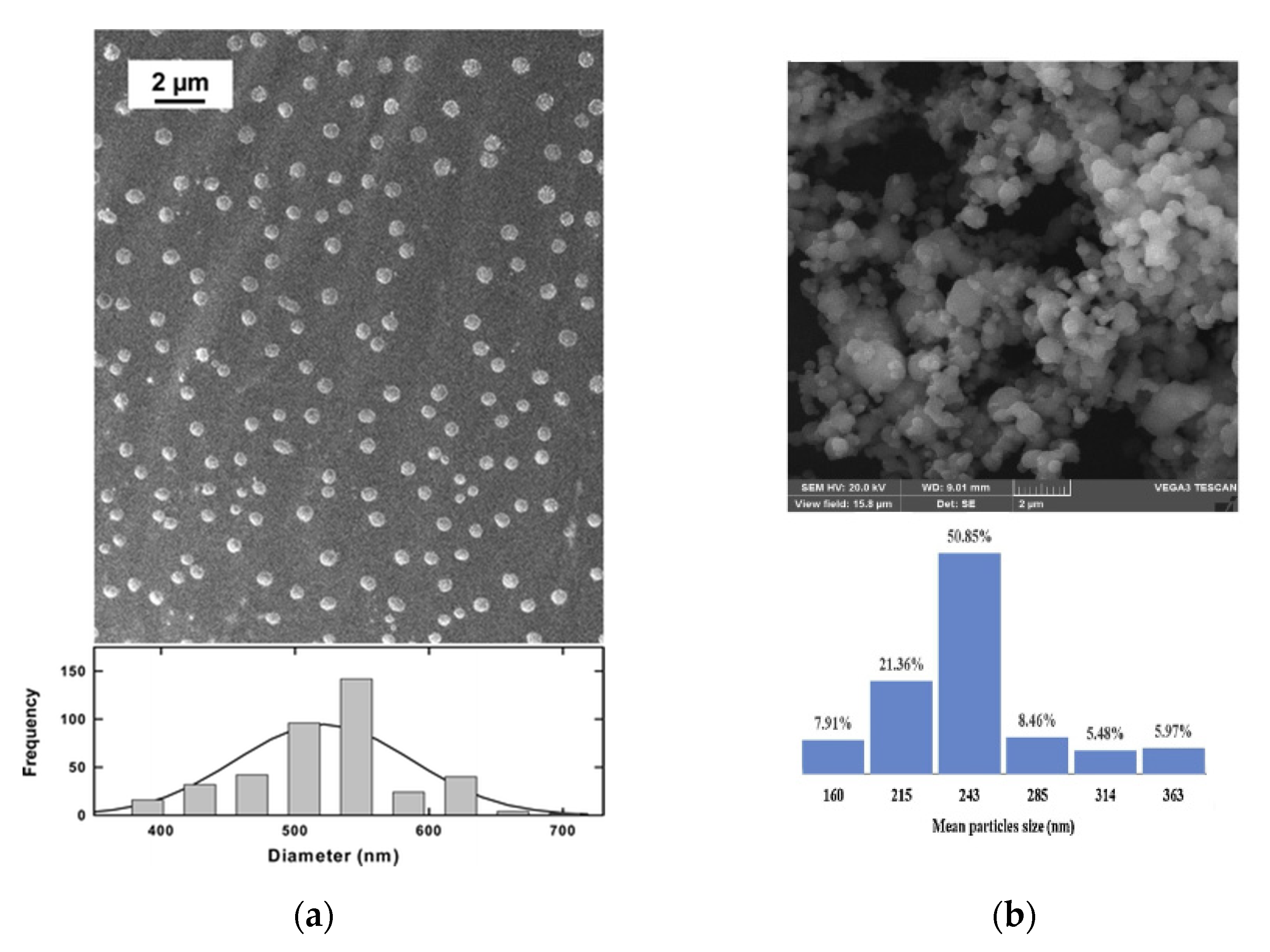
| RESOLV | GA | lung, breast, and other CC | P = 25 MPa; T = 55 °C | md = 251.2 nm ± 85.6 nm; enhanced anticancer efficacy of the NPs compared to that of commercial GA | [56] |
| PTX | ovarian, breast, lung, colon, head, leukemia, and neck CC | P = 31 MPa; T = 40 °C | md = 530 nm ± 85 nm; paclitaxel NPs are effective, with an antineoplastic activity comparable to that of the commercial paclitaxel formulation | [57] | |
| RESS | CIS | head, neck, bladder, ovarian, and lung CC | P = 30 MPa; T = 40 °C | md = 200–300 nm; reduced toxic side effects in cancer patients undergoing CIS-based therapy | [58] |
| Piroxicam | bladder, colon, prostate, and nonmelanoma skin CC | P = 20–35 MPa; T = 60–70 °C | md = 176 nm ± 53 nm; the results on different batches confirmed the robustness and reproducibility of the process | [59] | |
| RESS-SC | Aprepidant | prevention of nausea and vomiting caused by chemotherapic drugs | P = 15–33 MPa; T = 35–65 °C | md = 23–523 nm; the dissolution rate coefficient was up to 8.2 times higher than that of the unprocessed API | [60] |
| scCO2 used as the antisolvent with respect to the API | |||||
| GAS | CPC | breast, colorectal, and gastric CC | P = 12–16 MPa; T = 35–55 °C | md = 243 nm at the optimal operating conditions; lower crystallinity of processed NPs leading to higher solubility and faster dissolution | [61] |
| SAS | 5-FU | lung CC | P = 10–15 MPa; T = 40 °C | md = 248 nm using methanol-DCM 50:50 as the organic solvent; dry powder inhaler formulations with lactose as the carrier with an increase up to 21% of the respiratory fraction | [62] |
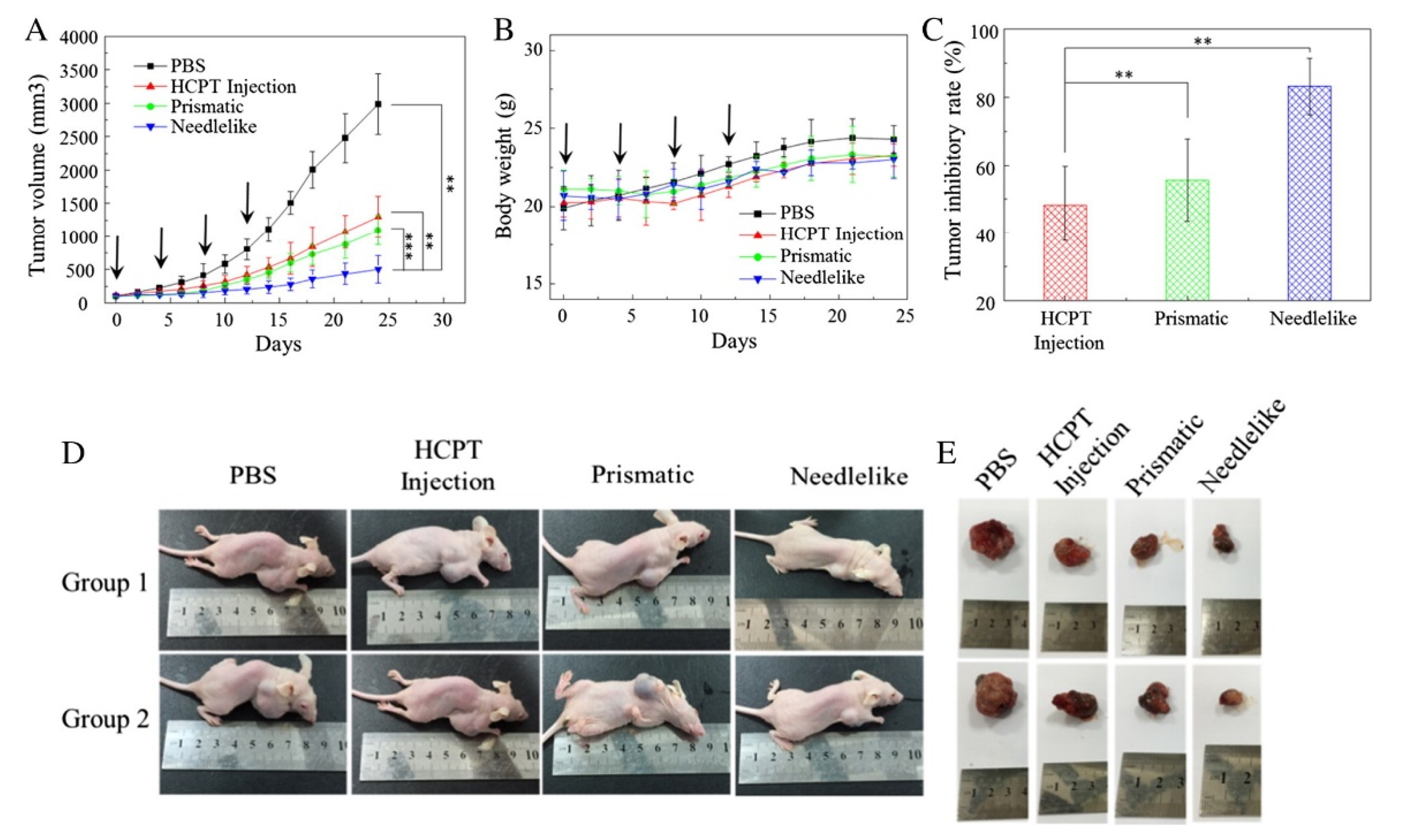
| HCPT | broad spectrum of human CC | P = 10–14 MPa; T = 30–40 °C | md = 600 nm; shape- and polymorph-dependent tumor suppression observed in vitro and in vivo; the tumor inhibitory rate was 68% in needle-shape NPs | [63] | |
| CPT | broad spectrum of human CC | P = 10–25 MPa; T = 35 °C | md = 250 nm ± 20 nm; optimization of the micronization conditions and demonstration of no API degradation | [64] | |
| CPT | broad spectrum of human CC | P = 20 MPa; T = 35 °C | md = 251 nm ± 20 nm; with respect to topotecan, 6 times in vitro cytotoxicity activeagainst cell lines MCF-7, nearly the same in vivo antitumor activity with lower toxicity | [65] | |
| CPT | broad spectrum of human CC | P = 14 MPa; T = 40 °C | md = 390–870 nm depending on the organic solvent used; smaller particles obtained by using EtOH/DMSO because the solubility of CPT in the solution was lower | [66] | |
| Curcumin | colorectal CC | P = 22–22.5 MPa; T = 31–32.5 °C | md = 230–240 nm; enhanced anticancer effect on colorectal cancer cells (HCT116); reduced cytotoxicity on normal cells (NCM460) compared to curcumin-DMSO and 5-Fu | [67] | |
| Genistein | breast, ovarian, and prostate CC | P = 8.5–12 MPa; T = 40 °C | md = 254 nm; increased 24 h-plasma concentration by 2.6-fold after orally administrated to rats | [68] | |
| Taxol | ovarian, breast, and lung CC | P = 10–25 MPa; T = 35–68 °C | md = 150 nm; not induced degradation of the API | [69] | |
| Carrier | API | Operating Conditions | Main Results | Reference |
|---|---|---|---|---|
| scCO2 with the rule of the antisolvent | ||||
| HP-β-CD+PVP | PTX | P = 8.3 MPa; T = 40 °C | In vivo tests showed excellent antitumor activity in female mice bearing human mammary tumor xenografts; high degree of tumor growth inhibition with 100% complete tumor regressions and 80% tumor-free survivors | [73] |
| BSA-PMMA | CPT | P = 10 MPa; T = 40 °C | md = 310 nm ± 27 nm; in vitro and in vivo anticancer activity against human colorectal cancer cells | [72] |
| ELR | Docetaxel | P = 9.5–11 MPa T = 35 °C | md = 40 nm; increased water solubility up to fifty orders of magnitude; controlled API release profile; in vitro measurements demonstrated an enhanced effect of the API in breast cancer cells compared to healthy endothelial cells | [74] |
| FA-CS | HCPT | P= 20 MPa; T = 35 °C | Particle size in the range of 123–182 nm, suitable for intravenous Injection, and useful in the treatment of HCPT-sensitive tumors | [75] |
| FA-DEX | CPT | P = 10–20 MPa; T = 40–60 °C | md = 182 nm; attainment of tumor-targeted NPs | [76] |
| FA-HSA | HCPT | P= 25 MPa; T = 35 °C | HCPT NPs with a md = 119 nm and FA-HSA-HCPT NPs with a md = 234 nm; sustained release of the API and high affinity for tumor cells in vitro | [77] |
| PCL | Rosemary extract | P = 30 MPa; T = 40 °C | md = 255 nm; in vitro release exhibited an initial burst release within the first 15 min | [78] |
| PLGA | 5-FU | P = 11 MPa; T = 36 °C | In vitro release profiles revealed a burst effect on the first day, followed by a sustained-release phase; an MTT assay performed on human lung carcinoma demonstrated the activity of the API when coprecipitated with PLGA | [79] |
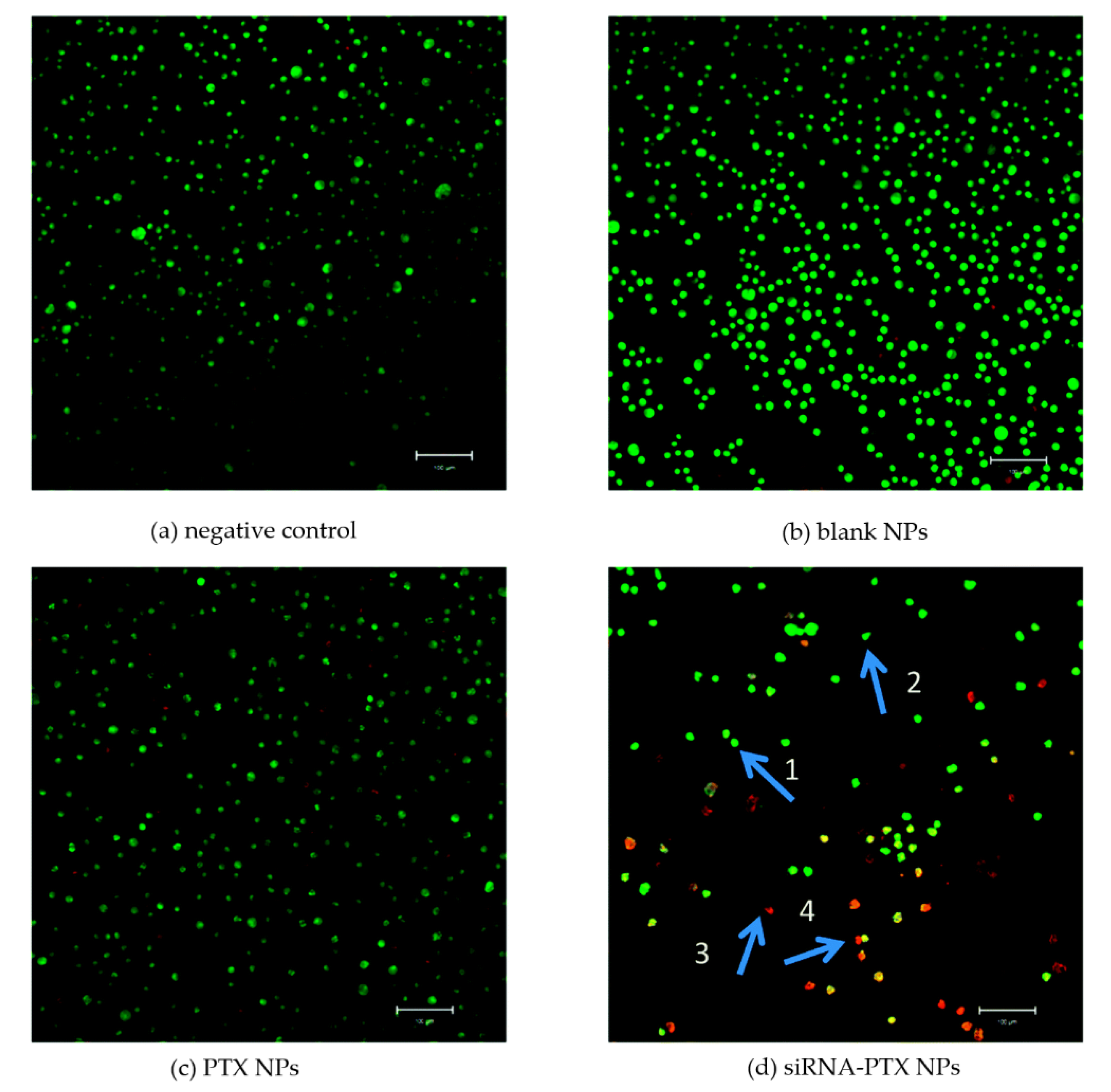
| PLLA | DOX+ siRNA-CS | P = 8 MPa; T = 30 °C | Sustained release of DOX and higher anticancer efficacy in drug-resistant cells (human small cell lung cancer) than those treated with free DOX or DOX-PLLA | [80] |
| PLLA-PEG-PLLA | PTX+ siRNA-CS | P = 12 MPa; T = 35 °C | md = 323 nm; in vitro antitumor effect demonstrated by AO/EB assay; the drug-loaded NPs induced the apoptosis and death of cells | [81] |
| PLLA | PTX | P = 12 MPa; T = 33 °C | In vitro prolonged cytotoxicity against the proliferation of nonsmall-cell lung cancer A549 and ovarian cancer SKOV3 cell lines | [82] |
| PLLA | Tamoxifen | T = 13 MPa; T = 38 °C | Controlled delivery aimed at reducing the side effects of the API | [83] |
| PVP | β-Carotene | P = 8.5–10 MPa; T = 40 °C | md = 250 nm ± 50 nm; 10 times increase of the API dissolution rate | [84] |
| PVP | Folic acid | P = 9–15 MPa T = 35–40 °C | md in the range 50–810 nm depending on the process conditions; shorter dissolution time than the unprocessed API | [85] |
| PVP | Quercetin | P = 9 MPa T = 40 °C | md = 470 nm; dissolution rate from the coprecipitated NPs 10 times faster than the unprocessed API | [86] |
| PVP | Rutin | P = 9 MPa T = 40 °C | md = 840 nm; dissolution rate from the coprecipitated NPs 3.19 times faster than the unprocessed API | [86] |
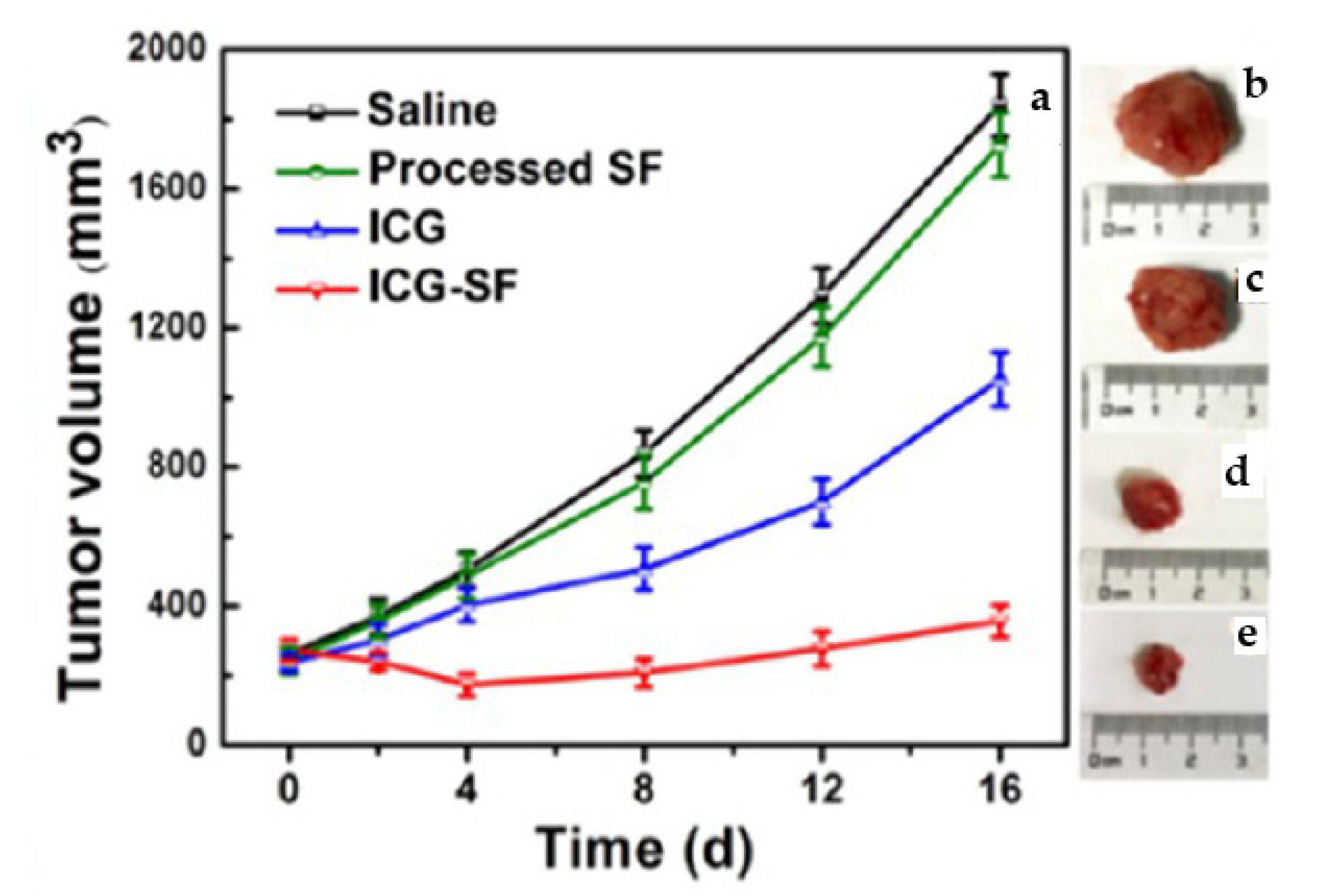
| SF | ICG | P = 10 MPa T = 35 °C | In vitro and in vivo photothermal experiments showed that ICG-SF NPs were capable of devastating tumor cells under light-induced hyperthermia | [87] |
| SF | Curcumin | P = 20 MPa | md lower than 100 nm; improved inhibition effect against colon cancer cells | [88] |
| scCO2 with the rule of the co-solute | ||||
| CS | DOX | P = 8–12 MPa; Tm = 70 °C; Tp = 90 °C | md in the range 120–250 nm; in vitro drug release profiles showed a pH-responsive release with an initial burst; the SAA process did not alter the activity of DOX | [89] |
| CS | siRNA | P = 10 MPa; Tm = 70 °C; Tp = 90 °C | Controlled and sustained release of the API; in vivo biodistribution assessment of the powders in healthy mice showed deep lung diffusion | [90] |
| DXT | Curcumin | P = 9.2 MPa; Tm = 85 °C; Tp = 100 °C | md = 400 nm ± 130 nm; complete dissolution of the API in about 30 min | [91] |
| HP-β-CD | Curcumin | P = 9 MPa; Tm = 80 °C; Tp = 100 °C | md = 510 nm ± 160 nm; complete dissolution of the API in about 30 min | [91] |
| PVP | Curcumin | P = 8 MPa; Tm = 80 °C; Tp = 80 °C | md = 430 nm ± 140 nm; complete dissolution of the API in a reduced time (from a few minutes to 70 min depending on the PVP/API ratio) with respect to the unprocessed API | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, I. Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines 2022, 13, 1449. https://doi.org/10.3390/mi13091449
De Marco I. Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines. 2022; 13(9):1449. https://doi.org/10.3390/mi13091449
Chicago/Turabian StyleDe Marco, Iolanda. 2022. "Supercritical Fluids and Nanoparticles in Cancer Therapy" Micromachines 13, no. 9: 1449. https://doi.org/10.3390/mi13091449
APA StyleDe Marco, I. (2022). Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines, 13(9), 1449. https://doi.org/10.3390/mi13091449






