Double Immunochromatographic Test System for Sensitive Detection of Phycotoxins Domoic Acid and Okadaic Acid in Seawater and Seafood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of OA–Protein and DA–Protein Conjugates
2.3. Production of MAbs
2.4. ELISAs of OA and DA
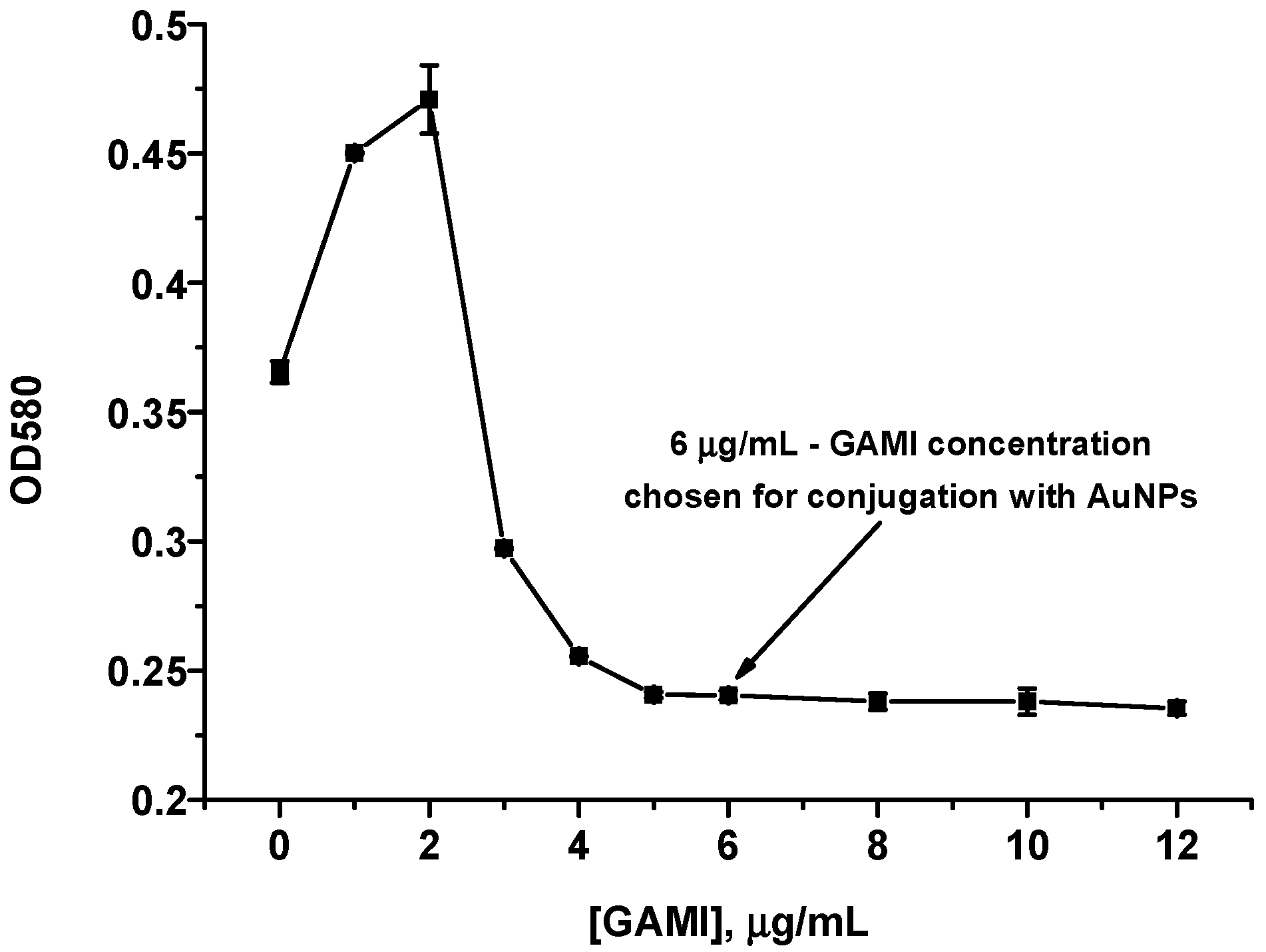
2.5. Synthesis of AuNPs and Choice of GAMI Concentration for Conjugation
2.6. Conjugation of AuNPs with GAMI
2.7. Preparation of Test Strips
2.8. Pretreatment of Seawater and Seafood Samples
2.9. Single ICAs of OA and DA
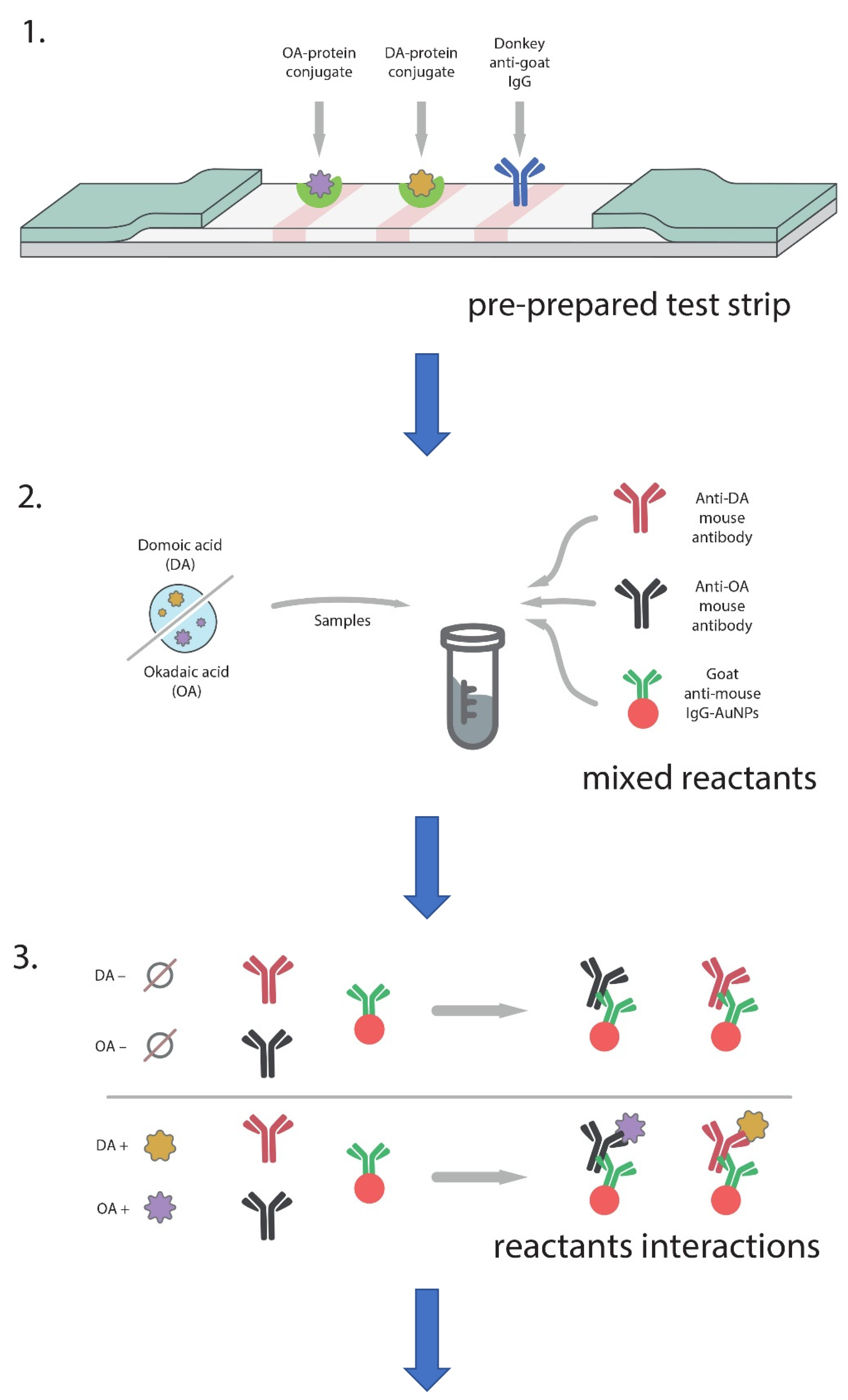
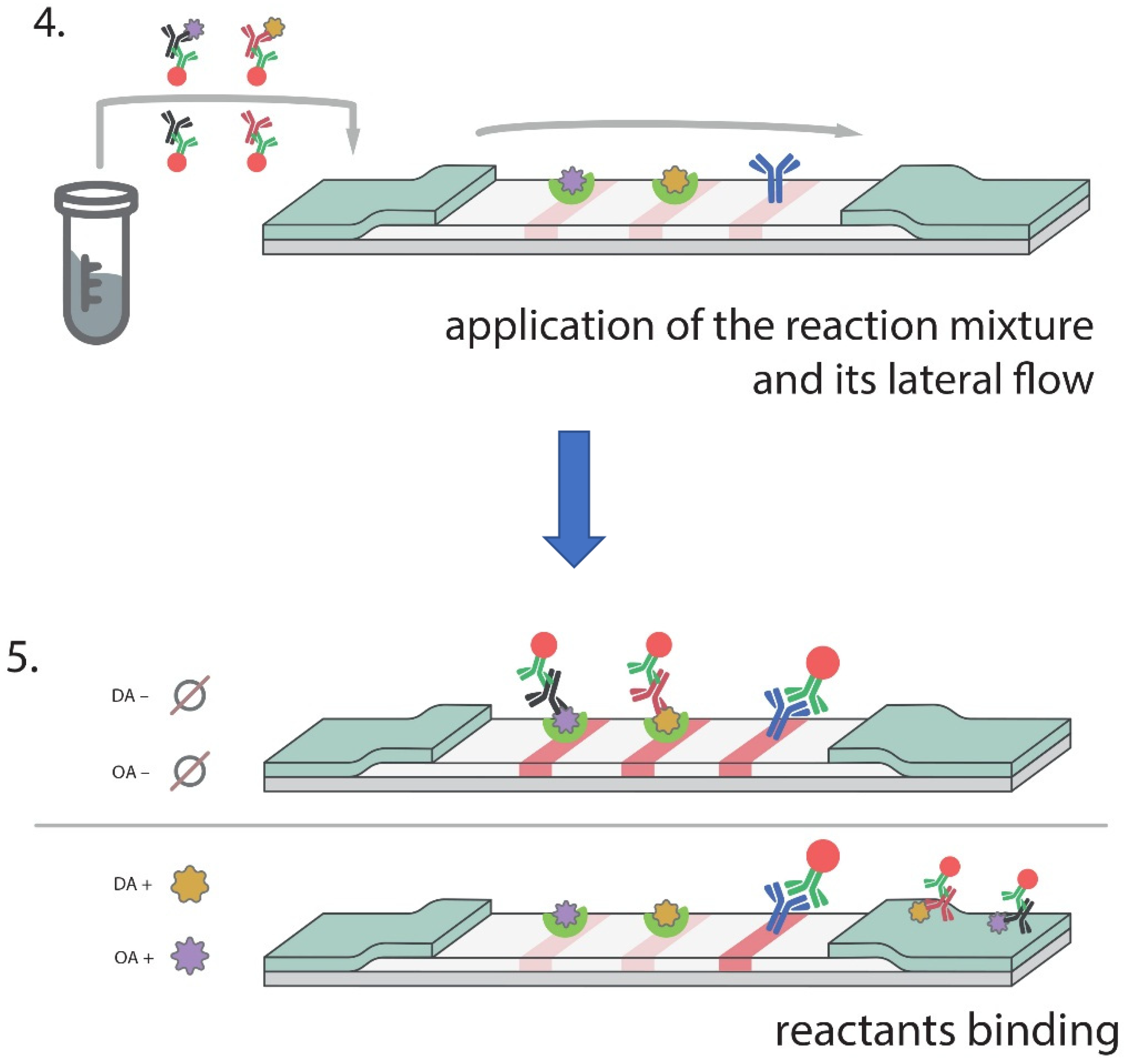
2.10. Double ICA of OA and DA
2.11. Evaluation of the ICA and ELISA Results
3. Results and Discussion
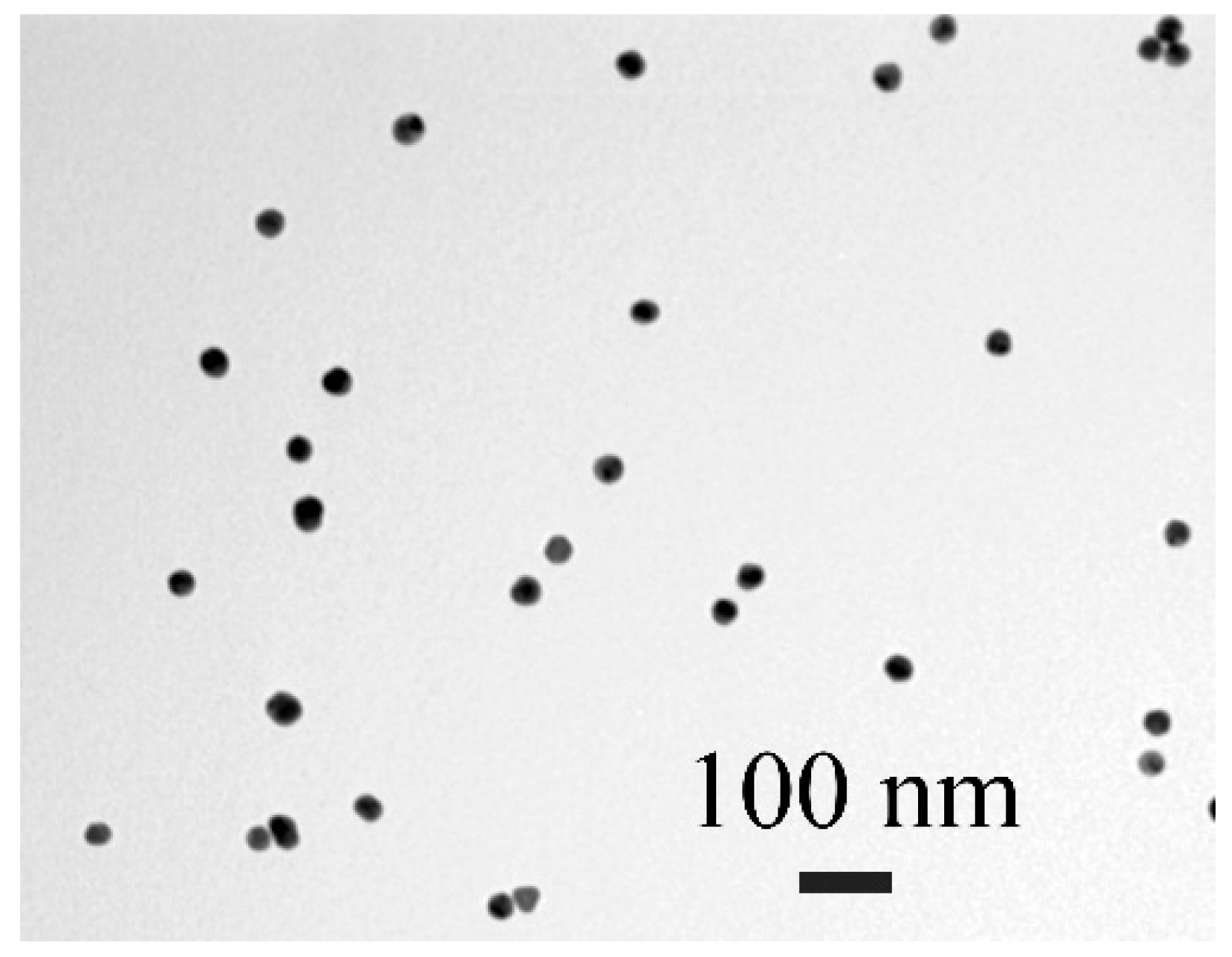
3.1. Obtaining the Immunoreagents
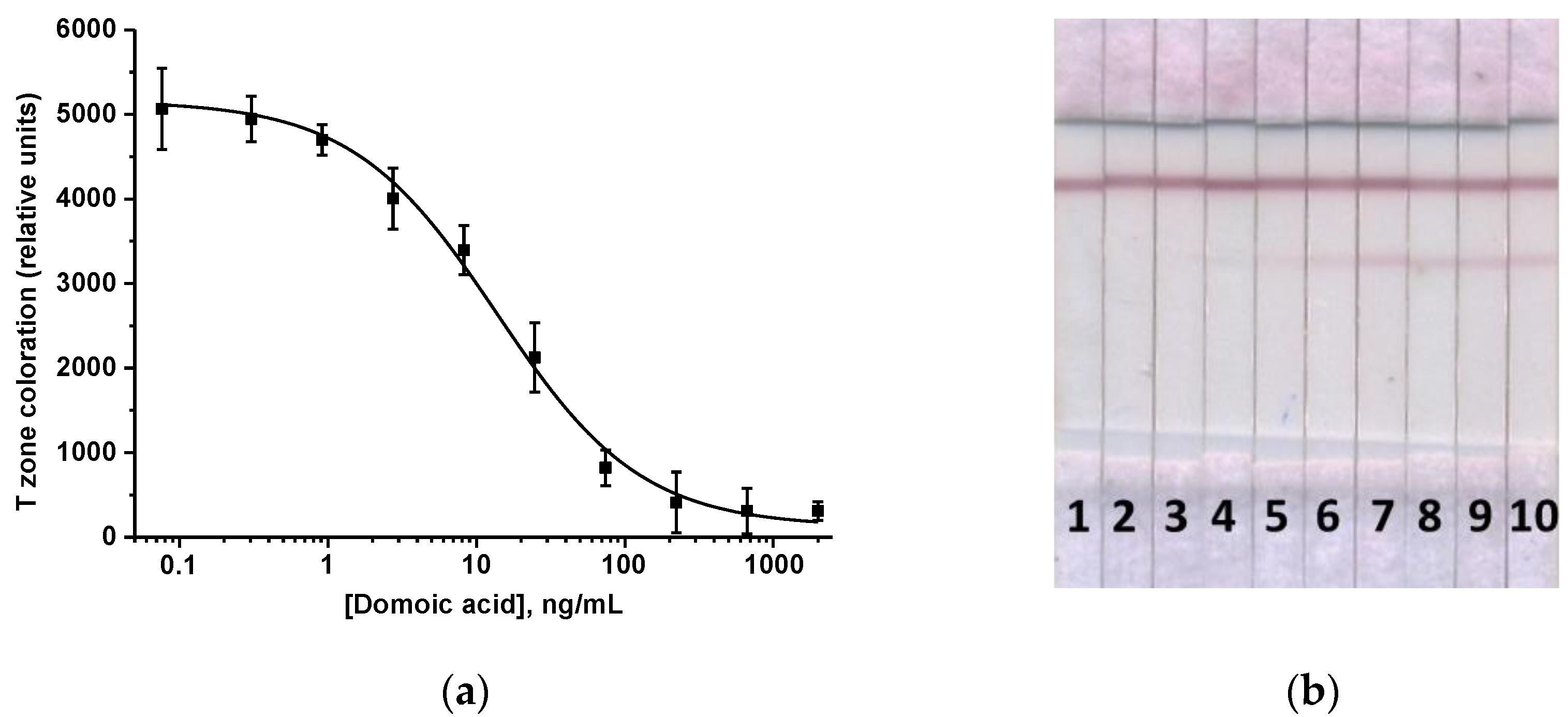
3.2. ICA of DA
3.3. ICA of OA
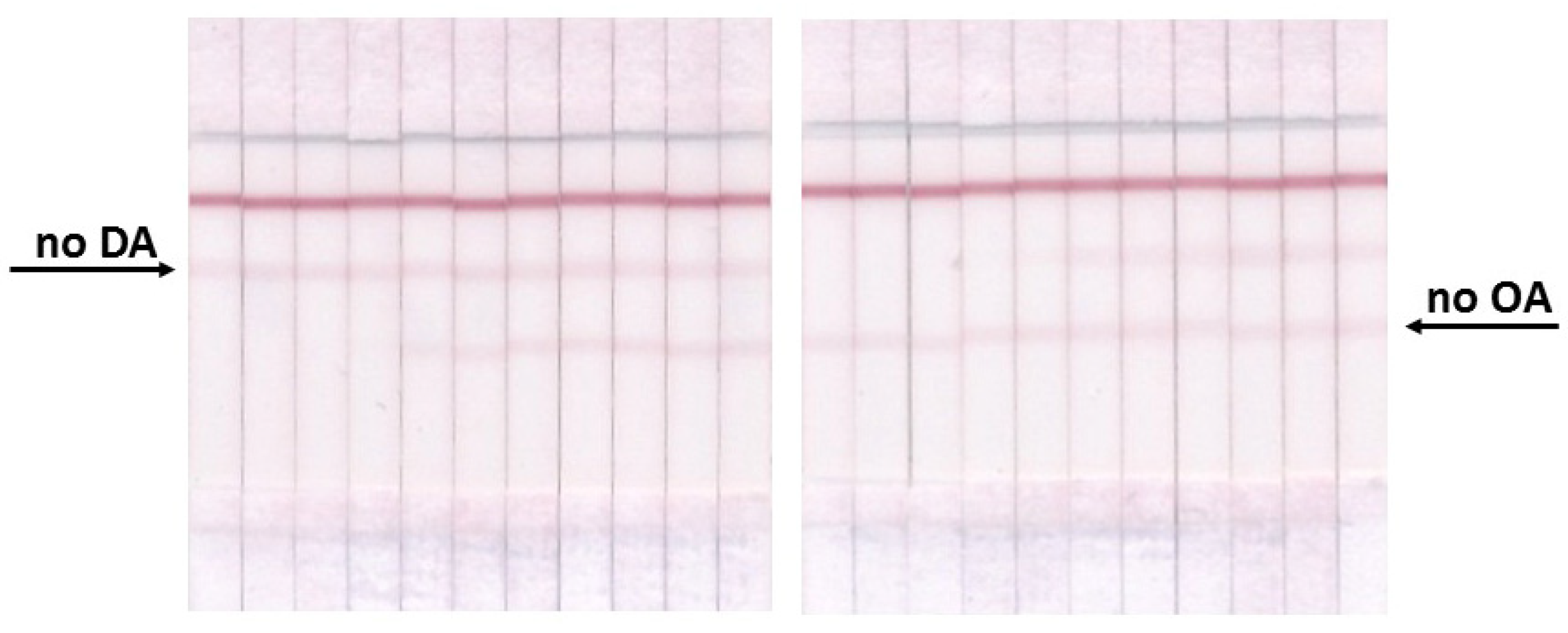
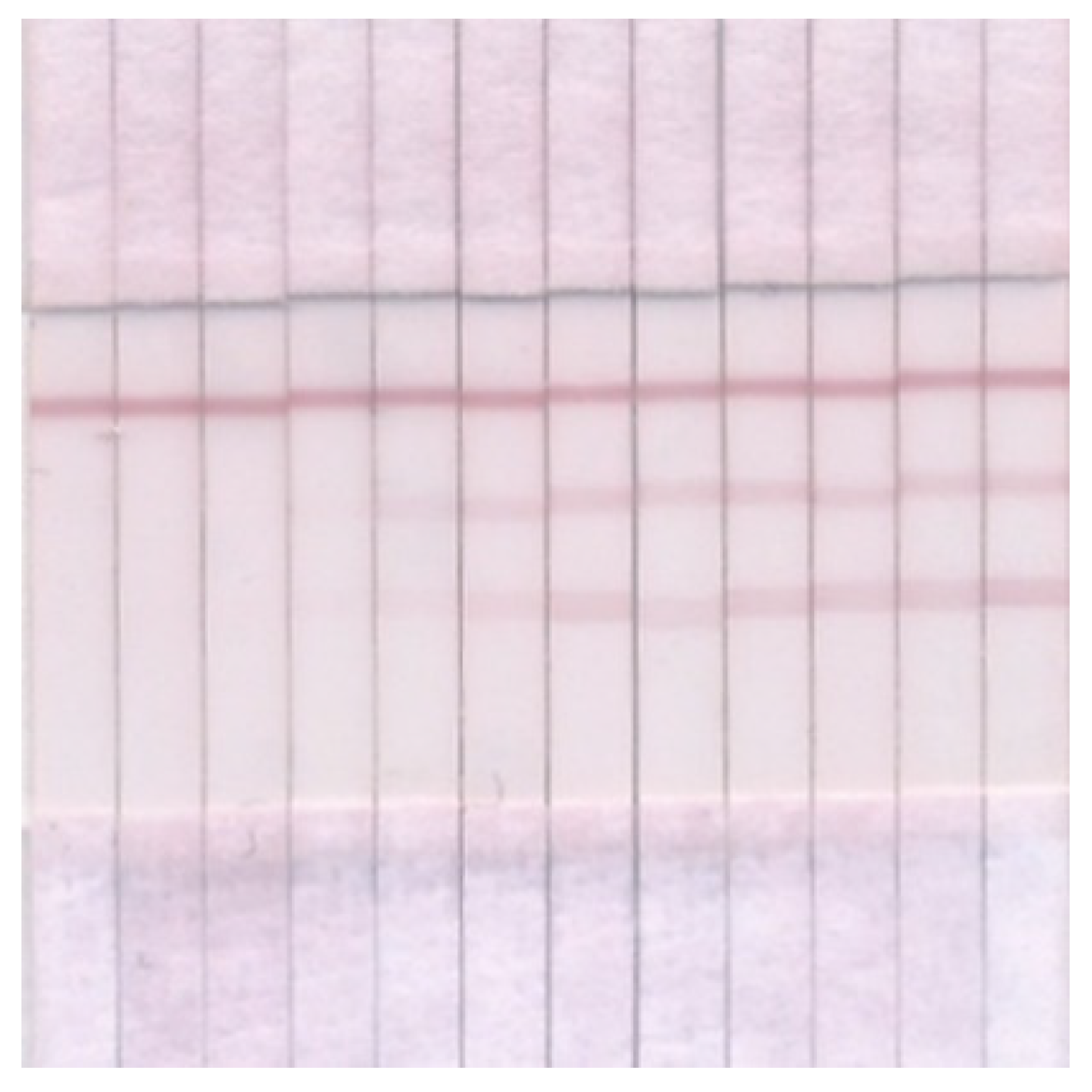
3.4. Double ICA of OA and DA
3.5. ICA of OA and DA in Seawater
3.6. ICA of OA and DA in Seafood
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daguer, H.; Hoff, R.B.; Molognoni, L.; Kleemann, C.R.; Felizardo, L.V. Outbreaks, toxicology, and analytical methods of marine toxins in seafood. Curr. Opin. Food Sci. 2018, 24, 43–55. [Google Scholar] [CrossRef]
- Vilarino, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human poisoning from marine toxins: Unknowns for optimal consumer protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Blanco, L.; Rodriguez, L.P.; Vieites, J.M.; Cabado, A.G. Phycotoxins in marine shellfish: Origin, occurrence and effects on humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef]
- Murk, A.J.; Nicolas, J.; Smulders, F.J.M.; Burk, C.; Gerssen, A. Marine biotoxins: Types of poisonig, underlying mechanisms of action and risk management programmes. Food Saf. Assur. Vet. Public Health Chem. Hazards Foods Anim. Orig. 2019, 7, 207–239. [Google Scholar]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pasaro, E.; Mendez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Marine Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhao, X.Y.; Ji, L.D.; Xu, J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Petroff, R.; Hendrix, A.; Shum, S.; Grant, K.S.; Lefebvre, K.A.; Burbacher, T.M. Public health risks associated with chronic, low-level domoic acid exposure: A review of the evidence. Pharmacol. Ther. 2021, 227, 107865. [Google Scholar] [CrossRef]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Kumar, K.P.; Kumar, S.P.; Nair, G.A. Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J. Environ. Biol. 2009, 30, 319–325. [Google Scholar]
- Marine biotoxins in shellfish—Summary on regulated marine biotoxins. Scientific opinion of the panel on contaminants in the food chain in feed and food. EFSA J. 2009, 1306, 1–23. [Google Scholar]
- Neves, R.A.F.; Nascimento, S.M.; Santos, L.N. Harmful algal blooms and shellfish in the marine environment: An overview of the main molluscan responses, toxin dynamics, and risks for human health. Environ. Sci. Pollut. Res. 2021, 28, 55846–55868. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Vehniäinen, M.; Kankaanpää, H.T.; Lamminmäki, U. Rapid and highly sensitive non-competitive immunoassay for specific detection of nodularin. Microorganisms 2017, 5, 58. [Google Scholar] [CrossRef]
- Dillon, M.; Zaczek-Moczydlowska, M.A.; Edwards, C.; Turner, A.D.; Miller, P.I.; Moore, H.; McKinney, A.; Lawton, L.; Campbell, K. Current trends and challenges for rapid smart diagnostics at point-of-site testing for marine toxins. Sensors 2021, 21, 2499. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Ultrasensitive lateral flow immunoassay of phycotoxin microcystin-LR in seafood based on magnetic particles and peroxidase signal amplification. Food Control. 2022, 133, 108655. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst 2020, 145, 2143–2151. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, J.; Li, Y.; Wu, Y.; Huang, T.; Tang, D. Hollow nanogold microsphere-signalized lateral flow immunodipstick for the sensitive determination of the neurotoxin brevetoxin B. Microchim. Acta 2014, 181, 1447–1454. [Google Scholar] [CrossRef]
- Hu, L.; Liu, J.; Wang, Q.; Zhang, Y.; Jia, R.; Cai, C.; Wu, W.; Chen, S.-F. Development of an immunochromatographic strip test for the rapid detection of okadaic acid in shellfish sample. J. Appl. Phycol. 2013, 25, 1091–1099. [Google Scholar] [CrossRef]
- Jawaid, W.; Meneely, J.; Campbell, K.; Hooper, M.; Melville, K.; Holmes, S.; Rice, J.; Elliott, C. Development and validation of the first high performance-lateral flow immunoassay (HP-LFIA) for the rapid screening of domoic acid from shellfish extracts. Talanta 2013, 116, 663–669. [Google Scholar] [CrossRef]
- Liu, B.-H.; Hung, C.-T.; Lu, C.-C.; Chou, H.-N.; Yu, F.-Y. Production of monoclonal antibody for okadaic acid and its utilization in an ultrasensitive enzyme-linked immunosorbent assay and one-step immunochromatographic strip. J. Agric. Food Chem. 2014, 62, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Lin, C.; Li, Y.-S.; Zhou, Y.; Meng, X.-M.; Yu, S.-Y.; Li, Z.-H.; Li, L.; Ren, H.-L.; Liu, Z.-S. A screening lateral flow immunochromatographic assay for on-site detection of okadaic acid in shellfish products. Anal. Biochem. 2012, 422, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Z.-J.; Liao, Y.-C.; Liu, B.-H.; Su, C.-C.; Yu, F.-Y. Development of a monoclonal antibody against domoic acid and its application in enzyme-linked immunosorbent assay and colloidal gold immunostrip. J. Agric. Food Chem. 2007, 55, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-Y.; Liu, B.-H.; Wu, T.-S.; Chi, T.-F.; Su, M.-C. Development of a sensitive enzyme-linked immunosorbent assay for the determination of domoic acid in shellfish. J. Agric. Food Chem. 2004, 52, 5334–5339. [Google Scholar] [CrossRef]
- Ling, S.; Li, X.; Zhang, D.; Wang, K.; Zhao, W.; Zhao, Q.; Wang, R.; Yuan, J.; Xin, S.; Wang, S. Detection of okadaic acid (OA) and tetrodotoxin (TTX) simultaneously in seafood samples using colloidal gold immunoassay. Toxicon 2019, 165, 103–109. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, J.; Beloglazova, N.; Yang, S.; De Saeger, S.; Mujtaba Mari, G.; Zhang, S.; Shen, J.; Wang, Z.; Yu, X. Portable multiplex immunochromatographic assay for quantitation of two typical algae toxins based on dual-color fluorescence microspheres. J. Agric. Food Chem. 2019, 67, 6041–6047. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Song, S.; Feng, M.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, L.; Yang, H.; Zhong, Y.; Wang, J.; Ling, S.; Farhan Saeed, A.; Yuan, J.; Wang, S. Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. J. Hazard. Mater. 2017, 339, 54–160. [Google Scholar] [CrossRef]
- Finlay, W.J.J.; Shaw, I.; Reilly, J.P.; Kane, M. Generation of high-affinity chicken single-chain Fv antibody fragments for measurement of the Pseudonitzschia pungens toxin domoic acid. Appl. Environ. Microbiol. 2006, 72, 3343–3349. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Shanin, I.A.; Zherdev, A.V.; Tarannum, N.; Dzantiev, B.B. Highly sensitive immunochromatographic detection of antibiotic ciprofloxacin in milk. Appl. Biochem. Microbiol. 2018, 54, 670–676. [Google Scholar] [CrossRef]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Uhrovcik, J. Strategy for determination of LOD and LOQ values—some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Pierce Biotechnology, Thermo Fisher Scientific: Rockford, IL, USA, 2013. [Google Scholar]
- Urusov, A.E.; Petrakova, A.V.; Zherdev, A.V.; Dzantiev, B.B. “Multistage in one touch” design with a universal labelling conjugate for high-sensitive lateral flow immunoassays. Biosens. Bioelectron. 2016, 86, 575–579. [Google Scholar] [CrossRef]

| Phycotoxin | Added Quantity (ng/g) | Measured Quantity ± SD (ng/g) | Recovery ± 1 SD (%) |
|---|---|---|---|
| DA | 20 | 23.6 ± 0.96 | 118.2 ± 4.8 |
| 8 | 8.75 ± 0.35 | 109.4 ± 4.4 | |
| OA | 0.4 | 0.49 ± 0.01 | 121.5 ± 2.9 |
| 0.2 | 0.24 ± 0.009 | 118.1 ± 4.6 |
| Domoic Acid | |||
|---|---|---|---|
| Matrix | Added Quantity (µg/g) | Measured Quantity ± SD (µg/g) | Recovery ± 1 SD (%) |
| Scallops | 20 | 17.4 ± 1.3 | 87.4 ± 6.4 |
| 8 | 6.7 ± 0.7 | 83.4 ± 9.2 | |
| 3.2 | 2.8 ± 0.2 | 88.8 ± 7.3 | |
| Tiger shrimps | 20 | 23.2 ± 1.6 | 116.2 ± 7.8 |
| 8 | 9.2 ± 0.4 | 114.7 ± 5.3 | |
| 3.2 | 2.8 ± 0.3 | 115.1 ± 8.8 | |
| Whelks | 20 | 18.2 ± 1.2 | 91.0 ± 5.8 |
| 8 | 7.1 ± 1.1 | 88.8 ± 13.9 | |
| 3.2 | 2.6 ± 0.2 | 80.8 ± 5.0 | |
| Octopuses | 20 | 20.6 ± 0.9 | 102.9 ± 4.5 |
| 8 | 9.9 ± 0.2 | 123.3 ± 2.7 | |
| 3.2 | 3.7 ± 0.3 | 116.4 ± 8.2 | |
| Mussels | 20 | 22.1 ± 0.3 | 110.6 ± 1.7 |
| 8 | 9.6 ± 0.3 | 119.8 ± 3.2 | |
| 3.2 | 4.0 ± 0.3 | 124.1 ± 7.8 | |
| Mussels | 20 | 22.9 ± 3.0 | 114.5 ± 15.0 |
| 8 | 8.9 ± 1.2 | 111.9 ± 14.7 | |
| 3.2 | 3.8 ± 0.1 | 120.0 ± 3.1 | |
| Okadaic acid | |||
| Added quantity (ng/g) | Measured quantity ± SD (ng/g) | Recovery ± 1 SD (%) | |
| Scallops | 320 | 265.3 ± 32.0 | 82.9 ± 10 |
| 160 | 130.7 ± 6.2 | 81.7 ± 3.9 | |
| 80 | 71.9 ± 5.2 | 89.9 ± 6.5 | |
| Tiger shrimps | 320 | 295.7 ± 46.4 | 92.4 ± 14.5 |
| 160 | 165.3 ± 9.1 | 103.3 ± 5.7 | |
| 80 | 89.3 ± 1.9 | 111.6 ± 2.4 | |
| Whelks | 320 | 291.5 ± 25.6 | 91.1 ± 8.0 |
| 160 | 171.8 ± 9.3 | 107.4 ± 5.8 | |
| 80 | 66.2 ± 6.1 | 82.7 ± 7.6 | |
| Octopuses | 320 | 376.6 ± 48 | 117.7 ± 15.0 |
| 160 | 190.2 ± 22.9 | 118.9 ± 14.3 | |
| 80 | 88.4 ± 7.4 | 110.5 ± 9.3 | |
| Mussels | 320 | 389.4 ± 18.2 | 121.7 ± 5.7 |
| 160 | 184.3 ± 2.4 | 115.2 ± 1.5 | |
| 80 | 97.4 ± 3.7 | 121.8 ± 4.6 | |
| Crabs | 320 | 394.9 ± 13.4 | 123.4 ± 4.2 |
| 160 | 162.4 ± 18.9 | 101.5 ± 11.8 | |
| 80 | 84.2 ± 10.1 | 105.2 ± 12.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickson, O.D.; Zvereva, E.A.; Solopova, O.N.; Zherdev, A.V.; Sveshnikov, P.G.; Eremin, S.A.; Dzantiev, B.B. Double Immunochromatographic Test System for Sensitive Detection of Phycotoxins Domoic Acid and Okadaic Acid in Seawater and Seafood. Micromachines 2022, 13, 1506. https://doi.org/10.3390/mi13091506
Hendrickson OD, Zvereva EA, Solopova ON, Zherdev AV, Sveshnikov PG, Eremin SA, Dzantiev BB. Double Immunochromatographic Test System for Sensitive Detection of Phycotoxins Domoic Acid and Okadaic Acid in Seawater and Seafood. Micromachines. 2022; 13(9):1506. https://doi.org/10.3390/mi13091506
Chicago/Turabian StyleHendrickson, Olga D., Elena A. Zvereva, Olga N. Solopova, Anatoly V. Zherdev, Peter G. Sveshnikov, Sergei A. Eremin, and Boris B. Dzantiev. 2022. "Double Immunochromatographic Test System for Sensitive Detection of Phycotoxins Domoic Acid and Okadaic Acid in Seawater and Seafood" Micromachines 13, no. 9: 1506. https://doi.org/10.3390/mi13091506






