Advances in BBB on Chip and Application for Studying Reversible Opening of Blood–Brain Barrier by Sonoporation
Abstract
:1. Introduction
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Intranasal drug delivery | Non-invasive; direct reach to the brain | Affected by the nature of nasal mucosa; low efficiency | [15,16,17] |
| Chemical agents (osmotic procedure) | Well-developed; reversible disruption of the BBB | Pre-conditions needed; the whole BBB is exposed; neurotoxins influx | [8,9,10,18] |
| Local delivery (surgery) | Highly effective; repeatable; minimized systemic exposure; controlled drug releases time | Invasive; limited drug concentration and distribution; infection risks | [19,20] |
| Sonoporation | Non-invasive; minimized systemic exposure; reversible opening; well controlled; highly effective | Equipment needed; neurotoxins influx; | [21,22,23] |
2. Structure of BBB and Substance Transportation
2.1. Cellular Structure of BBB
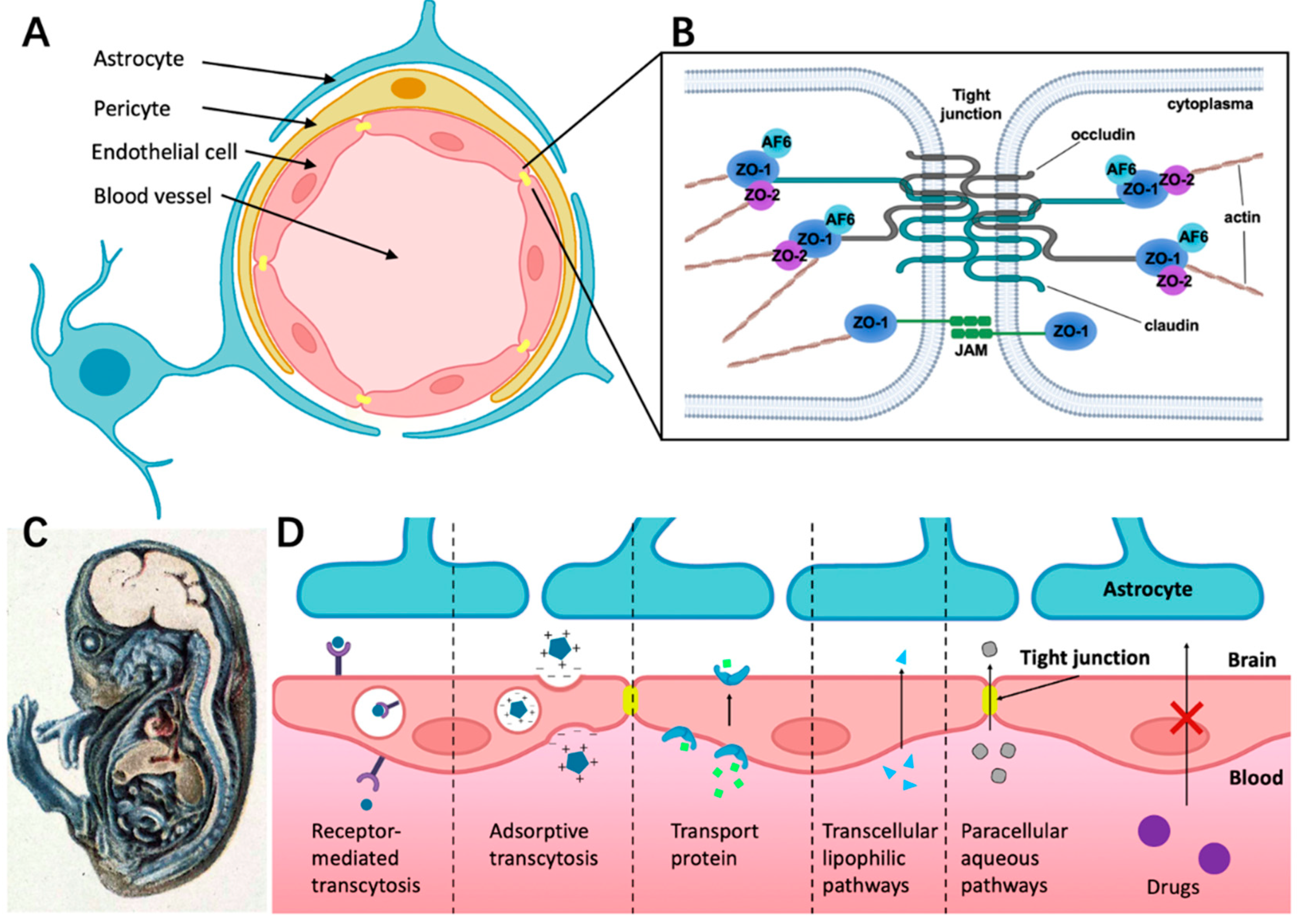
2.2. Structure Foundation of Tight Junctions (TJs)
2.3. Different Ways for Substances Crossing the BBB under Physiological Conditions
3. Recent Work in BOC Development
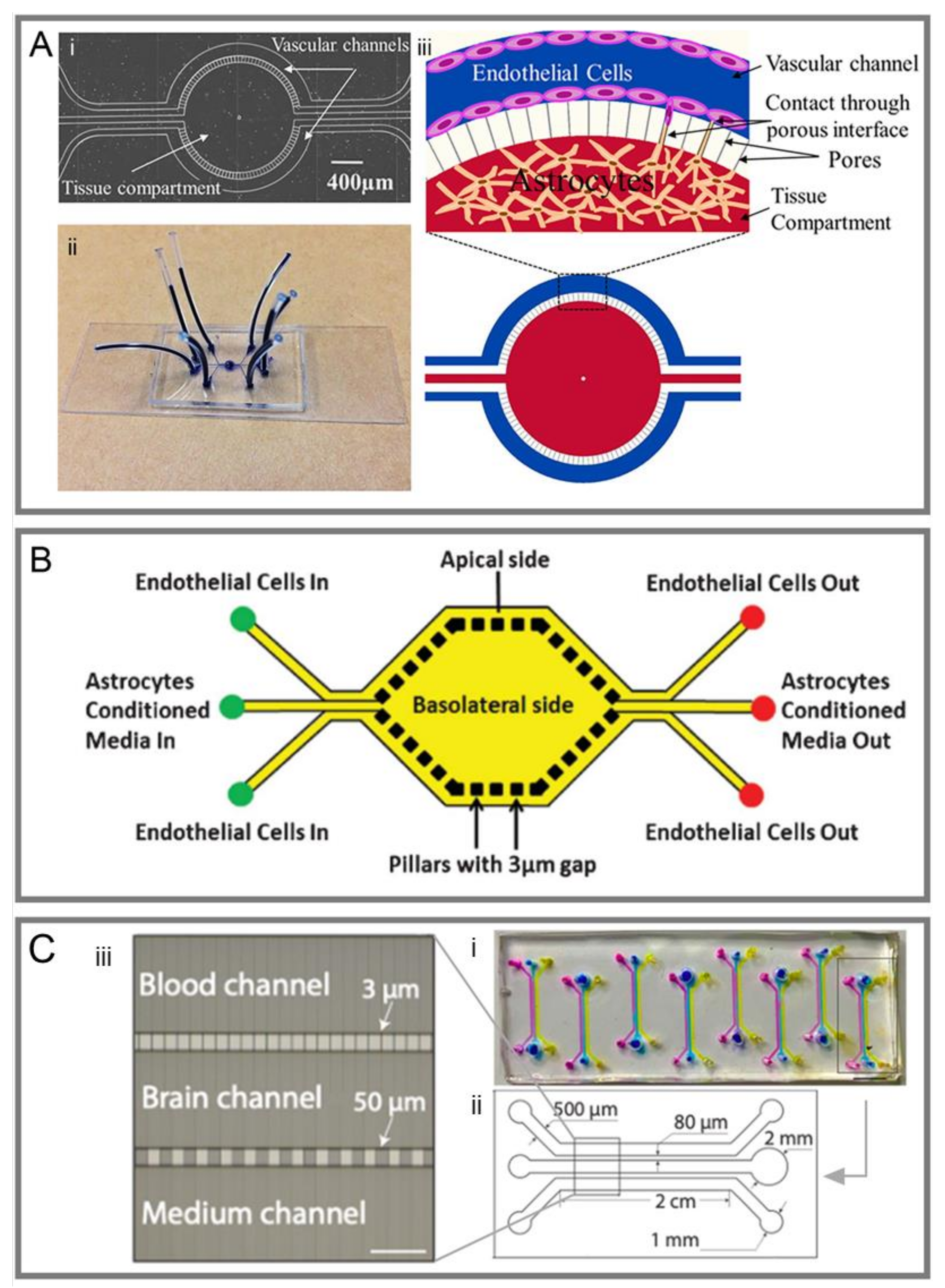
3.1. Sandwich Models
3.2. Two-Dimensional Models
3.3. Three-Dimensional Models

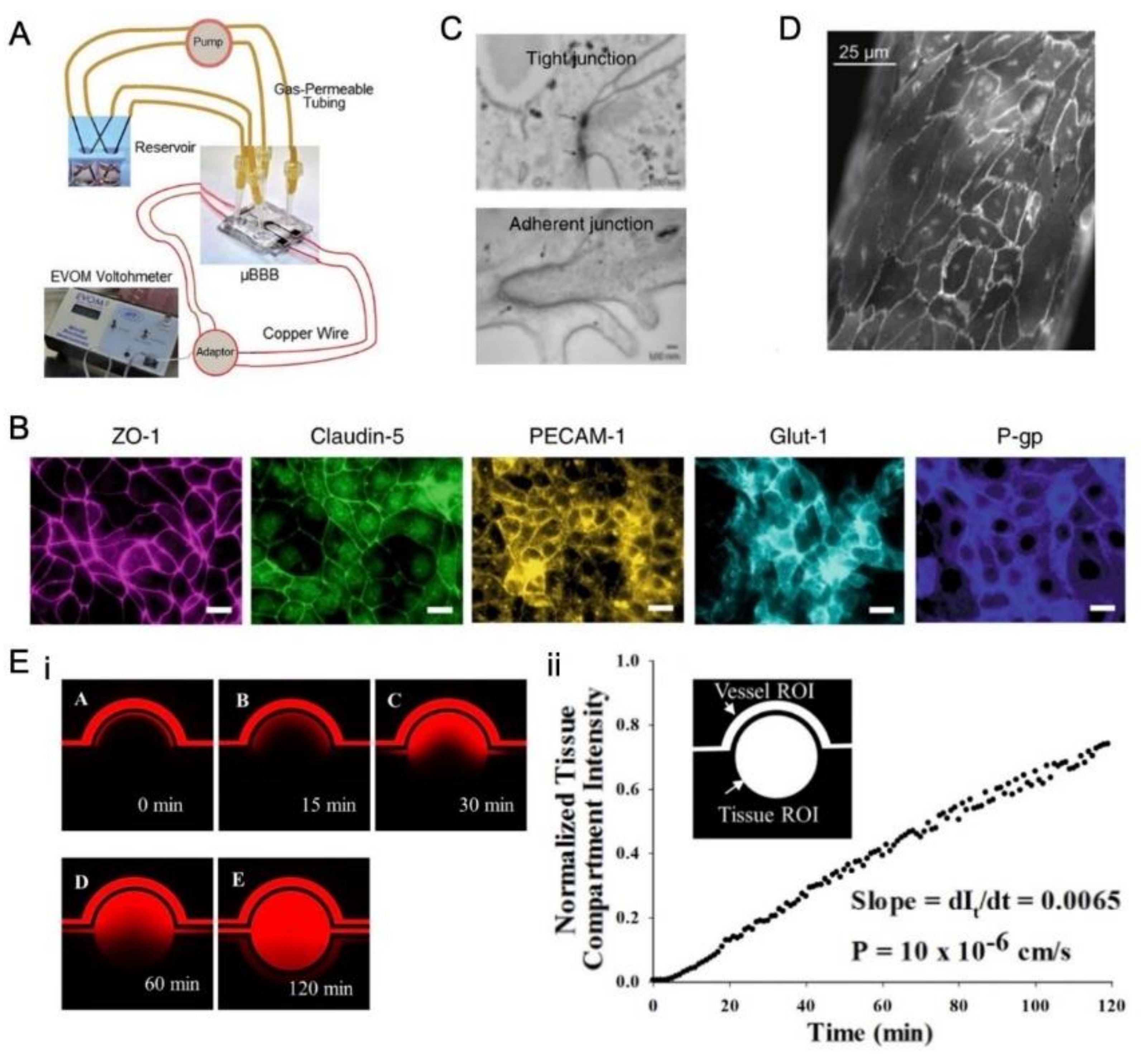
3.4. Different Means for Evaluating BBB Integrity In Vitro
4. Ultrasound-Driven Microbubbles Reversibly Open BBB
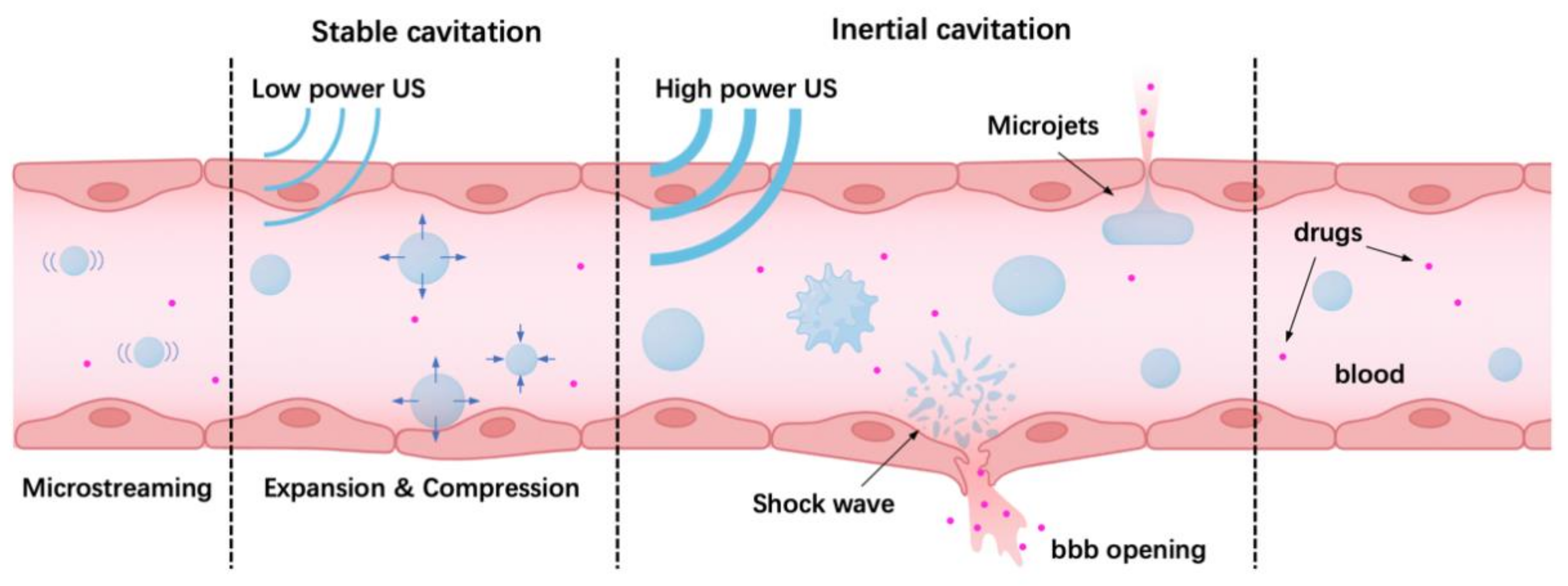
4.1. Oscillation and Cavitation of Microbubbles Driven by Ultrasound
4.2. BBB Opening by Ultrasound-Driven Microbubbles In Vivo and In Vitro
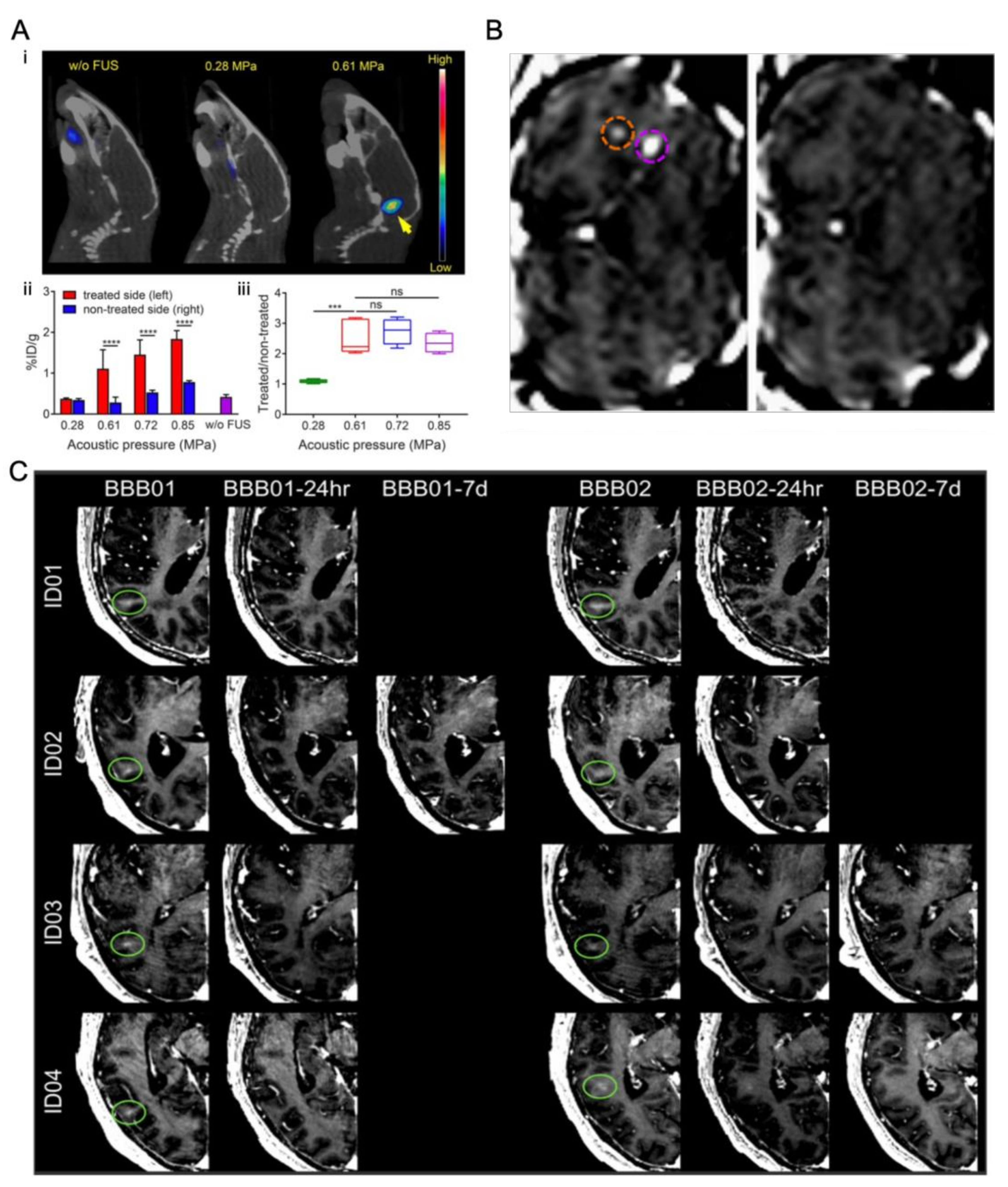
4.2.1. In Vivo Experiments
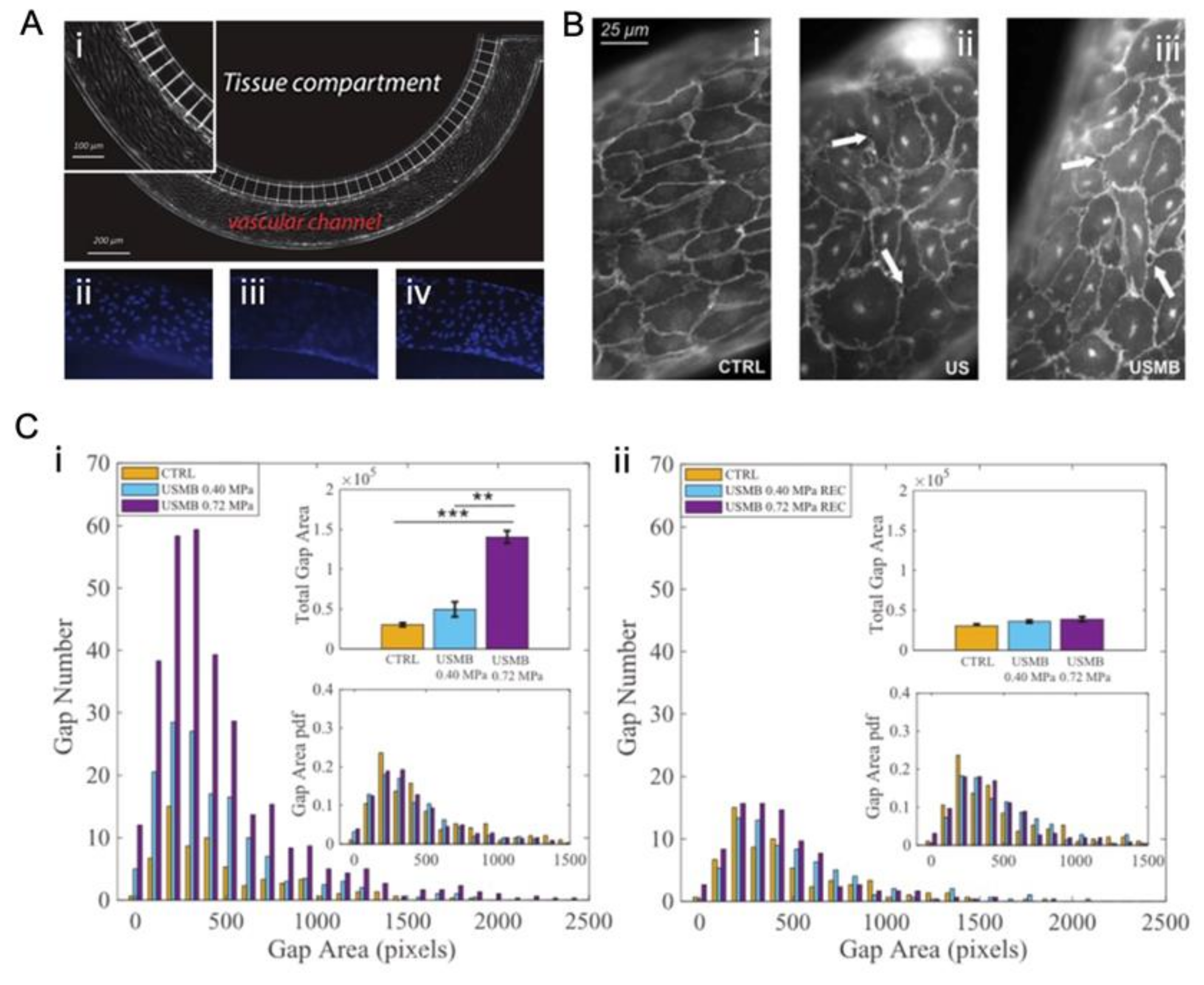
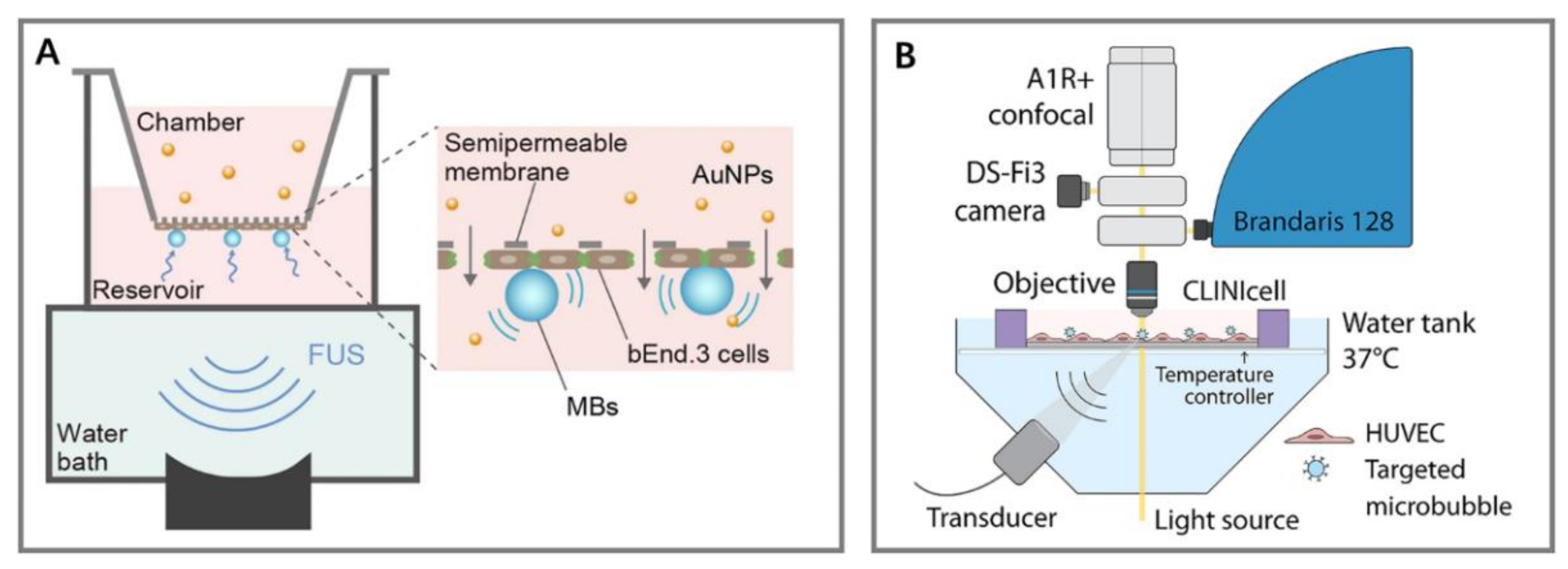
4.2.2. In Vitro Static System
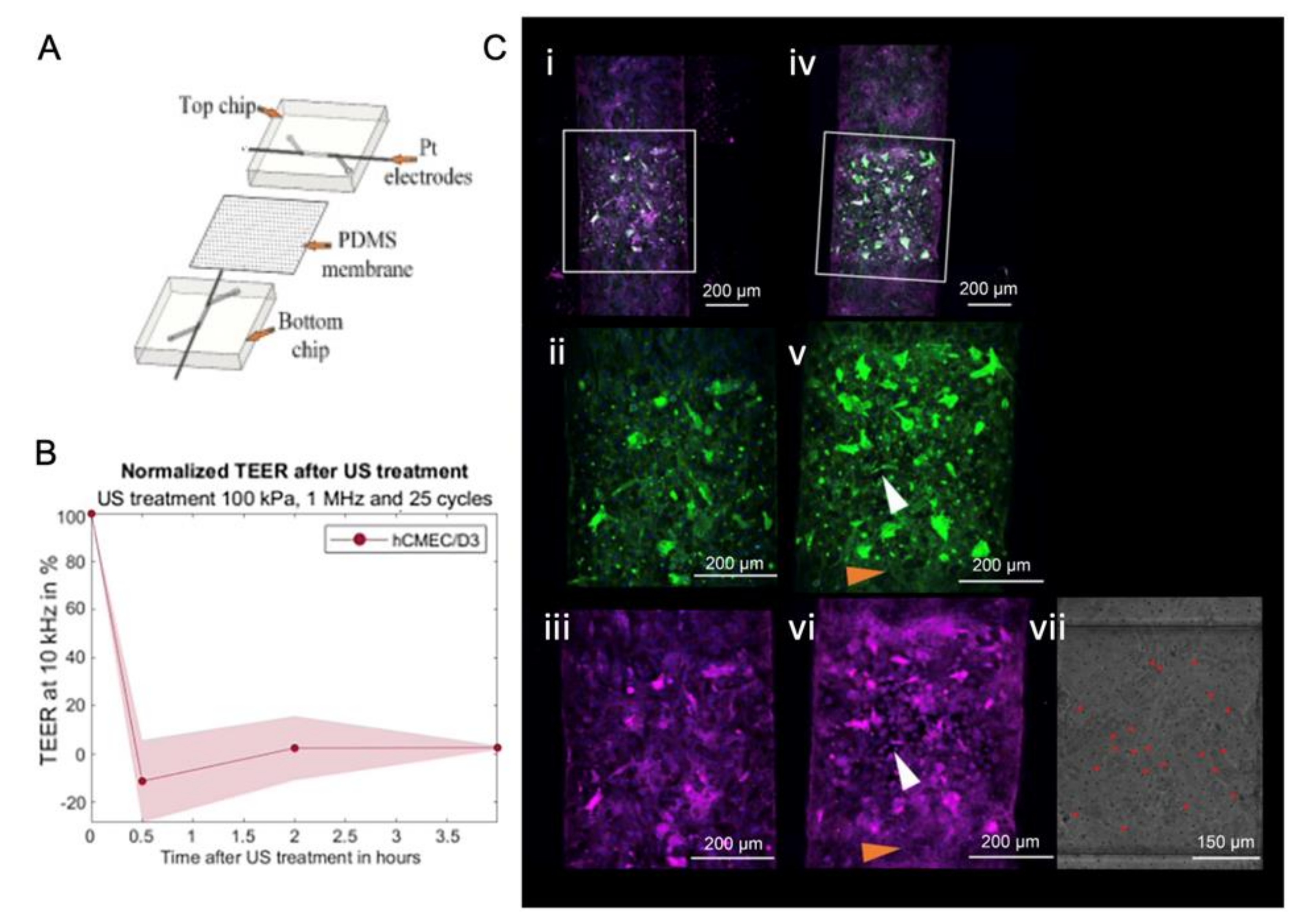
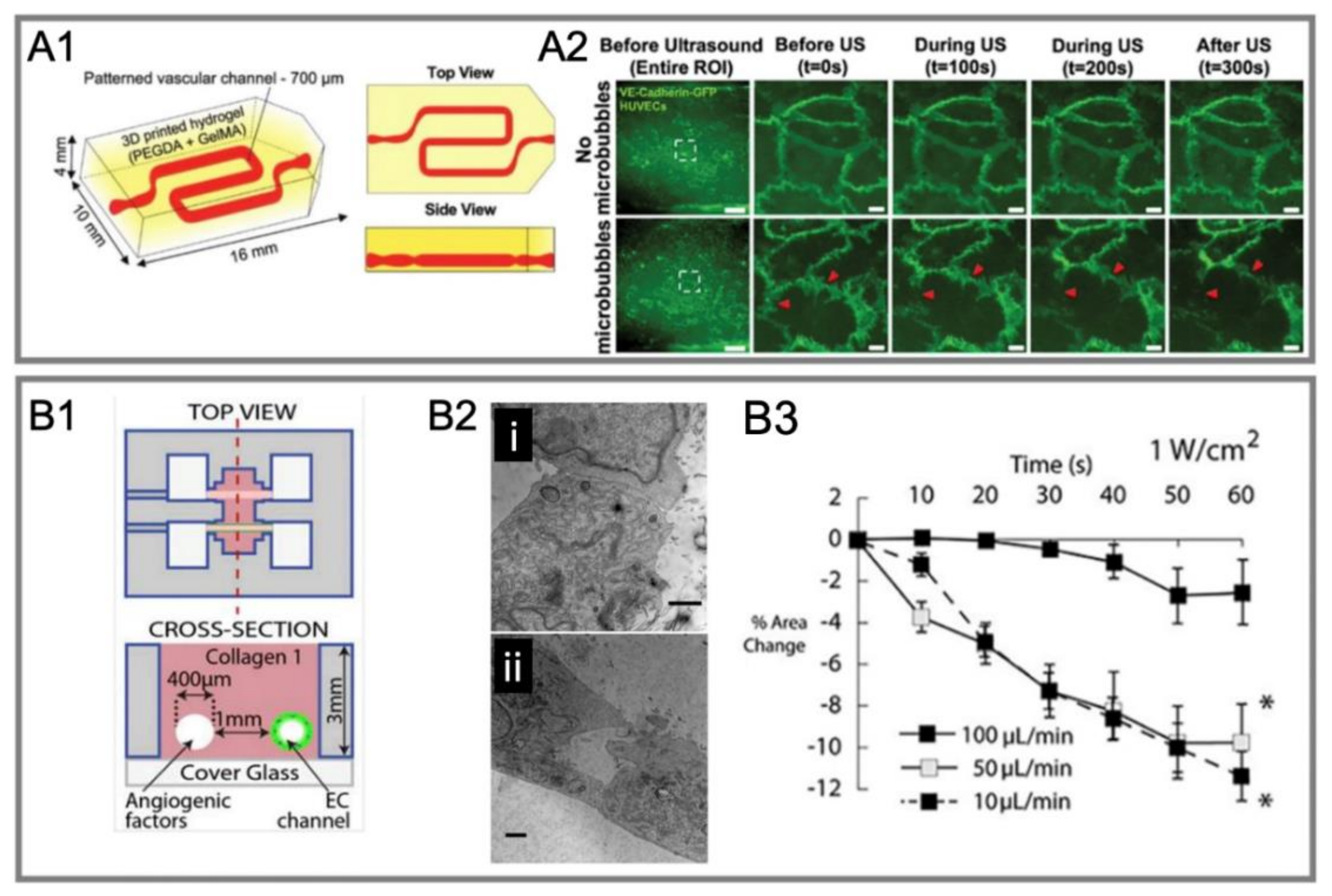
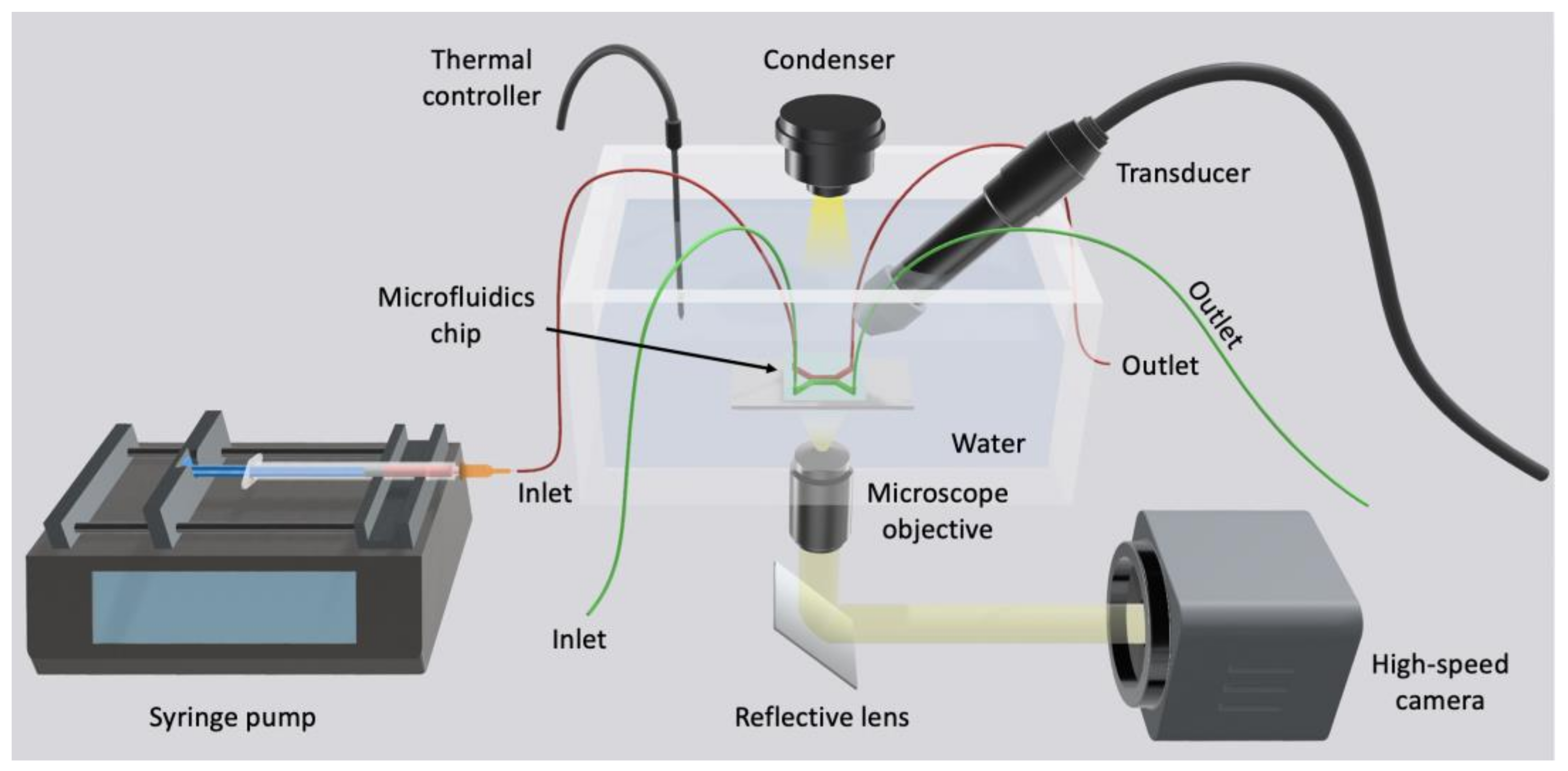
4.2.3. In Vitro Dynamic System

5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 14, 367–429. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier drug targeting: The future of brain drug development. Mol. Interv. 2003, 3, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidenfeller, C.; Svendsen, C.N.; Shusta, E.V. Differentiating embryonic neural progenitor cells induce blood–brain barrier properties. J. Neurochem. 2007, 101, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Aday, S.; Cecchelli, R.; Hallier-Vanuxeem, D.; Dehouck, M.P.; Ferreira, L. Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefano, P.; Bianchi, E.; Dubini, G. The impact of microfluidics in high-throughput drug-screening applications. Biomicrofluidics 2022, 16, 031501. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M. Brain-on-a-Chip: Characterizing the next generation of advanced in vitro platforms for modeling the central nervous system. APL Bioeng. 2021, 5, 030902. [Google Scholar] [CrossRef]
- Matsukado, K.; Sugita, M.; Black, K.L. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 1998, 792, 10–15. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic Opening of the Blood–Brain Barrier: Principles, Mechanism, and Therapeutic Applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef]
- Rapoport, S.I. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs 2001, 10, 1809–1818. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Alkins, R.; Schwartz, M.L.; Hynynen, K. Opening the Blood-Brain Barrier with MR Imaging-guided Focused Ultrasound: Preclinical Testing on a Trans-Human Skull Porcine Model. Radiology 2017, 282, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ye, D.; Yang, L.; Yue, Y.; Sultan, D.; Pacia, C.P.; Pang, H.; Detering, L.; Heo, G.S.; Luehmann, H.; et al. Magnetic Resonance Imaging-Guided Focused Ultrasound-Based Delivery of Radiolabeled Copper Nanoclusters to Diffuse Intrinsic Pontine Glioma. ACS Appl. Nano. Mater. 2020, 3, 11129–11134. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef] [PubMed]
- Erdo, F.; Bors, L.A.; Farkas, D.; Bajza, A.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Posadas, I.; Monteagudo, S.; Ceña, V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine 2016, 11, 833–849. [Google Scholar] [CrossRef] [Green Version]
- Chaichana, K.L.; Pinheiro, L.; Brem, H. Delivery of local therapeutics to the brain: Working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015, 6, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Malviya, R.; K Sharma, P. Brain targeted drug delivery: Factors, approaches and patents. Recent Pat. Nanomed. 2014, 4, 2–14. [Google Scholar]
- Silvani, G.; Scognamiglio, C.; Caprini, D.; Marino, L.; Chinappi, M.; Sinibaldi, G.; Peruzzi, G.; Kiani, M.F.; Casciola, C.M. Reversible Cavitation-Induced Junctional Opening in an Artificial Endothelial Layer. Small 2019, 15, e1905375. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, K.; Cui, J.; Ye, J.Y.; Deng, C.X. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release 2012, 157, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood–brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Ridet, J.; Privat, A.; Malhotra, S.; Gage, F. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.K.; D’Amore, P.A. Pericytes in the microvasculature. Cardiovasc. Res. 1996, 32, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001, 153, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [Green Version]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, M.; Palma do Carmo, M.A.; van der Helm, M.W.; Le-The, H.; de Graaf, M.N.S.; Orlova, V.; van den Berg, A.; van der Meer, A.D.; Broersen, K.; Segerink, L.I. Multiplexed blood-brain barrier organ-on-chip. Lab Chip 2020, 20, 3132–3143. [Google Scholar] [CrossRef]
- Biemans, E.A.; Jäkel, L.; de Waal, R.M.; Kuiperij, H.B.; Verbeek, M.M. Limitations of the hCMEC/D3 cell line as a model for Aβ clearance by the human blood-brain barrier. J. Neurosci. Res. 2017, 95, 1513–1522. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [Green Version]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef] [Green Version]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef]
- Kim, D.; Eom, S.; Park, S.M.; Hong, H.; Kim, D.S. A collagen gel-coated, aligned nanofiber membrane for enhanced endothelial barrier function. Sci. Rep. 2019, 9, 14915. [Google Scholar] [CrossRef] [Green Version]
- Juang, E.K.; De Cock, I.; Keravnou, C.; Gallagher, M.K.; Keller, S.B.; Zheng, Y.; Averkiou, M. Engineered 3D Microvascular Networks for the Study of Ultrasound-Microbubble-Mediated Drug Delivery. Langmuir 2019, 35, 10128–10138. [Google Scholar] [CrossRef]
- Qian, T.; Maguire, S.E.; Canfield, S.G.; Bao, X.; Olson, W.R.; Shusta, E.V.; Palecek, S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017, 3, e1701679. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Mollgard, K.; Bauer, H.C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 2017, 94, 581–594.e585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Smart strategies for therapeutic agent delivery into brain across the blood–brain barrier using receptor-mediated transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, I.; Oliver, J.A.; Xu, H.; Fu, B.M.; Zhu, D. Blood-Brain Barrier Integrity and Clearance of Amyloid-β from the BBB. Adv. Exp. Med. Biol. 2018, 1097, 261–278. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef]
- Joseph, E.; Saha, R.N. Advances in brain targeted drug delivery: Nanoparticulate systems. J. PharmaSciTech 2013, 3, 1–8. [Google Scholar]
- Piantino, M.; Louis, F.; Shigemoto-Mogami, Y.; Kitamura, K.; Sato, K.; Yamaguchi, T.; Kawabata, K.; Yamamoto, S.; Iwasaki, S.; Hirabayashi, H.; et al. Brain microvascular endothelial cells derived from human induced pluripotent stem cells as in vitro model for assessing blood-brain barrier transferrin receptor-mediated transcytosis. Mater. Today Bio 2022, 14, 100232. [Google Scholar] [CrossRef]
- Goodwin-Trotman, M.; Patel, K.; Granata, A. An hiPSC-Derived In Vitro Model of the Blood–Brain Barrier. In The Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 2022; pp. 103–116. [Google Scholar]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Establishment of an In Vitro Model of Human Blood–Brain Barrier to Study the Impact of Ischemic Injury. In The Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 2022; pp. 143–155. [Google Scholar]
- Gericke, B.; Romermann, K.; Noack, A.; Noack, S.; Kronenberg, J.; Blasig, I.E.; Loscher, W. A face-to-face comparison of claudin-5 transduced human brain endothelial (hCMEC/D3) cells with porcine brain endothelial cells as blood-brain barrier models for drug transport studies. Fluids Barriers CNS 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Nery, E.D.; Ebner, D.; Montoya, M.C.; Ostling, P.; Pietiainen, V.; Price, L.S.; et al. Screening out irrelevant cell-based models of disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, R.; Kim, H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood-brain barrier model. Ann. Biomed. Eng. 2014, 42, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Griep, L.M.; Wolbers, F.; de Wagenaar, B.; ter Braak, P.M.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A. BBB on chip: Microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices 2013, 15, 145–150. [Google Scholar] [CrossRef]
- Achyuta, A.K.H.; Conway, A.J.; Crouse, R.B.; Bannister, E.C.; Lee, R.N.; Katnik, C.P.; Behensky, A.A.; Cuevas, J.; Sundaram, S.S. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 2013, 13, 542–553. [Google Scholar] [CrossRef]
- Walter, F.R.; Valkai, S.; Kincses, A.; Petnehazi, A.; Czeller, T.; Veszelka, S.; Ormos, P.; Deli, M.A.; Der, A. A versatile lab-on-a-chip tool for modeling biological barriers. Sens. Actuators B-Chem. 2016, 222, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A. Shuttle-mediated nanoparticle transport across an in vitro brain endothelium under flow conditions. Biotechnol. Bioeng. 2017, 114, 1087–1095. [Google Scholar] [CrossRef]
- Shao, X.; Gao, D.; Chen, Y.; Jin, F.; Hu, G.; Jiang, Y.; Liu, H. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal. Chim. Acta 2016, 934, 186–193. [Google Scholar] [CrossRef]
- Mossu, A.; Rosito, M.; Khire, T.; Chung, H.L.; Nishihara, H.; Gruber, I.; Luke, E.; Dehouck, L.; Sallusto, F.; Gosselet, F.; et al. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood-brain barrier under flow. J. Cereb. Blood Flow Metab. 2019, 39, 395–410. [Google Scholar] [CrossRef] [Green Version]
- Hudecz, D.; Khire, T.; Chung, H.L.; Adumeau, L.; Glavin, D.; Luke, E.; Nielsen, M.S.; Dawson, K.A.; McGrath, J.L.; Yan, Y. Ultrathin Silicon Membranes for in Situ Optical Analysis of Nanoparticle Translocation across a Human Blood-Brain Barrier Model. ACS Nano 2020, 14, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem. Cell 2019, 24, 995–1005.e1006. [Google Scholar] [CrossRef] [PubMed]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.; Seo, J.H.; Garud, K.S.; Park, S.W.; Lee, M.Y. Numerical approach-based simulation to predict cerebrovascular shear stress in a blood-brain barrier organ-on-a-chip. Biosens. Bioelectron. 2021, 183, 113197. [Google Scholar] [CrossRef]
- Tu, K.H.; Yu, L.S.; Sie, Z.H.; Hsu, H.Y.; Al-Jamal, K.T.; Wang, J.T.; Chiang, Y.Y. Development of Real-Time Transendothelial Electrical Resistance Monitoring for an In Vitro Blood-Brain Barrier System. Micromachines 2020, 12, 37. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Hawkins, B.T.; Grego, S. An optically transparent membrane supports shear stress studies in a three-dimensional microfluidic neurovascular unit model. Biomicrofluidics 2015, 9, 061102. [Google Scholar] [CrossRef] [Green Version]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Domansky, K.; Leslie, D.C.; McKinney, J.; Fraser, J.P.; Sliz, J.D.; Hamkins-Indik, T.; Hamilton, G.A.; Bahinski, A.; Ingber, D.E. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab Chip 2013, 13, 3956–3964. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Onoe, H.; Osaki, T.; Kawano, R.; Takeuchi, S. Parylene-coating in PDMS microfluidic channels prevents the absorption of fluorescent dyes. Sens. Actuators B Chem. 2010, 150, 478–482. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; AP, I.J.; Mummery, C.L. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Kumar, N.; Foo, L.C.; Ng, S.H.; Hunziker, W.; Choudhury, D. A pump-free tricellular blood-brain barrier on-a-chip model to understand barrier property and evaluate drug response. Biotechnol. Bioeng. 2020, 117, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A Novel Dynamic Neonatal Blood-Brain Barrier on a Chip. PLoS ONE 2015, 10, e0142725. [Google Scholar] [CrossRef] [PubMed]
- Prabhakarpandian, B.; Shen, M.C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic Blood Brain Barrier model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, A.M.; Lutton, E.M.; Cannella, L.A.; Reichenbach, N.; Razmpour, R.; Seasock, M.J.; Kaspin, S.J.; Merkel, S.F.; Langford, D.; Persidsky, Y.; et al. Characterization of human fetal brain endothelial cells reveals barrier properties suitable for in vitro modeling of the BBB with syngenic co-cultures. J. Cereb. Blood Flow Metab. 2018, 38, 888–903. [Google Scholar] [CrossRef]
- Brown, T.D.; Nowak, M.; Bayles, A.V.; Prabhakarpandian, B.; Karande, P.; Lahann, J.; Helgeson, M.E.; Mitragotri, S. A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. Transl. Med. 2019, 4, e10126. [Google Scholar] [CrossRef] [Green Version]
- Terrell-Hall, T.B.; Ammer, A.G.; Griffith, J.I.; Lockman, P.R. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, Y.B.; Charlebois, C.; Nguyen, T.; Liu, Z.Y.; Bloemberg, D.; Zafer, A.; Baumann, E.; Sodja, C.; Leclerc, S.; et al. Application of blood brain barrier models in pre-clinical assessment of glioblastoma-targeting CAR-T based immunotherapies. Fluids Barriers CNS 2022, 19, 38. [Google Scholar] [CrossRef]
- Peng, B.; Tong, Z.; Tong, W.Y.; Pasic, P.J.; Oddo, A.; Dai, Y.; Luo, M.; Frescene, J.; Welch, N.G.; Easton, C.D.; et al. In situ surface modification of microfluidic blood-brain-barriers for improved screening of small molecules and nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 56753–56766. [Google Scholar] [CrossRef]
- van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [Green Version]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delince, M.; Di Caprio, G.; Upadhyayula, S.; de Nola, G.; Hunt, R.; Ohashi, K.G.; Gray, T.; et al. Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Nah, S.Y.; Lee, K.; Choi, N.; Kim, H.N. Triculture Model of In Vitro BBB and its Application to Study BBB-Associated Chemosensitivity and Drug Delivery in Glioblastoma. Adv. Funct. Mater. 2021, 32, 2106860. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, H.N.; Im, S.K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [Green Version]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Chung, B.; Kim, J.; Nam, J.; Kim, H.; Jeong, Y.; Liu, H.W.; Cho, Y.; Kim, Y.H.; Oh, H.J.; Chung, S. Evaluation of Cell-Penetrating Peptides Using Microfluidic In Vitro 3D Brain Endothelial Barrier. Macromol. Biosci. 2020, 20, e1900425. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Partyka, P.P.; Godsey, G.A.; Galie, J.R.; Kosciuk, M.C.; Acharya, N.K.; Nagele, R.G.; Galie, P.A. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials 2017, 115, 30–39. [Google Scholar] [CrossRef]
- He, Y.N.; Yu, Y.; Yang, Y.Q.; Gu, Y.X.; Mao, T.J.; Shen, Y.; Liu, Q.; Liu, R.L.; Ding, J.D. Design and aligner-assisted fast fabrication of a microfluidic platform for quasi-3D cell studies on an elastic polymer. Bioact. Mater. 2022, 15, 288–304. [Google Scholar] [CrossRef]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef] [Green Version]
- Collins, N.T.; Cummins, P.M.; Colgan, O.C.; Ferguson, G.; Birney, Y.A.; Murphy, R.P.; Meade, G.; Cahill, P.A. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: Influence on intercellular tight junction assembly and function. Arter. Thromb. Vasc. Biol. 2006, 26, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, C.W., Jr.; McIntire, L.V. Shear stress and cyclic strain modulation of gene expression in vascular endothelial cells. Blood Purif. 1995, 13, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.H.; Duong, D.D.; Kwun, J.D.; Lee, N.Y. Hybrid elastomer-plastic microfluidic device as a convenient model for mimicking the blood-brain barrier in vitro. Biomed. Microdevices 2019, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.J.; Huang, C.C.; Fan, C.H.; Liu, H.L.; Yeh, C.K. Ultrasonic technologies in imaging and drug delivery. Cell Mol. Life Sci. 2021, 78, 6119–6141. [Google Scholar] [CrossRef]
- Presset, A.; Bonneau, C.; Kazuyoshi, S.; Nadal-Desbarats, L.; Mitsuyoshi, T.; Bouakaz, A.; Kudo, N.; Escoffre, J.-M.; Sasaki, N. Endothelial cells, first target of drug delivery using microbubble-assisted ultrasound. Ultrasound Med. Biol. 2020, 46, 1565–1583. [Google Scholar] [CrossRef]
- De Cock, I.; Zagato, E.; Braeckmans, K.; Luan, Y.; de Jong, N.; De Smedt, S.C.; Lentacker, I. Ultrasound and microbubble mediated drug delivery: Acoustic pressure as determinant for uptake via membrane pores or endocytosis. J. Control. Release 2015, 197, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.C.; Zhang, C.; Kim, S.; Mohamedi, G.; Beigie, C.; Nagy, J.O.; Holt, R.G.; Cleveland, R.O.; Jeon, N.L.; Wong, J.Y. Microvessels-on-a-Chip to Assess Targeted Ultrasound-Assisted Drug Delivery. ACS Appl. Mater Interfaces 2016, 8, 31541–31549. [Google Scholar] [CrossRef]
- Stride, E.P.; Coussios, C.C. Cavitation and contrast: The use of bubbles in ultrasound imaging and therapy. Proc. Inst. Mech. Eng. H 2010, 224, 171–191. [Google Scholar] [CrossRef]
- Li, F.; Yang, C.; Yuan, F.; Liao, D.; Li, T.; Guilak, F.; Zhong, P. Dynamics and mechanisms of intracellular calcium waves elicited by tandem bubble-induced jetting flow. Proc. Natl. Acad. Sci. USA 2018, 115, E353–E362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadzadeh, M.; Li, F.; Ohl, C.-D. Shearing flow from transient bubble oscillations in narrow gaps. Phys. Rev. Fluids 2017, 2, 014301. [Google Scholar] [CrossRef]
- Li, F.; Park, T.H.; Sankin, G.; Gilchrist, C.; Liao, D.; Chan, C.U.; Mao, Z.; Hoffman, B.D.; Zhong, P. Mechanically induced integrin ligation mediates intracellular calcium signaling with single pulsating cavitation bubbles. Theranostics 2021, 11, 6090–6104. [Google Scholar] [CrossRef]
- Li, F.; Yuan, F.; Sankin, G.; Yang, C.; Zhong, P. A Microfluidic System with Surface Patterning for Investigating Cavitation Bubble(s)-Cell Interaction and the Resultant Bioeffects at the Single-cell Level. J. Vis. Exp. 2017, 119, e55106. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snipstad, S.; Sulheim, E.; de Lange Davies, C.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to improve drug delivery to tumors: From fundamental understanding to clinical translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Hynynen, K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin. Drug Deliv. 2014, 11, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.H.; Liu, H.L.; Ting, C.Y.; Lee, Y.H.; Huang, C.Y.; Ma, Y.J.; Wei, K.C.; Yen, T.C.; Yeh, C.K. Submicron-bubble-enhanced focused ultrasound for blood-brain barrier disruption and improved CNS drug delivery. PLoS ONE 2014, 9, e96327. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- Ting, C.Y.; Fan, C.H.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712. [Google Scholar] [CrossRef]
- Marquet, F.; Tung, Y.S.; Teichert, T.; Ferrera, V.P.; Konofagou, E.E. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo. PLoS ONE 2011, 6, e22598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDannold, N.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M.S. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012, 72, 3652–3663. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Boska, M.D.; Lof, J.; Uberti, M.G.; Tsutsui, J.M.; Porter, T.R. Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model. Ultrasound Med. Biol. 2008, 34, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Salas, C.; Fernandez-Rodriguez, B.; Pineda-Pardo, J.A.; Rodriguez-Rojas, R.; Obeso, I.; Hernandez-Fernandez, F.; Del Alamo, M.; Mata, D.; Guida, P.; Ordas-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef] [Green Version]
- D’Haese, P.F.; Ranjan, M.; Song, A.; Haut, M.W.; Carpenter, J.; Dieb, G.; Najib, U.; Wang, P.; Mehta, R.I.; Chazen, J.L.; et al. beta-Amyloid Plaque Reduction in the Hippocampus After Focused Ultrasound-Induced Blood-Brain Barrier Opening in Alzheimer’s Disease. Front. Hum. Neurosci. 2020, 14, 593672. [Google Scholar] [CrossRef]
- Conway, G.E.; Paranjape, A.; Chen, X.; Villanueva, F.S. Development of an in vitro System to Study Mechanisms of Ultrasound-Targeted Microbubble Cavitation-Mediated Blood Brain Barrier Opening. Circulation 2021, 144, A12472. [Google Scholar]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. oting the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Chen, L.; Sutharsan, R.; Lee, J.L.; Cruz, E.; Asnicar, B.; Palliyaguru, T.; Wasielewska, J.M.; Gaudin, A.; Song, J.; Leinenga, G. Claudin-5 binder enhances focused ultrasound-mediated opening in an in vitro blood-brain barrier model. Theranostics 2022, 12, 1952. [Google Scholar] [CrossRef]
- Beekers, I.; Vegter, M.; Lattwein, K.R.; Mastik, F.; Beurskens, R.; van der Steen, A.F.W.; de Jong, N.; Verweij, M.D.; Kooiman, K. Opening of endothelial cell-cell contacts due to sonoporation. J. Control. Release 2020, 322, 426–438. [Google Scholar] [CrossRef]
- Karshafian, R.; Bevan, P.D.; Williams, R.; Samac, S.; Burns, P.N. Sonoporation by ultrasound-activated microbubble contrast agents: Effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med. Biol. 2009, 35, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Karshafian, R.; Samac, S.; Bevan, P.D.; Burns, P.N. Microbubble mediated sonoporation of cells in suspension: Clonogenic viability and influence of molecular size on uptake. Ultrasonics 2010, 50, 691–697. [Google Scholar] [CrossRef] [PubMed]
- DeOre, B.J.; Galie, P.A.; Sehgal, C.M. Fluid flow rate dictates the efficacy of low-intensity anti-vascular ultrasound therapy in a microfluidic model. Microcirculation 2019, 26, e12576. [Google Scholar] [CrossRef] [PubMed]
- Royse, M.K.; Means, A.K.; Calderon, G.A.; Kinstlinger, I.S.; He, Y.; Durante, M.R.; Procopio, A.T.; Veiseh, O.; Xu, J. A 3D printable perfused hydrogel vascular model to assay ultrasound-induced permeability. Biomater. Sci. 2022, 10, 3158–3173. [Google Scholar] [CrossRef] [PubMed]
- Wichers Schreur, J. Transient Disruption of a Blood-Brain Barrier On-Chip Using Focused Ultrasound and Microbubbles. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2022. [Google Scholar]
- Nguyen, D.H.; Stapleton, S.C.; Yang, M.T.; Cha, S.S.; Choi, C.K.; Galie, P.A.; Chen, C.S. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 6712–6717. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Kurosawa, T.; Sako, D.; Tega, Y.; Debori, Y.; Tomihara, Y.; Aoyama, K.; Kubo, Y.; Amano, N.; Deguchi, Y. Construction and Functional Evaluation of a Three-Dimensional Blood–Brain Barrier Model Equipped with Human Induced Pluripotent Stem Cell-Derived Brain Microvascular Endothelial Cells. Pharm. Res. 2022, 39, 1535–1547. [Google Scholar] [CrossRef]
- Miura, S.; Morimoto, Y.; Furihata, T.; Takeuchi, S. Functional analysis of human brain endothelium using a microfluidic device integrating a cell culture insert. APL Bioeng. 2022, 6, 016103. [Google Scholar] [CrossRef]
- Seo, Y.; Bang, S.; Son, J.; Kim, D.; Jeong, Y.; Kim, P.; Yang, J.; Eom, J.H.; Choi, N.; Kim, H.N. Brain physiome: A concept bridging in vitro 3D brain models and in silico models for predicting drug toxicity in the brain. Bioact. Mater. 2022, 13, 135–148. [Google Scholar] [CrossRef]

| Frequency | Acoustic Pressure /Intensity | Pulse Duration | Duty Cycle | Flow Rate | Reference |
|---|---|---|---|---|---|
| 1 MHz | 0.1 MPa or 0.5MPa | 25 μs | Not mentioned | Not mentioned | [127] |
| 1 MHz | 0.4 MPa | 1 ms | 20 % | 10 µL /min | [37] |
| 1 MHz | 1.4 MPa | 500 μs | 5% | ||
| 1 MHz | 2.0 W/cm2 | Not mentioned | 50 % | 100 µL /min | [126] |
| 1 MHz | 0.4 MPa | 500 μs | 0.1 % | 0.5 µL/min | [21] |
| 0.72 MPa | 25 µL/min | ||||
| 1.1 MHz | 0.8 MPa | 10 μs | 0.1 % | Not mentioned | [101] |
| 3 MHz | 0.5 w/cm2 | N.A. | Continuous | 10, 50, and 100 μL/min | [125] |
| 1 w/cm2 | N.A. | Continuous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Fan, K.; Lin, J.; Ma, L.; Li, F. Advances in BBB on Chip and Application for Studying Reversible Opening of Blood–Brain Barrier by Sonoporation. Micromachines 2023, 14, 112. https://doi.org/10.3390/mi14010112
Cai Y, Fan K, Lin J, Ma L, Li F. Advances in BBB on Chip and Application for Studying Reversible Opening of Blood–Brain Barrier by Sonoporation. Micromachines. 2023; 14(1):112. https://doi.org/10.3390/mi14010112
Chicago/Turabian StyleCai, Yicong, Kexin Fan, Jiawei Lin, Lin Ma, and Fenfang Li. 2023. "Advances in BBB on Chip and Application for Studying Reversible Opening of Blood–Brain Barrier by Sonoporation" Micromachines 14, no. 1: 112. https://doi.org/10.3390/mi14010112
APA StyleCai, Y., Fan, K., Lin, J., Ma, L., & Li, F. (2023). Advances in BBB on Chip and Application for Studying Reversible Opening of Blood–Brain Barrier by Sonoporation. Micromachines, 14(1), 112. https://doi.org/10.3390/mi14010112






