Poly(1-Napthylamine) Nanoparticles as Potential Scaffold for Supercapacitor and Photocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(1-Naphthylamine) Nanoparticles
2.3. Characterizations of Poly(1-Naphthylamine) Nanoparticles
2.4. Fabrication of Poly(1-Naphthylamine) Nanoparticle-Based Electrodes for Supercapacitor Applications
2.5. Photocatalytic Activity of Poly(1-Naphthylamine) Nanoparticles
3. Results and Discussion
3.1. Characterizations and Properties of PNA Nanoparticles
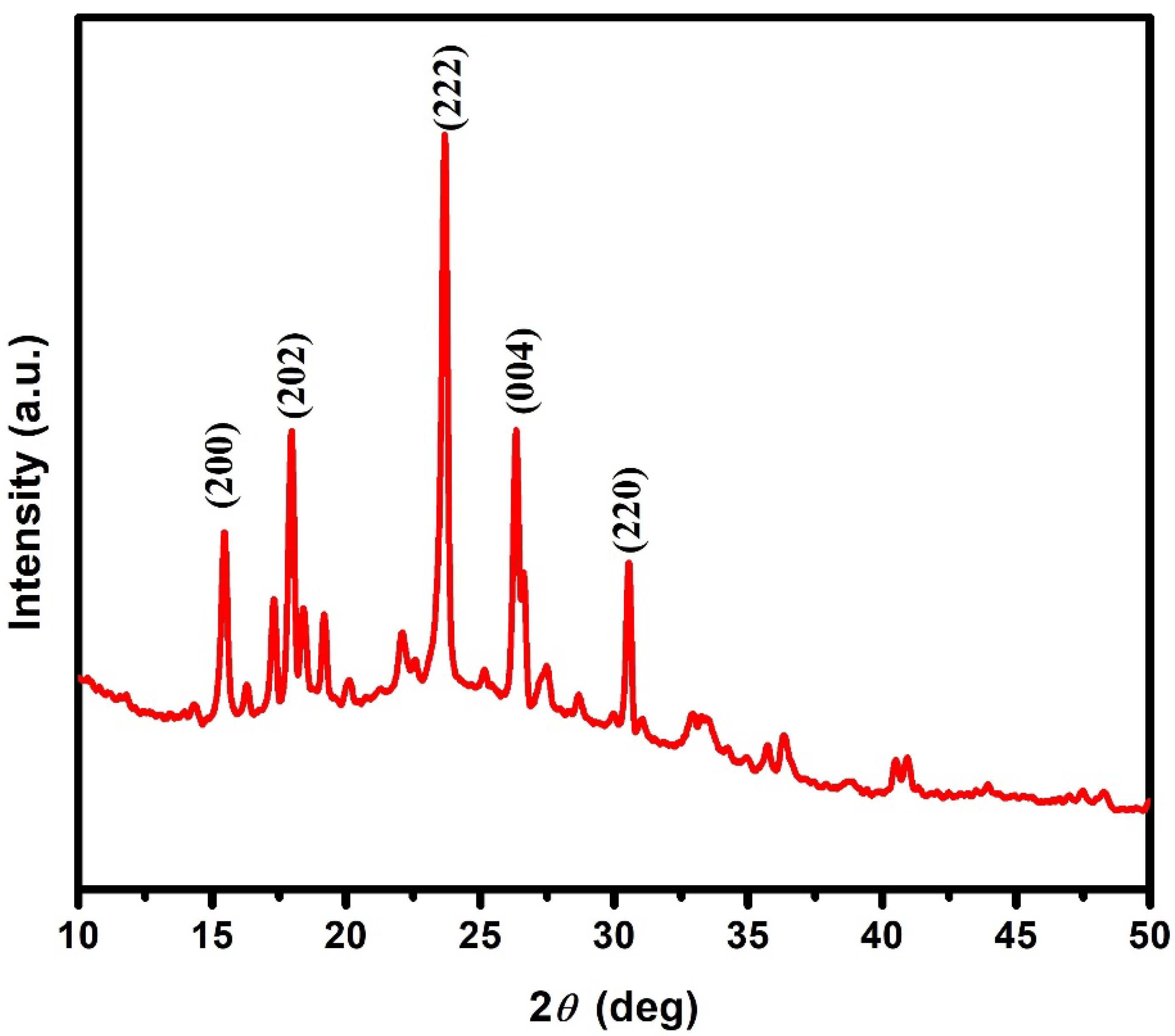
3.1.1. XRD Studies
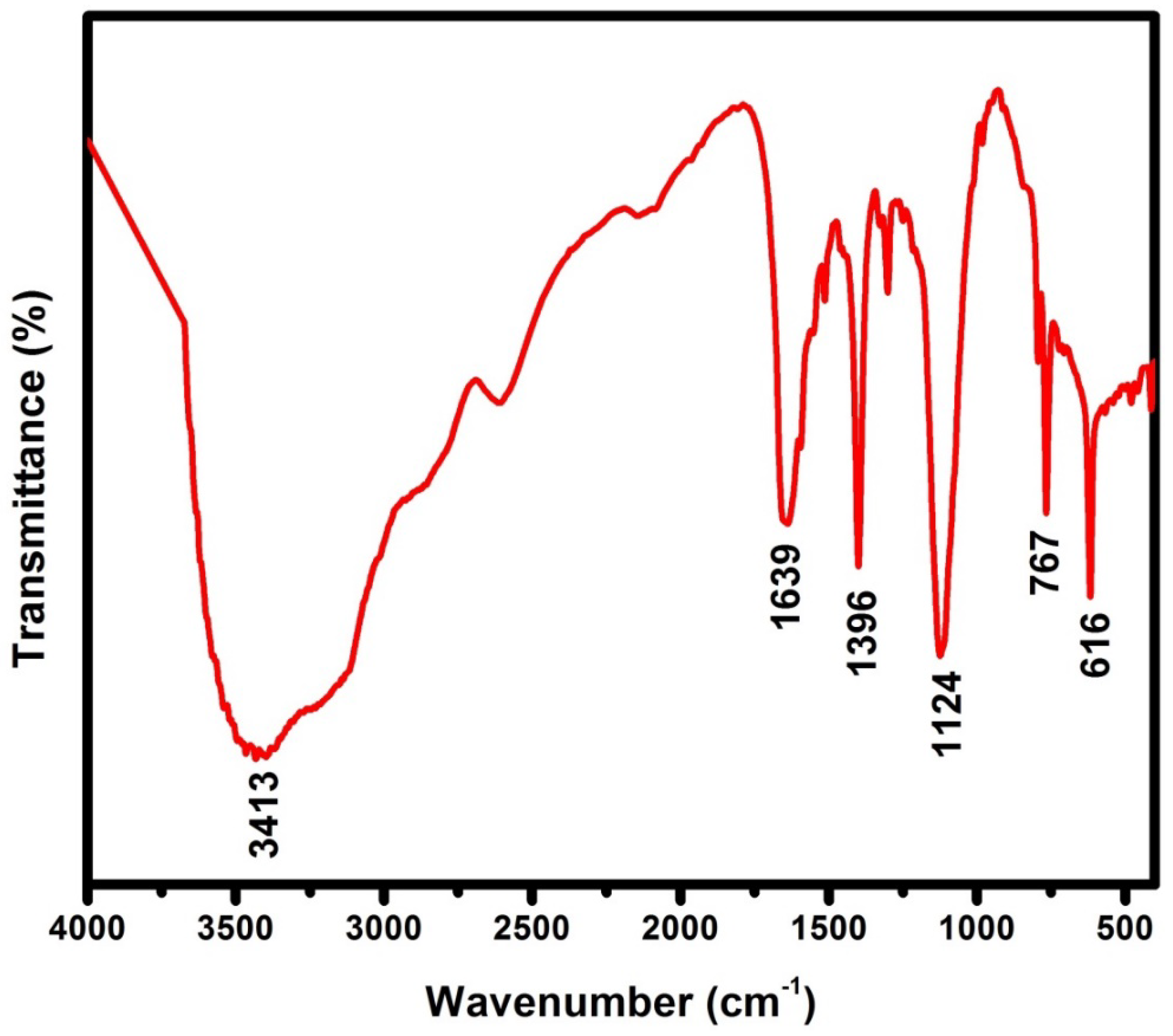
3.1.2. FTIR Studies
3.1.3. FESEM Analysis
3.1.4. UV-Visible Analysis
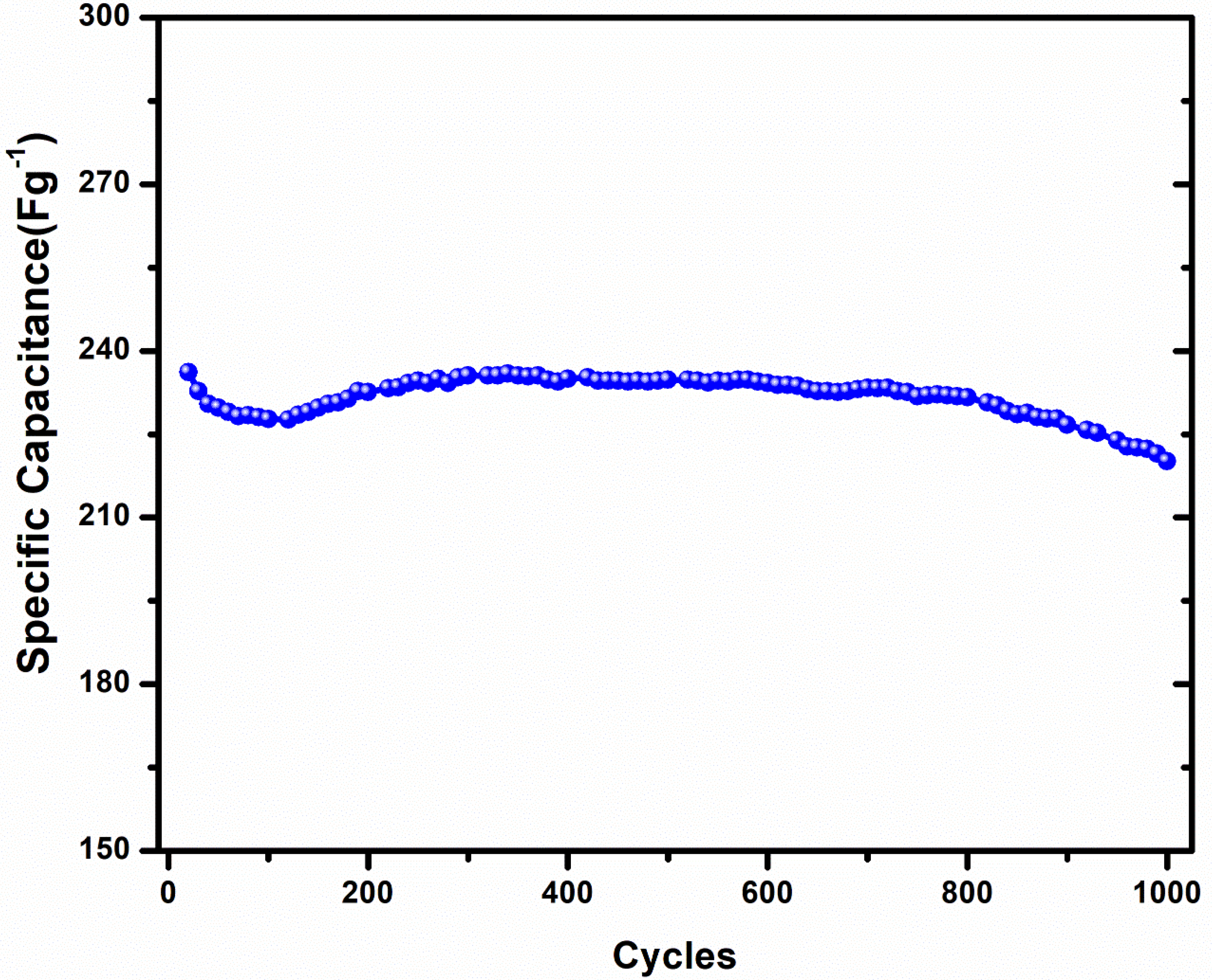
3.2. Supercapacitor Application of PNA Nanoparticles
3.3. Photocatalytic Properties of PNA Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Xu, F.; Hu, Z.A.; Han, S.Y.; Ge, Y.H. Photocatalytic Degradation of Pharmaceutically Active Compounds with Nano-TiO2: Recent Advances and Future Trends. Sci. Adv. Mater. 2021, 13, 2259–2264. [Google Scholar] [CrossRef]
- Alanazi, H.S.; Alotaibi, H.; Al-Shehri, H.S.; Alharthi, F.A. Green Synthesis of NiO Nanoparticle Using Ziziphus Spina-Christi Leaves Extract for Photocatalytic Methylene Blue Dye Degradation. Sci. Adv. Mater. 2021, 13, 1756–1763. [Google Scholar] [CrossRef]
- Won, S.; Kim, J.S. Spinach Extract Derived Carbon Dots Decorated on ZnO Nanorods for Photocatalytic Dye Degradation. Sci. Adv. Mater. 2021, 13, 922–926. [Google Scholar] [CrossRef]
- Michael, M.S.; Prabaharan, S.R.S. High voltage electrochemical double layer capacitors using conductive carbons as additives. J. Power Sources 2004, 136, 250–256. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.-L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Amaresh, S.; Aravindan, V.; Lee, Y.S. Microwave assisted green synthesis of MgO–carbon nanotube composites as electrode material for high power and energy density supercapacitors. J. Mater. Chem. A 2013, 1, 4105–4111. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, J.S.; Jun, Y. Molybdenum diselenide/reduced graphene oxide based hybrid nanosheets for supercapacitor applications. Dalton Trans. 2016, 45, 9646–9653. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, M.; Kim, B.H.; Lee, J.S.; Jun, Y. Freeze-dried MoS 2 sponge electrodes for enhanced electrochemical energy storage. Dalton Trans. 2017, 46, 2122–2128. [Google Scholar] [CrossRef]
- Balasingam; Kannan, S.; Thirumurugan, A.; Lee, J.S.; Jun, Y. Amorphous MoS x thin-film-coated carbon fiber paper as a 3D electrode for long cycle life symmetric supercapacitors. Nanoscale 2016, 8, 11787–11791. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Packiaraj, R.; Devendran, P.; Venkatesh, K.S.; Manikandan, A.; Nallamuthu, N. Electrochemical investigations of magnetic Co 3 O 4 nanoparticles as an active electrode for supercapacitor applications. J. Supercond. Nov. Magn. 2019, 32, 2427–2436. [Google Scholar] [CrossRef]
- Packiaraj, R.; Devendran, P.; Bahadur, S.A.; Nallamuthu, N. Structural and electrochemical studies of Scheelite type BiVO 4 nanoparticles: Synthesis by simple hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 13265–13276. [Google Scholar] [CrossRef]
- Kesavan, K.S.; Surya, K.; Michael, M.S. High powered hybrid supercapacitor with microporous activated carbon. Solid State Ion. 2018, 321, 15–22. [Google Scholar] [CrossRef]
- Jeyabanu, K.; Devendran, P.; Manikandan, A.; Packiaraj, R.; Ramesh, K.; Nallamuthu, N. Preparation and characterization studies of La doped CuS nanospheres by microwave irradiation for high performance supercapacitors. Phys. B Condens. Matter 2019, 573, 92–101. [Google Scholar] [CrossRef]
- Yumak, T.; Dustin Bragg Edward, M. Sabolsky. Effect of synthesis methods on the surface and electrochemical characteristics of metal oxide/activated carbon composites for supercapacitor applications. Appl. Surf. Sci. 2019, 469, 983–993. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. The development supercapacitor from activated carbon by electroless plating—A review. Renew. Sustain. Energy Rev. 2015, 42, 823–834. [Google Scholar] [CrossRef]
- Simon, R.; Chakraborty, S.; Darshini, K.S.; Mary, N.L. Electrolyte dependent performance of graphene–mixed metal oxide composites for enhanced supercapacitor applications. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Wang, B.; Fang, X.; Sun, H.; He, S.; Ren, J.; Zhang, Y.; Peng, H. Fabricating continuous supercapacitor fibers with high performances by integrating all building materials and steps into one process. Adv. Mater. 2015, 27, 7854–7860. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of conducting bifunctional polyaniline@ Mn-TiO2 nanocomposites for supercapacitor electrode and visible light driven photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Jadoun, S.; Sharma, V.; Ashraf, S.M.; Riaz, U. Sonolytic doping of poly (1-naphthylamine) with luminol: Influence on spectral, morphological and fluorescent characteristics. Colloid Polym. Sci. 2017, 295, 715–724. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Ahmad, S.; Riaz, U. Synthesis and Characterization of Novel Poly (1-Naphthylamine)-Montmorillonite Nanocomposites Intercalated by Emulsion Polymerization. J. Macromol. Sci. Part B Phys. 2006, 45, 1109–1123. [Google Scholar] [CrossRef]
- Kashyap, J.; Gautam, S.; Ashraf, S.M.; Riaz, U. Synergistic Performance of Sonolytically Synthesized Poly (1-naphthylamine)/TiO2 Nanohybrids: Degradation Studies of Amido Black-10B Dye. Chem. Sel. 2018, 3, 11926–11934. [Google Scholar]
- Meng, L.; Fan, H.; Lane, J.M.D.; Qin, Y. Bottom-up approaches for precisely nanostructuring hybrid organic/inorganic multi-component composites for organic photovoltaics. MRS Adv. 2020, 41, 2055–2065. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting polymer based nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Yazawa, N.; Tanaka, Y.; Chiba, T.; Izumizawa, T.; Kubo, M. Polycarbazole nanocomposites with conducting metal oxides for transparent electrode applications. ACS Appl. Mater. Interfaces 2010, 2, 413–424. [Google Scholar] [CrossRef]
- Jadoun, S.; Chauhan, N.P.S.; Chinnam, S.; Aepuru, R.; Sathish, M.; Chundawat, N.S.; Rahdar, A. A Short Review on Conducting Polymer Nanocomposites. Biomed. Mater. Devices 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Saidu, F.K.; Joseph, A.; Varghese, E.V.; Thomas, G.V. Characterization and electrochemical studies on poly (1-naphthylamine)-graphene oxide nanocomposites prepared by in situ chemical oxidative polymerization. J. Solid State Electrochem. 2019, 23, 2897–2906. [Google Scholar] [CrossRef]
- Riaz, U.; Ahmad, S.; Ashraf, S.M. Template free synthesis of nanoparticles of poly (1-naphthylamine): Influence of alcoholic medium on polymerization. Colloid Polym. Sci. 2008, 286, 459–462. [Google Scholar] [CrossRef]
- Saidu, K.; Femina, A.J.; Thomas, G.V. Synthesis of novel poly (1-naphthylamine)-silver nanocomposites and its catalytic studies on reduction of 4-nitrophenol and methylene blue. J. Appl. Polym. Sci. 2020, 137, 48318. [Google Scholar]
- Jadoun, S.; Verma, A.; Ashraf, S.M.; Riaz, U. A short review on the synthesis, characterization, and application studies of poly (1-naphthylamine): A seldom explored polyaniline derivative. Colloid Polym. Sci. 2017, 295, 1443–1453. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Kim, Y.S.; Yang, O.-B.; Shin, H.-S. Synthesis and characterization of novel poly (1-naphthylamine)/zinc oxide nanocomposites: Application in catalytic degradation of methylene blue dye. Colloid Polym. Sci. 2010, 288, 1633–1638. [Google Scholar] [CrossRef]
- Gvozdenović, M.M.; Jugović, B.Z.; Jokić, B.M.; Džunuzović, E.S.; Grgur, B.N. Electrochemical synthesis and characterization of poly (o-toluidine) as high energy storage material. Electrochim. Acta 2019, 317, 746–752. [Google Scholar] [CrossRef]
- Hou, Z.Z.; Yang, Q.H. Porous poly (1-naphthylamine) synthesized by interfacial polymerization. Mater. Sci. Forum. 2015, 809, 319–322. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Umar, A.; Shin, H.S. Effective modified electrode of poly (1-naphthylamine) nanoglobules for ultra-high sensitive ethanol chemical sensor. Chem. Eng. J. 2013, 229, 267–275. [Google Scholar] [CrossRef]
- Andriianova, A.N.; Biglova, Y.N.; Mustafin, A.G. Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv. 2020, 10, 7468–7491. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Wei, Z. Electrochemical properties of poly-1-naphthylamine synthesized in the presence of ferrocenesulfonic acid. Synth. Met. 2005, 155, 45–50. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Salmany-Afagh, P. Electrocatalytic oxidation of some carbohydrates by poly (1-naphthylamine)/nickel modified carbon paste electrode. J. Electroanal. Chem. 2004, 571, 1–8. [Google Scholar] [CrossRef]
- Ishaq, S.; Moussa, M.; Kanwal, F.; Ehsan, M.; Saleem, M.; Van, T.N.; Losic, D. Facile synthesis of ternary graphene nanocomposites with doped metal oxide and conductive polymers as electrode materials for high performance supercapacitors. Sci. Rep. 2019, 9, 5974. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Budhiraja, V.; Aleem, S.; Kashyap, J. Comparative studies of the photocatalytic and microwave–assisted degradation of alizarin red using ZnO/poly (1-naphthylamine) nanohybrids. J. Mol. Liq. 2016, 216, 259–267. [Google Scholar] [CrossRef]
- Rosaline, D.R.; Suganthi, A.; Vinodhkumar, G.; Inbanathan, S.S.R.; Umar, A.; Ameen, S.; Akhtar, M.S.; LuizFoletto, E. Enhanced sunlight-driven photocatalytic activity of SnO2-Sb2O3 composite towards emerging contaminant degradation in water. J. Alloy. Compd. 2022, 897, 162935. [Google Scholar] [CrossRef]
- Silvestri, S.; de Oliveira, J.F.; Foletto, E.L. Degradation of methylene blue using Zn2SnO4 catalysts prepared with pore-forming agents. Mater. Res. Bull. 2019, 117, 56–62. [Google Scholar] [CrossRef]
- Saidu, F.K.; Thomas, G.V. Synthesis, characterization and dielectric studies of poly (1-naphthylamine)–tungsten disulphide nanocomposites. SN Appl. Sci. 2020, 2, 1158. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Packiaraj, R.; Nallamuthu, N.; Bahadur, S.A. Electrochemical performance and optimization of α-NiMoO4 by different facile synthetic approach for supercapacitor application. J. Mater. Sci. Mater. Electron. 2019, 30, 3305–3315. [Google Scholar] [CrossRef]
- Chauhan, M.; Kaur, N.; Bansal, P.; Kumar, R.; Srinivasan, S.; Chaudhary, G.R. Proficient photocatalytic and sonocatalytic degradation of organic pollutants using CuO nanoparticles. J. Nanomater. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Kim, Y.S.; Shin, H.S. Nanocomposites of poly (1-naphthylamine)/SiO2 and poly (1-naphthylamine)/TiO2: Comparative photocatalytic activity evaluation towards methylene blue dye. Appl. Catal. B Environ. 2011, 2, 136–142. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Hexagonal ZnO Nanorods Assembled Flowers for Photocatalytic Dye Degradation: Growth, Structural and Optical Properties. Superlattices Microstruct. 2014, 64, 495–506. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, A.; Kumar, S.A.; Rosaline, D.R.; Algadi, H.; Ibrahim, A.A.; Ahmed, F.; Foletto, E.L.; Inbanathan, S.S.R. Poly(1-Napthylamine) Nanoparticles as Potential Scaffold for Supercapacitor and Photocatalytic Applications. Micromachines 2022, 13, 1528. https://doi.org/10.3390/mi13091528
Umar A, Kumar SA, Rosaline DR, Algadi H, Ibrahim AA, Ahmed F, Foletto EL, Inbanathan SSR. Poly(1-Napthylamine) Nanoparticles as Potential Scaffold for Supercapacitor and Photocatalytic Applications. Micromachines. 2022; 13(9):1528. https://doi.org/10.3390/mi13091528
Chicago/Turabian StyleUmar, Ahmad, Sundararajan Ashok Kumar, Daniel Rani Rosaline, Hassan Algadi, Ahmed A. Ibrahim, Faheem Ahmed, Edson Luiz Foletto, and Savariroyan Stephen Rajkumar Inbanathan. 2022. "Poly(1-Napthylamine) Nanoparticles as Potential Scaffold for Supercapacitor and Photocatalytic Applications" Micromachines 13, no. 9: 1528. https://doi.org/10.3390/mi13091528
APA StyleUmar, A., Kumar, S. A., Rosaline, D. R., Algadi, H., Ibrahim, A. A., Ahmed, F., Foletto, E. L., & Inbanathan, S. S. R. (2022). Poly(1-Napthylamine) Nanoparticles as Potential Scaffold for Supercapacitor and Photocatalytic Applications. Micromachines, 13(9), 1528. https://doi.org/10.3390/mi13091528







