Porous Structural Microfluidic Device for Biomedical Diagnosis: A Review
Abstract
:1. Introduction
2. Different Materials and Preparation Methods for Microfluidic Devices Based on Porous Structures
2.1. Fabrication of PDMS and PMMA-Based Microfluidics
2.2. Fabrication of Paper-Based Microfluidics
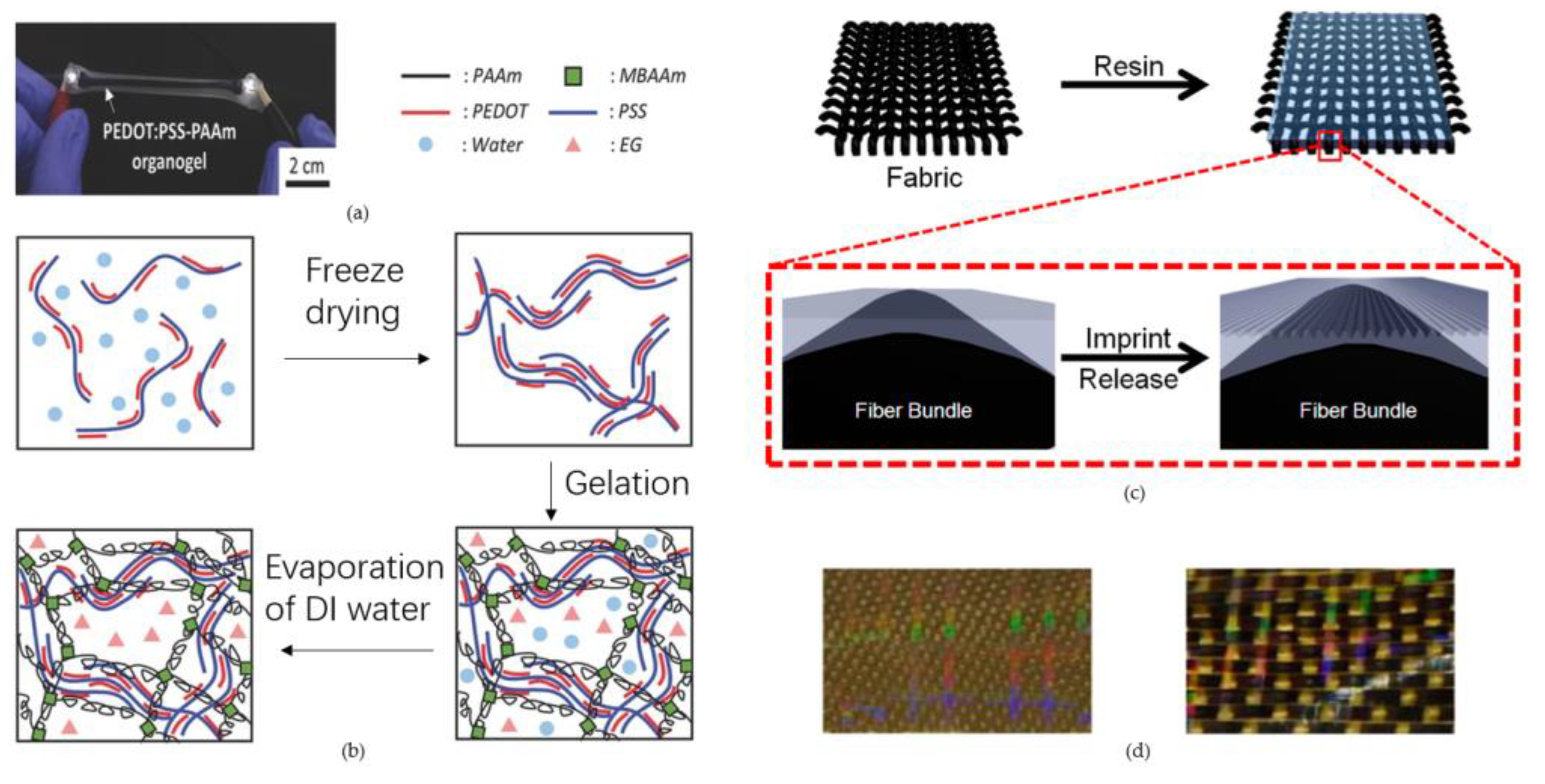
2.3. Fabrication of Three-Dimensional Hydrogels and Textile Fabrics-Based Microfluidics
3. Relevant Principles in Microchannels and Hydrodynamically Relevant Models for Porous Structures
3.1. Inertial Effect of Microfluidics
3.2. Electrorheological Effect of Microfluidics
3.3. Fluid Behavior in Porous Media
3.4. Other Relative Models
4. Microfluidic Devices Based on Porous Media for Biomedical Analysis
4.1. Porous Media-Based Microfluidic Devices for Biomedical Analysis
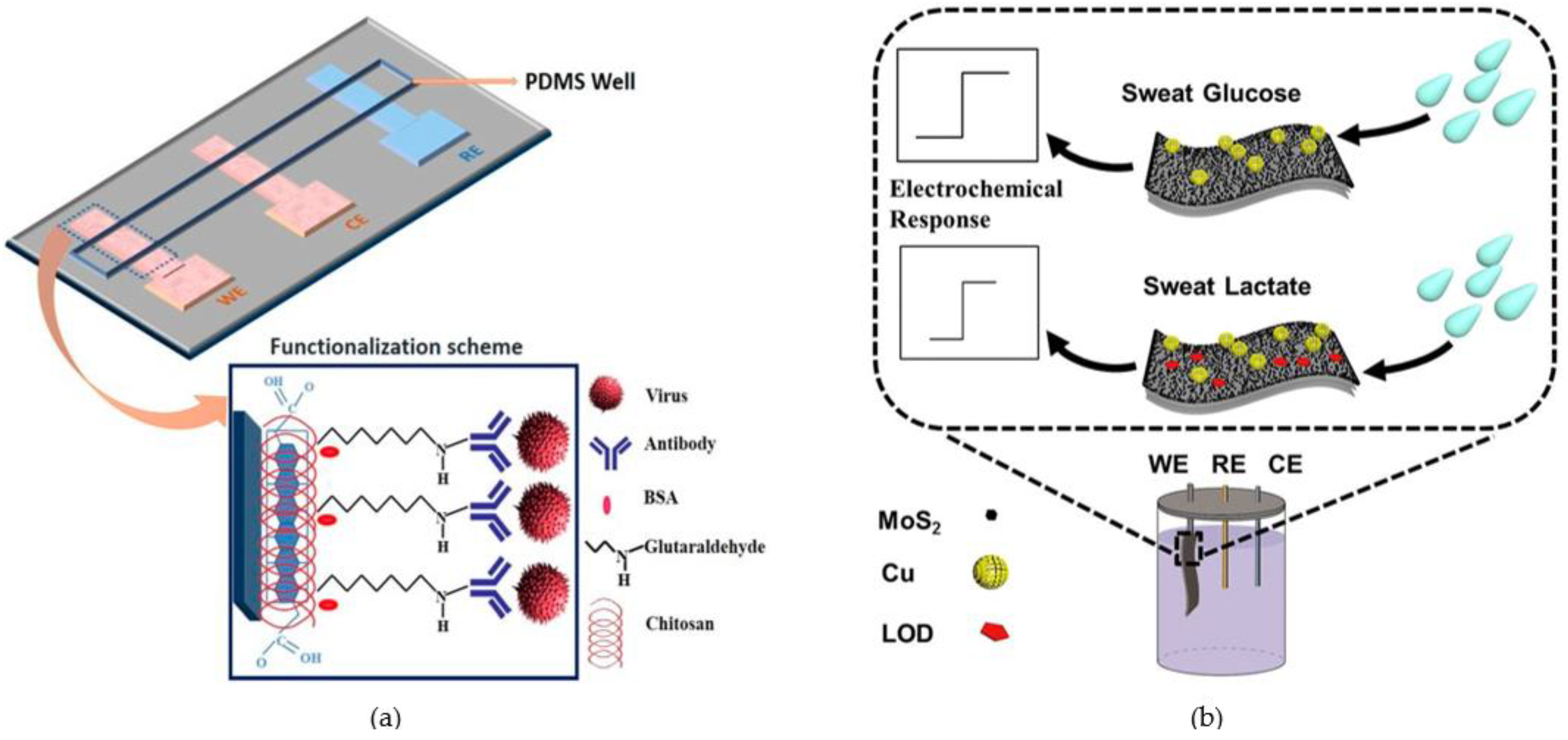
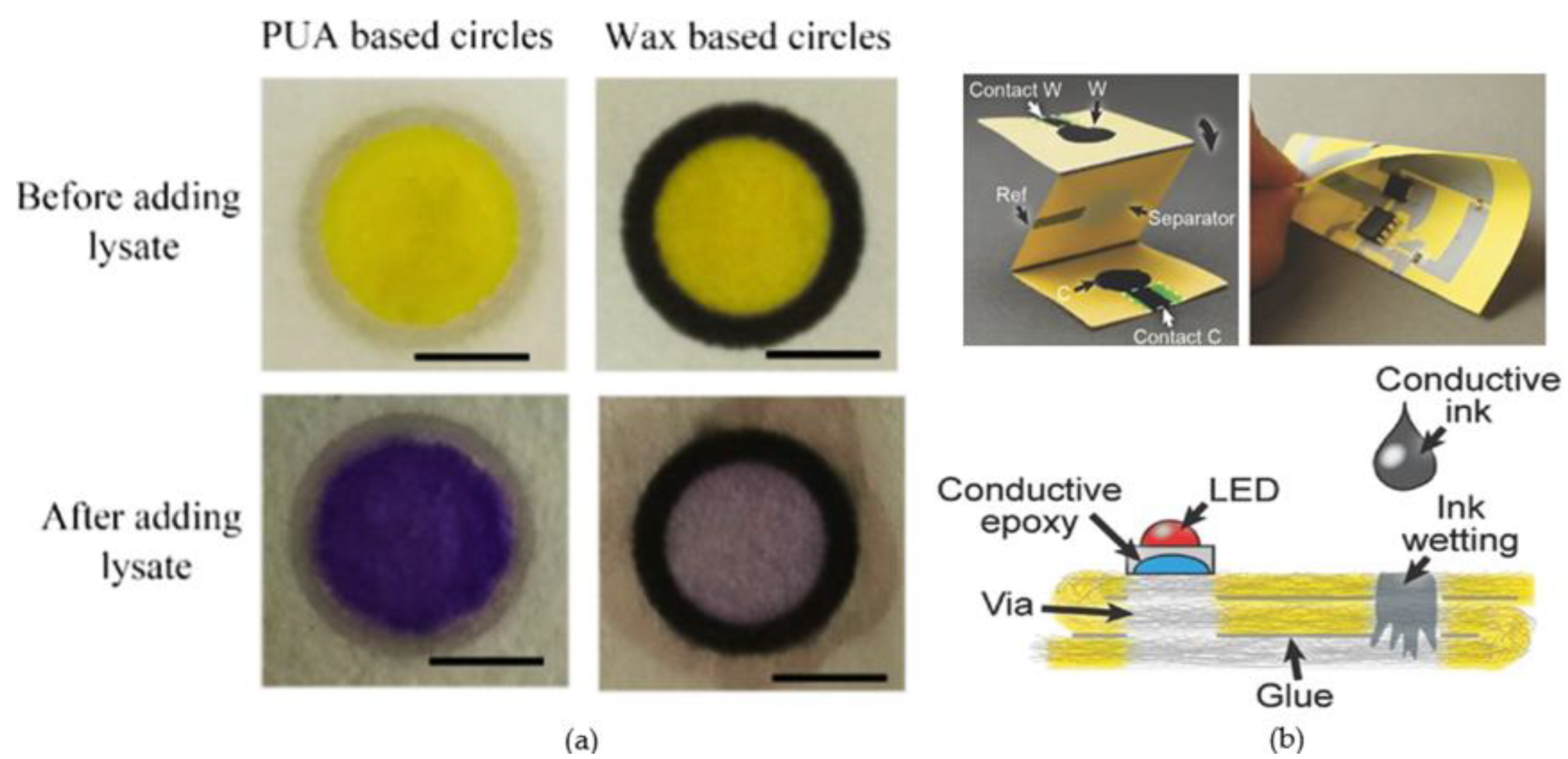
4.2. Microfluidic Devices for Other Biomedical Analysis Applications
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Yan, S.; Miao, C.; Li, L.; Shi, W.; Liu, X.; Luo, Y.; Liu, T.; Lin, B.; Wu, W.; et al. Paper Microfluidics for Cell Analysis. Adv. Healthc. Mater. 2019, 8, 1801084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, Q.; Fan, J.; Sun, C.; Luo, Y.; Liang, R.; Qiu, J. Microfluidics for chiral separation of biomolecules. TrAC Trends Anal. Chem. 2023, 158, 116842. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; VirumbralesMuñoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Dev. 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Yang, S.M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Tseng, C.; Kung, C.; Chen, R.; Tsai, M.; Chao, H.; Wang, Y.; Fu, L. Recent advances in microfluidic paper-based assay devices for diagnosis of human diseases using saliva, tears and sweat samples. Sens. Actuators B Chem. 2021, 342, 130078. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.; Si, T.; Zhou, Z.; Zhou, L.; Chin, Y.R.; Zhang, L.; Guan, X.; Yang, M. High Throughput Confined Migration Microfluidic Device for Drug Screening. Small 2023, 2207194, online ahead of print. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced Wearable Microfluidic Sensors for Healthcare Monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Park, J.; Park, J. Finger-Actuated Microfluidic Display for Smart Blood Typing. Anal. Chem. 2019, 91, 11636–11642. [Google Scholar] [CrossRef]
- Zhang, T.; Ratajczak, A.M.; Chen, H.; Terrell, J.A.; Chen, C. A Step Forward for Smart Clothes─Fabric-Based Microfluidic Sensors for Wearable Health Monitoring. ACS Sens. 2022, 7, 3857–3866. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.M.; Park, S.; Choi, C.; Kim, H.; Kim, J.; Mahmood, M.; Lee, Y.; Kim, J.; Yeo, W. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosens. Bioelectron. 2022, 210, 114329. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Microfluidic spinning of editable polychromatic fibers. J. Colloid Interface Sci. 2020, 558, 115–122. [Google Scholar] [CrossRef]
- Yu, Y.; Shang, L.; Guo, J.; Wang, J.; Zhao, Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc. 2018, 13, 2557–2579. [Google Scholar] [CrossRef]
- Cheng, R.; Liang, Z.B.; Zhu, L.; Li, H.; Zhang, Y.; Wang, C.F.; Chen, S. Fibrous Nanoreactors from Microfluidic Blow Spinning for Mass Production of Highly Stable Ligand-Free Perovskite Quantum Dots. Angew. Chem. Int. Ed. 2022, 61, e202204371. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Zhang, H.; Chen, H.; Gu, Z.; Zhang, D.; Zhao, Y. Jellyfish Tentacle-Inspired Hydrogel Microfibers Implanted with Discrete Structural Color Microsphere Tactile Sensing Units. Adv. Fiber Mater. 2022, 4, 1209–1218. [Google Scholar] [CrossRef]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef]
- Chen, T.; Huang, C.; Wang, Y.; Wu, J. Microfluidic methods for cell separation and subsequent analysis. Chin. Chem. Lett. 2022, 33, 1180–1192. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Lim, J.W.; Raman, R.; Seah, A.W.R.; Tan, M.P.; Zhang, Y. Portable Smartphone-Based Platform for Real-Time Particle Detection in Microfluidics. Adv. Mater. Technol. 2019, 4, 1800359. [Google Scholar] [CrossRef]
- Deng, Y.; Davis, S.P.; Yang, F.; Paulsen, K.S.; Kumar, M.; Sinnott Devaux, R.; Wang, X.; Conklin, D.S.; Oberai, A.; Herschkowitz, J.I.; et al. Inertial Microfluidic Cell Stretcher (iMCS): Fully Automated, High-Throughput, and Near Real-Time Cell Mechanotyping. Small 2017, 13, 1700705. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Schoeler, U.; Huang, C.B.; Vollmer, F. Combining Whispering-Gallery Mode Optical Biosensors with Microfluidics for Real-Time Detection of Protein Secretion from Living Cells in Complex Media. Small 2018, 14, 1703705. [Google Scholar] [CrossRef]
- Zhao, W.; van den Berg, A. Lab on paper. Lab A Chip 2008, 8, 1988–1991. [Google Scholar]
- Holman, J.B.; Shi, Z.; Fadahunsi, A.A.; Li, C.; Ding, W. Advances on microfluidic paper-based electroanalytical devices. Biotechnol. Adv. 2023, 63, 108093. [Google Scholar] [CrossRef]
- Banik, S.; Uchil, A.; Kalsang, T.; Chakrabarty, S.; Ali, M.A.; Srisungsitthisunti, P.; Mahato, K.K.; Surdo, S.; Mazumder, N. The revolution of PDMS microfluidics in cellular biology. Crit. Rev. Biotechnol. 2022, 1–19, online ahead of print. [Google Scholar] [CrossRef]
- Shangguan, J.; Liu, Y.; Pan, J.; Xu, B.; Xu, J.; Chen, H. Microfluidic PDMS on paper (POP) devices. Lab A Chip 2017, 17, 120–127. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. PDMS lab-on-a-chip fabrication using 3D printed templates. Lab A Chip 2014, 14, 424–430. [Google Scholar] [CrossRef]
- Chen, C.; Meng, H.; Guo, T.; Deshpande, S.; Chen, H. Development of Paper Microfluidics with 3D-Printed PDMS Barriers for Flow Control. ACS Appl. Mater. Interfaces 2022, 14, 40286–40296. [Google Scholar] [CrossRef]
- Curto, V.F.; Fay, C.; Coyle, S.; Byrne, R.; O’Toole, C.; Barry, C.; Hughes, S.; Moyna, N.; Diamond, D.; BenitoLopez, F. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 2012, 171–172, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Tan, P.; Wu, X.; Peng, L.; Cheng, H.; Wang, C.F.; Chen, W.; Yu, Z.; Chen, S. High-Performance Wearable Micro-Supercapacitors Based on Microfluidic-Directed Nitrogen-Doped Graphene Fiber Electrodes. Adv. Funct. Mater. 2017, 27, 1702493. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Montazerian, H.; Mohamed, M.G.A.; Montazeri, M.M.; Kheiri, S.; Milani, A.S.; Kim, K.; Hoorfar, M. Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater 2019, 96, 149–160. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Ainla, A.; Guder, F.; Christodouleas, D.C.; FernandezAbedul, M.T.; Whitesides, G.M. Integrating Electronics and Microfluidics on Paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.Y.; Kang, H.Y.; Gwon, S.H.; Choi, G.M.; Lim, S.M.; Sun, J.Y.; Joo, Y.C. A Strain-Insensitive Stretchable Electronic Conductor: PEDOT:PSS/Acrylamide Organogels. Adv. Mater. 2016, 28, 1636–1643. [Google Scholar] [CrossRef]
- Pendergraph, S.A.; Bartlett, M.D.; Carter, K.R.; Crosby, A.J. Opportunities with fabric composites as unique flexible substrates. ACS Appl. Mater. Interfaces 2012, 4, 6640–6645. [Google Scholar] [CrossRef]
- Rattanarat, P.; Dungchai, W.; Cate, D.; Volckens, J.; Chailapakul, O.; Henry, C.S. Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal. Chem. 2014, 86, 3555–3562. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Gumus, A.; Nassar, J.M.; Hussain, M.M. CMOS Enabled Microfluidic Systems for Healthcare Based Applications. Adv. Mater. 2018, 30, e1705759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, X.; Cheng, J. Bionic Enzyme-Assisted Ion-Selective Amperometric Biosensor Based on 3D Porous Conductive Matrix for Point-of-Care Nitrite Testing. ACS Nano 2022, 16, 14849–14859. [Google Scholar] [CrossRef]
- Vaquer, A.; AdroverJaume, C.; Clemente, A.; Iglesias, A.; López, M.; Martínez, R.; Roig, I.M.; Cosío, B.G.; de la Rica, R. Immunosensors made of polymer-infused porous paper for the non-invasive detection of airways cytokines trapped by porous face masks. Sens. Actuators B Chem. 2023, 379, 133233. [Google Scholar] [CrossRef]
- Xu, D.; Shen, Z.; Wang, G.; Wei, L.; Gao, X.; Dong, H.; Wang, G.; Sun, X.; Li, F.; Guo, Y. Dual-catalytic colorimetric biosensor based on double-active Fe@Co-N stellate porous carbon and DNAzyme for simultaneous detection of tetracycline antibiotics. Sens. Actuators B Chem. 2023, 376, 133024. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Duan, Z.; Wu, Y.; Zhao, Q.; Liu, B.; Huang, Q.; Yuan, Z.; Li, X.; Tai, H. Edge-enriched MoS2 nanosheets modified porous nanosheet-assembled hierarchical In2O3 microflowers for room temperature detection of NO2 with ultrahigh sensitivity and selectivity. J. Hazard. Mater. 2022, 434, 128836. [Google Scholar] [CrossRef]
- Baretta, R.; Raucci, A.; Cinti, S.; Frasconi, M. Porous hydrogel scaffolds integrating Prussian Blue nanoparticles: A versatile strategy for electrochemical (bio)sensing. Sens. Actuators B Chem. 2023, 376, 132985. [Google Scholar] [CrossRef]
- Li, J.; Gao, J.; Guo, T.; Huang, X.; Zhang, X.; Xu, C.; Xue, H. Hierarchically Porous Copolymer Film as Immobilization Matrix for Phenol Biosensor with High Sensitivity. ACS Appl. Polym. Mater. 2019, 1, 3148–3156. [Google Scholar] [CrossRef]
- Zhou, X.; Li, P.; Wu, X.; Lin, X.; Zhao, L.; Huang, H.; Wu, J.; Cai, H.; Xu, M.; Zhou, H.; et al. Multifunctional biosensor constructed by Ag-coating magnetic-assisted unique urchin core porous shell structure for dual SERS enhancement, enrichment, and quantitative detection of multi-components inflammatory markers. Biosens. Bioelectron. 2022, 210, 114257. [Google Scholar] [CrossRef]
- Mu, X.; Bertron, T.; Dunn, C.; Qiao, H.; Wu, J.; Zhao, Z.; Saldana, C.; Qi, H. Porous polymeric materials by 3D printing of photocurable resin. Mater. Horiz. 2017, 4, 442–449. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Zhang, L. Isolation of plasma from whole blood using a microfludic chip in a continuous cross-flow. Chin. Sci. Bull. 2009, 54, 324–327. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Faheem, M.; Zou, X.; Ma, X.; Wang, Z.; Meng, Q.; Wang, L.; Zhao, S.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks Serving as Porous Artificial Enzymes. Adv. Mater. 2018, 30, 1800069. [Google Scholar] [CrossRef]
- Balakrishnan, H.K.; Dumée, L.F.; Merenda, A.; Aubry, C.; Yuan, D.; Doeven, E.H.; Guijt, R.M. 3D Printing Functionally Graded Porous Materials for Simultaneous Fabrication of Dense and Porous Structures in Membrane-Integrated Fluidic Devices. Small Struct. 2023, 2200314, online ahead of print. [Google Scholar] [CrossRef]
- Tu, T.; Liang, B.; Zhang, S.; Li, T.; Zhang, B.; Xu, S.; Mao, X.; Cai, Y.; Fang, L.; Ye, X. Controllable Patterning of Porous MXene (Ti3C2) by Metal-Assisted Electro-Gelation Method. Adv. Funct. Mater. 2021, 31, 2101374. [Google Scholar] [CrossRef]
- Hua, E.; Zhang, Y.; Yun, K.; Pan, W.; Liu, Y.; Li, S.; Wang, Y.; Tu, R.; Wang, M. Whole-Cell Biosensor and Producer Co-cultivation-Based Microfludic Platform for Screening Saccharopolyspora erythraea with Hyper Erythromycin Production. ACS Synth. Biol. 2022, 11, 2697–2708. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Wang, Q.; Wang, F.; Li, J.; Xie, R.; Ju, X.; Wang, W.; Pan, D.; Chu, L. Porous functional materials with excellent solar-thermal and electro-thermal properties for desalination of saline water. Sep. Purif. Technol. 2023, 310, 123184. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, Z.; Yang, H.; Cai, Y.; Wang, S.; Chen, J.; Hu, B.; Huang, Q.; Shen, C.; et al. Advanced porous nanomaterials as superior adsorbents for environmental pollutants removal from aqueous solutions. Crit. Rev. Environ. Sci. Technol. 2023, 1–21. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Sun, L.; Wang, Y.; Zhao, Y. Engineering Human Brain Assembloids by Microfluidics. Adv. Mater. 2023, 2210083. [Google Scholar] [CrossRef]
- Hou, C.; Gu, Y.; Yuan, W.; Zhang, W.; Xiu, X.; Lin, J.; Gao, Y.; Liu, P.; Chen, X.; Song, L. Application of microfluidic chips in the simulation of the urinary system microenvironment. Mater. Today Bio 2023, 19, 100553. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Mery, E.; Ricoul, F.; Sarrut, N.; Constantin, O.; Delapierre, G.; Garin, J.; Vinet, F. A silicon microfluidic chip integrating an ordered micropillar array separation column and a nano-electrospray emitter for LC/MS analysis of peptides. Sens. Actuators B Chem. 2008, 134, 438–446. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 115097. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J.; Liu, L.; Xu, L. Application of Microfluidics in Wearable Devices. Small Methods 2019, 3, 1900688. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Nguyen, N. Microfluidic solutions for biofluids handling in on-skin wearable systems. Lab A Chip 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Lin, T.; Niu, H. A novel Janus fabric with stable amphibious directional oil transport function. Chem. Eng. J. 2022, 427, 131936. [Google Scholar] [CrossRef]
- Li, Y.; Fischer, R.; Zboray, R.; Boillat, P.; Camenzind, M.; Toncelli, C.; Rossi, R.M. Laser-Engraved Textiles for Engineering Capillary Flow and Application in Microfluidics. ACS Appl. Mater. Amp. Interfaces 2020, 12, 29908–29916. [Google Scholar] [CrossRef]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2022, 34, e2105063. [Google Scholar] [CrossRef]
- Gao, Y.; Ota, H.; Schaler, E.W.; Chen, K.; Zhao, A.; Gao, W.; Fahad, H.M.; Leng, Y.; Zheng, A.; Xiong, F.; et al. Wearable Microfluidic Diaphragm Pressure Sensor for Health and Tactile Touch Monitoring. Adv. Mater. 2017, 29, 1701985. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; White, A.; Ou, W.; Van Belleghem, S.; Stewart, S.; Shamul, J.G.; Rahaman, S.O.; Fisher, J.P.; He, X. Noncovalent reversible binding-enabled facile fabrication of leak-free PDMS microfluidic devices without plasma treatment for convenient cell loading and retrieval. Bioact. Mater. 2022, 16, 346–358. [Google Scholar] [CrossRef]
- Habibey, R. Incubator-independent perfusion system integrated with microfluidic device for continuous electrophysiology and microscopy readouts. Biofabrication 2023, 15. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2019, 31, e1802922. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Cui, L.; Song, Z.; Zhao, X.; Ma, Y.; Jiang, L. Facile Preparation of the Porous PDMS Oil-Absorbent for Oil/Water Separation. Adv. Mater. Interfaces 2017, 4, 1600862. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Kim, C.M.; Kim, T.; Jin, S.; Kim, G.M. Development of In Situ Microfluidic System for Preparation of Controlled Porous Microsphere for Tissue Engineering. Pharmaceutics 2022, 14, 2345. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, G.; Liu, J.; Li, J.; Chen, X.; Zhu, H.; Liu, G.; Zhang, G.; Jin, W. PDMS thin-film composite membrane fabricated by ultraviolet crosslinking acryloyloxy-terminated monomers. J. Membr. Sci. 2022, 658, 120763. [Google Scholar] [CrossRef]
- Chang, S.T.; Uçar, A.B.; Swindlehurst, G.R.; Bradley, R.O.; Renk, F.J.; Velev, O.D. Materials of Controlled Shape and Stiffness with Photocurable Microfluidic Endoskeleton. Adv. Mater. 2009, 21, 2803–2807. [Google Scholar] [CrossRef]
- Kong, T.; Zhou, J.; Nie, F.; Zhang, C.; Shen, F.; Dai, S.; Pan, H.; Gong, L.; Zhao, L. Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure. Polymers 2022, 14, 4809. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Thong, K.L.; Leo, B.F.; Madou, M. All-carbon suspended nanowire sensors as a rapid highly-sensitive label-free chemiresistive biosensing platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef]
- Liu, H.; Jing, Y.; Yu, X.; Pang, D.; Zhang, Z. Construction of CdSe/ZnS quantum dot microarray in a microfluidic chip. Sci. China Chem. 2012, 55, 543–549. [Google Scholar] [CrossRef]
- Van Giesen, L.; NeaguMaier, G.L.; Kwon, J.Y.; Sprecher, S.G. A microfluidics-based method for measuring neuronal activity in Drosophila chemosensory neurons. Nat. Protoc. 2016, 11, 2389–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Serien, D.; Hu, A.; Sugioka, K. 3D Microfluidic Surface-Enhanced Raman Spectroscopy (SERS) Chips Fabricated by All-Femtosecond-Laser-Processing for Real-Time Sensing of Toxic Substances. Adv. Funct. Mater. 2018, 28, 1706262. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, W.; Ding, H.; Sun, H. On-chip laser processing for the development of multifunctional microfluidic chips. Laser Photon. Rev. 2017, 11, 1600116. [Google Scholar] [CrossRef]
- Shin, J.; Ko, J.; Jeong, S.; Won, P.; Lee, Y.; Kim, J.; Hong, S.; Jeon, N.L.; Ko, S.H. Monolithic digital patterning of polydimethylsiloxane with successive laser pyrolysis. Nat. Mater. 2021, 20, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Mehrdel, P.; CasalsTerré, J.; FarréLlados, J. Cost-effective microfabrication of sub-micron-depth channels by femto-laser anti-stiction texturing. Biofabrication 2020, 12, 025021. [Google Scholar] [CrossRef]
- Bouchard, F.; Soldera, M.; Lasagni, A.F. PMMA Optical Diffusers with Hierarchical Surface Structures Imprinted by Hot Embossing of Laser-Textured Stainless Steel. Adv. Opt. Mater. 2023, 11, 2202091. [Google Scholar] [CrossRef]
- Volpe, A.; Krishnan, U.; Chiriacò, M.S.; Primiceri, E.; Ancona, A.; Ferrara, F. A Smart Procedure for the Femtosecond Laser-Based Fabrication of a Polymeric Lab-on-a-Chip for Capturing Tumor Cell. Engineering 2021, 7, 1434–1440. [Google Scholar] [CrossRef]
- Say, M.G.; Brett, C.J.; Edberg, J.; Roth, S.V.; Söderberg, L.D.; Engquist, I.; Berggren, M. Scalable Paper Supercapacitors for Printed Wearable Electronics. ACS Appl. Mater. Interfaces 2022, 14, 55850–55863. [Google Scholar] [CrossRef]
- Liu, H.; Qing, H.; Li, Z.; Han, Y.L.; Lin, M.; Yang, H.; Li, A.; Lu, T.J.; Li, F.; Xu, F. Paper: A promising material for human-friendly functional wearable electronics. Mater. Sci. Eng. R Rep. 2017, 112, 1–22. [Google Scholar] [CrossRef]
- Dong, L.; Xu, C.; Li, Y.; Pan, Z.; Liang, G.; Zhou, E.; Kang, F.; Yang, Q. Breathable and Wearable Energy Storage Based on Highly Flexible Paper Electrodes. Adv. Mater. 2016, 28, 9313–9319. [Google Scholar] [CrossRef]
- Cai, S.; Zuo, C.; Zhang, J.; Liu, H.; Fang, X. A Paper-Based Wearable Photodetector for Simultaneous UV Intensity and Dosage Measurement. Adv. Funct. Mater. 2021, 31, 2100026. [Google Scholar] [CrossRef]
- Huang, H.; Lin, C.; Hua, Z.; Guo, J.; Lu, D.; Ni, Y.; Cao, S.; Ma, X. Fabrication of ultrathin, flexible, all-in-one paper supercapacitor with high electrochemical performance based on multi-layer forming in paper sheet formation technology. Chem. Eng. J. 2022, 448, 137589. [Google Scholar] [CrossRef]
- Jia, C.; Jiang, F.; Hu, P.; Kuang, Y.; He, S.; Li, T.; Chen, C.; Murphy, A.; Yang, C.; Yao, Y.; et al. Anisotropic, Mesoporous Microfluidic Frameworks with Scalable, Aligned Cellulose Nanofibers. ACS Appl. Mater. Interfaces 2018, 10, 7362–7370. [Google Scholar] [CrossRef]
- Hallan, R.; Barkas, W.W. Physical Properties of Paper Pulps: ‘Freeness’ of Paper Pulps and the Forces of Capillary Water Retention. Nature 1953, 171, 166. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable paper microfluidic device based on single iron site-containing hydrogel nanozyme for efficient glucose biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, Y.; Chu, J.; He, B. Emerging paper microfluidic devices. Analyst 2019, 144, 6497–6511. [Google Scholar] [CrossRef]
- Fu, E.; Wentland, L. A survey of 3D printing technology applied to paper microfluidics. Lab A Chip 2022, 22, 9–25. [Google Scholar] [CrossRef]
- Jia, Z.; Luo, Y.; Wang, D.; Dinh, Q.N.; Lin, S.; Sharma, A.; Block, E.M.; Yang, M.; Gu, T.; Pearlstein, A.J.; et al. Nondestructive multiplex detection of foodborne pathogens with background microflora and symbiosis using a paper chromogenic array and advanced neural network. Biosens. Bioelectron. 2021, 183, 113209. [Google Scholar] [CrossRef]
- Raj, N.; Breedveld, V.; Hess, D. Fabrication of fully enclosed paper microfluidic devices using plasma deposition and etching. Lab A Chip 2019, 19, 3337–3343. [Google Scholar] [CrossRef]
- Postulka, N.; Striegel, A.; Krauße, M.; Mager, D.; Spiehl, D.; Meckel, T.; Worgull, M.; Biesalski, M. Combining Wax Printing with Hot Embossing for the Design of Geometrically Well-Defined Microfluidic Papers. ACS Appl. Mater. Interfaces 2019, 11, 4578–4587. [Google Scholar] [CrossRef]
- Lin, D.; Li, B.; Qi, J.; Ji, X.; Yang, S.; Wang, W.; Chen, L. Low cost fabrication of microfluidic paper-based analytical devices with water-based polyurethane acrylate and their application for bacterial detection. Sens. Actuators B Chem. 2020, 303, 127213. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Kurniawan, A.; Kao, C.; Wang, M. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Cui, K.; Zhang, L.; Xu, J.; Liu, H.; Yu, J. Highly conductive and bendable gold networks attached on intertwined cellulose fibers for output controllable power paper. J. Mater. Chem. A 2018, 6, 19611–19620. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Kadimisetty, K.; Malla, S.; Joshi, A.A.; Rusling, J.F. Paper-based electrochemiluminescent screening for genotoxic activity in the environment. Env. Sci. Technol. 2013, 47, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yu, Y.; Cai, L.; Wang, Y.; Shi, K.; Shang, L.; Pan, J.; Zhao, Y. Microfluidics for flexible electronics. Mater. Today 2021, 44, 105–135. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Matrix-Assisted Three-Dimensional Printing of Cellulose Nanofibers for Paper Microfluidics. ACS Appl. Mater. Interfaces 2017, 9, 26438–26446. [Google Scholar] [CrossRef]
- Khan, M.J.; Zhang, J.; Guo, Q. Covalent/crystallite cross-linked co-network hydrogels: An efficient and simple strategy for mechanically strong and tough hydrogels. Chem. Eng. J. 2016, 301, 92–102. [Google Scholar] [CrossRef]
- GilaVilchez, C.; RodriguezArco, L.; MañasTorres, M.C.; Álvarez de Cienfuegos, L.; LopezLopez, M.T. Self-assembly in magnetic supramolecular hydrogels. Curr. Opin. Colloid Interface Sci. 2022, 62, 101644. [Google Scholar] [CrossRef]
- Sontakke, V.A.; Yokobayashi, Y. Programmable Macroscopic Self-Assembly of DNA-Decorated Hydrogels. J. Am. Chem. Soc. 2022, 144, 2149–2155. [Google Scholar] [CrossRef]
- Wachendörfer, M.; Schräder, P.; Buhl, E.M.; Palkowitz, A.L.; Ben Messaoud, G.; Richtering, W.; Fischer, H. A defined heat pretreatment of gelatin enables control of hydrolytic stability, stiffness, and microstructural architecture of fibrin–gelatin hydrogel blends. Biomater. Sci. 2022, 10, 5552–5565. [Google Scholar] [CrossRef]
- Le-The, H.; Tibbe, M.; Loessberg-Zahl, J.; Palma do Carmo, M.; van der Helm, M.; Bomer, J.; van den Berg, A.; Leferink, A.; Segerink, L.; Eijkel, J. Large-scale fabrication of free-standing and sub-μm PDMS through-hole membranes. Nanoscale 2018, 10, 7711–7718. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Huntington, M.D.; Zhou, W.; Yang, J.-C.; Odom, T.W. Programmable Soft Lithography: Solvent-Assisted Nanoscale Embossing. Nano Lett. 2011, 11, 311–315. [Google Scholar] [CrossRef]
- Quirós-Solano, W.F.; Gaio, N.; Stassen, O.M.J.A.; Arik, Y.B.; Silvestri, C.; Van Engeland, N.C.A.; Van der Meer, A.; Passier, R.; Sahlgren, C.M.; Bouten, C.V.C.; et al. Microfabricated tuneable and transferable porous PDMS membranes for Organs-on-Chips. Sci. Rep. 2018, 8, 13524. [Google Scholar] [CrossRef] [Green Version]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Zargar, R.; Nourmohammadi, J.; Amoabediny, G. Preparation, characterization, and silanization of 3D microporous PDMS structure with properly sized pores for endothelial cell culture. Biotechnol. Appl. Biochem. 2016, 63, 190–199. [Google Scholar] [CrossRef]
- Qi, L.; Ruck, C.; Spychalski, G.; King, B.; Wu, B.; Zhao, Y. Writing Wrinkles on Poly(dimethylsiloxane) (PDMS) by Surface Oxidation with a CO2 Laser Engraver. ACS Appl. Mater. Interfaces 2018, 10, 4295–4304. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Zhang, J.; Hou, X. Femtosecond laser induced underwater superaerophilic and superaerophobic PDMS sheets with through microholes for selective passage of air bubbles and further collection of underwater gas. Nanoscale 2018, 10, 3688–3696. [Google Scholar] [CrossRef]
- Duan, S.; Yang, K.; Wang, Z.; Chen, M.; Zhang, L.; Zhang, H.; Li, C. Fabrication of Highly Stretchable Conductors Based on 3D Printed Porous Poly(dimethylsiloxane) and Conductive Carbon Nanotubes/Graphene Network. ACS Appl. Mater. Interfaces 2016, 8, 2187–2192. [Google Scholar] [CrossRef]
- Mathur, A.; Roy, S.S.; Tweedie, M.; Mukhopadhyay, S.; Mitra, S.K.; McLaughlin, J.A. Characterisation of PMMA microfluidic channels and devices fabricated by hot embossing and sealed by direct bonding. Curr. Appl. Phys. 2009, 9, 1199–1202. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Geschke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab A Chip 2002, 2, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sedghamiz, E.; Liu, M.; Wenzel, W. Challenges and limits of mechanical stability in 3D direct laser writing. Nat. Commun. 2022, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection molding and characterization of PMMA-based microfluidic devices. Microsyst. Technol. 2020, 26, 1317–1324. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Zhu, L.; Mei, X.; Peng, Z.; Liu, J.; Yang, J.; Li, Y. A rotating paper-based microfluidic sensor array combining Michael acceptors and carbon quantum dots for discrimination of biothiols. Chem. Eng. J. 2023, 454, 140065. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, A.K.; See, D.; Ying, J.Y. Enhancing Protein Adsorption for Improved Lateral Flow Assay on Cellulose Paper by Depleting Inert Additive Films Using Reactive Plasma. ACS Appl. Mater. Interfaces 2023, 15, 6561–6571. [Google Scholar] [CrossRef]
- Kalish, B.; Tan, M.K.; Tsutsui, H. Modifying Wicking Speeds in Paper-Based Microfluidic Devices by Laser-Etching. Micromachines 2020, 11, 773. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.; Park, J.-K. Organic Solvent and Surfactant Resistant Paper-Fluidic Devices Fabricated by One-Step Embossing of Nonwoven Polypropylene Sheet. Micromachines 2017, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Wang, Y.; Gao, B.; He, B. Shark Tooth-Inspired Microneedle Dressing for Intelligent Wound Management. ACS Nano 2021, 15, 15316–15327. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, J.; Lin, J.; Zhu, Y.; Xu, X.; Cui, W. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small 2023, 2207211, online ahead of print. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.; Ye, T.; Lu, G.; Li, R.; Deng, L.; Wang, L.; Cai, M.; Cui, W. Stem cell-laden injectable hydrogel microspheres for cancellous bone regeneration. Chem. Eng. J. 2020, 393, 124715. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Shang, L.; Zhao, Y. Histidine-Triggered GO Hybrid Hydrogels for Microfluidic 3D Printing. ACS Nano 2022, 16, 19533–19542. [Google Scholar] [CrossRef]
- Neiman, J.A.S.; Raman, R.; Chan, V.; Rhoads, M.G.; Raredon, M.S.B.; Velazquez, J.J.; Dyer, R.L.; Bashir, R.; Hammond, P.T.; Griffith, L.G. Photopatterning of hydrogel scaffolds coupled to filter materials using stereolithography for perfused 3D culture of hepatocytes. Biotechnol. Bioeng. 2015, 112, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Nilghaz, A.; Ballerini, D.R.; Shen, W. Exploration of microfluidic devices based on multi-filament threads and textiles: A review. Biomicrofluidics 2013, 7, 051501. [Google Scholar] [CrossRef] [Green Version]
- Agustini, D.; Caetano, F.R.; Quero, R.F.; Fracassi da Silva, J.A.; Bergamini, M.F.; Marcolino-Junior, L.H.; de Jesus, D.P. Microfluidic devices based on textile threads for analytical applications: State of the art and prospects. Anal. Methods 2021, 13, 4830–4857. [Google Scholar] [CrossRef]
- Feng, D.; Weng, D.; Wang, J. Interfacial tension gradient driven self-assembly of binary colloidal particles for fabrication of superhydrophobic porous films. J. Colloid Interface Sci. 2019, 548, 312–321. [Google Scholar] [CrossRef]
- He, B.; He, J.; Bi, E.; Zou, H.; Liu, T.; Liu, Z. Transport and retention of nano emulsified vegetable oil in porous media: Effect of pore straining, roughness wedging, and interfacial effects. J. Environ. Manag. 2022, 320, 115912. [Google Scholar] [CrossRef]
- Stoecklein, D.; Di Carlo, D. Nonlinear Microfluidics. Anal. Chem. 2019, 91, 296–314. [Google Scholar] [CrossRef]
- Youngren, G.K.; Acrivos, A. Stokes flow past a particle of arbitrary shape: A numerical method of solution. J. Fluid Mech. 2006, 69, 377–403. [Google Scholar] [CrossRef] [Green Version]
- Gou, Y.; Jia, Y.; Wang, P.; Sun, C. Progress of Inertial Microfluidics in Principle and Application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef] [Green Version]
- Segre, G.; Silberberg, A. Radial particle displacements in Poiseuille flow of suspensions. Nature 1961, 189, 209–210. [Google Scholar] [CrossRef]
- Segré, G.; Silberberg, A. Behaviour of macroscopic rigid spheres in Poiseuille flow Part 1. Determination of local concentration by statistical analysis of particle passages through crossed light beams. J. Fluid Mech. 2006, 14, 115–135. [Google Scholar] [CrossRef]
- Dean, W.R. XVI. Note on the motion of fluid in a curved pipe. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 4, 208–223. [Google Scholar] [CrossRef]
- Dean, W.R. LXXII. The stream-line motion of fluid in a curved pipe (Second paper). Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 5, 673–695. [Google Scholar] [CrossRef]
- Dinler, A.; Okumus, I. Inertial particle separation in curved networks: A numerical study. Chem. Eng. Sci. 2018, 182, 119–131. [Google Scholar] [CrossRef]
- Gossett, D.R.; Carlo, D.D. Particle focusing mechanisms in curving confined flows. Anal. Chem. 2009, 81, 8459–8465. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological Fluids: Mechanisms, Dynamics, and Microfluidics Applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Sun, J.M. Three-dimensional structure of induced electrorheological solid. Phys. Rev. Lett. 1991, 67, 398–401. [Google Scholar] [CrossRef]
- Yethiraj, A.; van Blaaderen, A. A colloidal model system with an interaction tunable from hard sphere to soft and dipolar. Nature 2003, 421, 513–517. [Google Scholar] [CrossRef]
- Hynninen, A.P.; Dijkstra, M. Phase diagram of dipolar hard and soft spheres: Manipulation of colloidal crystal structures by an external field. Phys. Rev. Lett. 2005, 94, 138303. [Google Scholar] [CrossRef] [Green Version]
- Bergman, D.J.; Stroud, D. Physical properties of macroscopically inhomogeneous media. In Solid State Physics; Elsevier: Amsterdam, The Netherlands, 1992; Volume 46, pp. 147–269. [Google Scholar]
- Ma, H.; Wen, W.; Tam, W.Y.; Sheng, P. Frequency dependent electrorheological properties: Origin and bounds. Phys. Rev. Lett. 1996, 77, 2499. [Google Scholar] [CrossRef] [Green Version]
- Tam, W.Y.; Yi, G.H.; Wen, W.; Ma, H.; Loy, M.M.; Sheng, P. New electrorheological fluid: Theory and experiment. Phys. Rev. Lett. 1997, 78, 2987. [Google Scholar] [CrossRef] [Green Version]
- Ulep, T.; Zenhausern, R.; Gonzales, A.; Knoff, D.S.; Lengerke Diaz, P.A.; Castro, J.E.; Yoon, J. Smartphone based on-chip fluorescence imaging and capillary flow velocity measurement for detecting ROR1+ cancer cells from buffy coat blood samples on dual-layer paper microfluidic chip. Biosens. Bioelectron. 2020, 153, 112042. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Mendez, S.; Fenton, E.M.; Gallegos, G.R.; Petsev, D.N.; Sibbett, S.S.; Stone, H.A.; Zhang, Y.; Lopez, G.P. Imbibition in porous membranes of complex shape: Quasi-stationary flow in thin rectangular segments. Langmuir 2010, 26, 1380–1385. [Google Scholar] [CrossRef]
- Kar, S.; Das, S.S.; Laha, S.; Chakraborty, S. Microfluidics on Porous Substrates Mediated by Capillarity-Driven Transport. Ind. Eng. Chem. Res. 2020, 59, 3644–3654. [Google Scholar] [CrossRef]
- Buser, J.R.; Byrnes, S.A.; Anderson, C.E.; Howell, A.J.; Kauffman, P.C.; Bishop, J.D.; Wheeler, M.H.; Kumar, S.; Yager, P. Understanding partial saturation in paper microfluidics enables alternative device architectures. Anal. Methods 2019, 11, 336–345. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon: Exposition et Application des Principes à Suivre et des Formules à Employer Dans les Questions de Distribution D’eau… Un Appendice Relatif aux Fournitures D’eau de Plusieurs Villes au Filtrage des Eaux; Dalmont, V., Ed.; Librarie Des Corps Imperiaux Des Ponts et Chaussées et Des Rines: Paris, France, 1856; Volume 1. [Google Scholar]
- Alcocer, F.; Kumar, V.; Singh, P. Permeability of periodic porous media. Phys. Rev. E 1999, 59, 711. [Google Scholar] [CrossRef]
- Alcocer, F.; Singh, P. Permeability of periodic arrays of cylinders for viscoelastic flows. Phys. Fluids 2002, 14, 2578–2581. [Google Scholar] [CrossRef]
- Chang, S.; Seo, J.; Hong, S.; Lee, D.-G.; Kim, W. Dynamics of liquid imbibition through paper with intra-fibre pores. J. Fluid Mech. 2018, 845, 36–50. [Google Scholar] [CrossRef]
- Cao, R.; Pan, Z.; Tang, H.; Wu, J.; Tian, J.; Nilghaz, A.; Li, M. Understanding the coffee-ring effect of red blood cells for engineering paper-based blood analysis devices. Chem. Eng. J. 2020, 391, 123522. [Google Scholar] [CrossRef]
- Park, S.; Zhang, Y.; Lin, S.; Wang, T.-H.; Yang, S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol. Adv. 2011, 29, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef]
- Hassan, U.; Ghonge, T.; Reddy, B., Jr.; Patel, M.; Rappleye, M.; Taneja, I.; Tanna, A.; Healey, R.; Manusry, N.; Price, Z.; et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat. Commun. 2017, 8, 15949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neoh, K.H.; Hassan, A.A.; Chen, A.; Sun, Y.; Liu, P.; Xu, K.; Wong, A.S.T.; Han, R.P.S. Rethinking liquid biopsy: Microfluidic assays for mobile tumor cells in human body fluids. Biomaterials 2018, 150, 112–124. [Google Scholar] [CrossRef]
- KékedyNagy, L.; Perry, J.M.; Little, S.R.; Llorens, O.Y.; Shih, S.C.C. An electrochemical aptasensor for Δ9-tetrahydrocannabinol detection in saliva on a microfluidic platform. Biosens. Bioelectron. 2023, 222, 114998. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, H.; Shi, X.; Tan, W.; Zhu, G. Fabrication for paper-based microfluidic analytical devices and saliva analysis application. Microfluid. Nanofluidics 2021, 25, 80. [Google Scholar] [CrossRef]
- Akarapipad, P.; Kaarj, K.; Breshears, L.E.; Sosnowski, K.; Baker, J.; Nguyen, B.T.; Eades, C.; Uhrlaub, J.L.; Quirk, G.; NikolichŽugich, J.; et al. Smartphone-based sensitive detection of SARS-CoV-2 from saline gargle samples via flow profile analysis on a paper microfluidic chip. Biosens. Bioelectron. 2022, 207, 114192. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. TrAC Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Viswanath, B.; Choi, C.S.; Lee, K.; Kim, S. Recent trends in the development of diagnostic tools for diabetes mellitus using patient saliva. TrAC Trends Anal. Chem. 2017, 89, 60–67. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dhar, S.; Deka, N.; Debnath, K.; Mondal, S.P. Non-enzymatic salivary glucose detection using porous CuO nanostructures. Sens. Actuators B Chem. 2020, 302, 127134. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, C. A microfluidic cloth-based photoelectrochemical analytical device for the detection of glucose in saliva. Talanta 2022, 238, 123052. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D porous graphene aerogel@ GOx based microfluidic biosensor for electrochemical glucose detection. Analyst 2020, 145, 5141–5147. [Google Scholar] [CrossRef]
- Vinoth, R.; Sangavi, P.; Nakagawa, T.; Jayaraman, M.; Mohan, A.M.V. All-in-one microfluidic device with an integrated porous filtration membrane for on-site detection of multiple salivary biomarkers. Sens. Actuators B Chem. 2023, 379, 133214. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.; Li, X.; Sun, D.; Hu, T.; Xiang, N.; Ni, Z. Based graphene oxide biosensor coupled with smartphone for the quantification of glucose in oral fluid. Biomed. Microdevices 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Fang, F.; Wu, Z. Sensitive paper-based analytical device for fast colorimetric detection of nitrite with smartphone. Anal. Bioanal. Chem. 2018, 410, 2665–2669. [Google Scholar] [CrossRef]
- Devarakonda, S.; Singh, R.; Bhardwaj, J.; Jang, J. Cost-Effective and Handmade Paper-Based Immunosensing Device for Electrochemical Detection of Influenza Virus. Sensors 2017, 17, 2597. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, I.; CapitánVallvey, L.F.; Erenas, M.M. Thread-based microfluidic sensor for lithium monitoring in saliva. Talanta 2023, 253, 124094. [Google Scholar] [CrossRef]
- Baker, L.B.; Seib, M.S.; Barnes, K.A.; Brown, S.D.; King, M.A.; De Chavez, P.J.D.; Qu, S.; Archer, J.; Wolfe, A.S.; Stofan, J.R.; et al. Skin-Interfaced Microfluidic System with Machine Learning-Enabled Image Processing of Sweat Biomarkers in Remote Settings. Adv. Mater. Technol. 2022, 7, 2200249. [Google Scholar] [CrossRef]
- Hong, X.; Wu, H.; Wang, C.; Zhang, X.; Wei, C.; Xu, Z.; Chen, D.; Huang, X. Hybrid Janus Membrane with Dual-Asymmetry Integration of Wettability and Conductivity for Ultra-Low-Volume Sweat Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9644–9654. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liang, B.; Ye, Z.; Zhang, L.; Xu, S.; Tu, T.; Zhang, Y.; Cai, Y.; Zhang, B.; Fang, L.; et al. An integrated and conductive hydrogel-paper patch for simultaneous sensing of Chemical–Electrophysiological signals. Biosens. Bioelectron. 2022, 198, 113855. [Google Scholar] [CrossRef] [PubMed]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Robinson, S.; Robinson, A.H. Chemical composition of sweat. Physiol. Rev. 1954, 34, 202–220. [Google Scholar] [CrossRef]
- TaghizadehBehbahani, M.; Hemmateenejad, B.; Shamsipur, M.; Tavassoli, A. A paper-based length of stain analytical device for naked eye (readout-free) detection of cystic fibrosis. Anal. Chim. Acta 2019, 1080, 138–145. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, S.; Gui, M.; Asif, M.; Wang, W.; Wang, F.; Liu, H. Graphene paper supported MoS(2) nanocrystals monolayer with Cu submicron-buds: High-performance flexible platform for sensing in sweat. Anal. Biochem. 2018, 543, 82–89. [Google Scholar] [CrossRef]
- Torul, H.; Çiftçi, H.; Çetin, D.; Suludere, Z.; Boyacı, I.H.; Tamer, U. Paper membrane-based SERS platform for the determination of glucose in blood samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- Bagheri, N.; Mazzaracchio, V.; Cinti, S.; Colozza, N.; Di Natale, C.; Netti, P.A.; Saraji, M.; Roggero, S.; Moscone, D.; Arduini, F. Electroanalytical Sensor Based on Gold-Nanoparticle-Decorated Paper for Sensitive Detection of Copper Ions in Sweat and Serum. Anal. Chem. 2021, 93, 5225–5233. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G.; et al. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A Portable 3D Microfluidic Origami Biosensor for Cortisol Detection in Human Sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef]
- Singh, A.; Hazarika, A.; Dutta, L.; Bhuyan, A.; Bhuyan, M. A fully handwritten-on-paper copper nanoparticle ink-based electroanalytical sweat glucose biosensor fabricated using dual-step pencil and pen approach. Anal. Chim. Acta 2022, 1227, 340257. [Google Scholar] [CrossRef]
- Fabiani, L.; Mazzaracchio, V.; Moscone, D.; Fillo, S.; De Santis, R.; Monte, A.; Amatore, D.; Lista, F.; Arduini, F. Paper-based immunoassay based on 96-well wax-printed paper plate combined with magnetic beads and colorimetric smartphone-assisted measure for reliable detection of SARS-CoV-2 in saliva. Biosens. Bioelectron. 2022, 200, 113909. [Google Scholar] [CrossRef]
- Moon, J.; Del Caño, R.; Moonla, C.; Sakdaphetsiri, K.; Saha, T.; Francine Mendes, L.; Yin, L.; Chang, A.; Seker, S.; Wang, J. Self-Testing of Ketone Bodies, along with Glucose, Using Touch-Based Sweat Analysis. ACS Sens. 2022, 7, 3973–3981. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; GarciaRey, S.; Ojeda, E.; BasabeDesmonts, L.; BenitoLopez, F. TiO2 Nanotubes Alginate Hydrogel Scaffold for Rapid Sensing of Sweat Biomarkers: Lactate and Glucose. ACS Appl. Mater. Interfaces 2021, 13, 37734–37745. [Google Scholar] [CrossRef]
- Guzman, J.M.C.C.; Hsu, S.; Chuang, H. Colorimetric Diagnostic Capillary Enabled by Size Sieving in a Porous Hydrogel. Biosensors 2020, 10, 130. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT:PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Somchob, B.; Rodthongkum, N.; Hoven, V.P. Bacterial cellulose-based re-swellable hydrogel: Facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr. Polym. 2021, 256, 117506. [Google Scholar] [CrossRef]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing Gradient Porous Graphene Substrate as the Solid-Contact Layer To Enhance Wearable Electrochemical Sweat Sensor Sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Wang, L.; Xu, T.; Fan, C.; Zhang, X. Wearable strain sensor for real-time sweat volume monitoring. iScience 2021, 24, 102028. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Fang, J.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Wearable Osmotic-Capillary Patch for Prolonged Sweat Harvesting and Sensing. ACS Appl. Mater. Interfaces 2021, 13, 8071–8081. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, W.; Tian, L.; Su, M.; Jiang, M.; Li, J.; Gu, H.; Yu, C. Preparation of nanostructured PDMS film as flexible immunosensor for cortisol analysis in human sweat. Anal. Chim. Acta 2021, 1184, 339010. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, X.; Shi, C.; Dai, Y.; Zhang, J.; Liu, W.; Wu, C.; Zhang, Y.; Huang, X.; Zeng, W. Flexible enzymatic biosensor based on graphene sponge for glucose detection in human sweat. Surf. Interfaces 2023, 36, 102525. [Google Scholar] [CrossRef]
- Xuan, X.; Kim, J.Y.; Hui, X.; Das, P.S.; Yoon, H.S.; Park, J. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018, 120, 160–167. [Google Scholar] [CrossRef]
- Kil, M.S.; Kim, S.J.; Park, H.J.; Yoon, J.H.; Jeong, J.; Choi, B.G. Highly Stretchable Sensor Based on Fluid Dynamics-Assisted Graphene Inks for Real-Time Monitoring of Sweat. ACS Appl. Mater. Interfaces 2022, 14, 48072–48080. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Lin, Z.; Meng, Z.; Shi, C.; Xu, Z.; Yang, L.; Liu, X.Y. Coupling of Silk Fibroin Nanofibrils Enzymatic Membrane with Ultra-Thin PtNPs/Graphene Film to Acquire Long and Stable On-Skin Sweat Glucose and Lactate Sensing. Small Methods 2021, 5, 2000926. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, Y.; Gao, S.; Zhang, Z.; Gu, Y.; Liu, X. Reduced Graphene Oxide-Coated Silica Nanospheres as Flexible Enzymatic Biosensors for Detection of Glucose in Sweat. ACS Appl. Nano Mater. 2021, 4, 12442–12452. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, J.S.; Han, D.K. Platinum nanozyme-hydrogel composite (PtNZHG)-impregnated cascade sensing system for one-step glucose detection in serum, urine, and saliva. Sens. Actuators B Chem. 2022, 359, 131585. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Prakasham, K.; Eswaran, M.; Duraisamy, M.; Ganesan, S.; Lee, S.L.; Ponnusamy, V.K. Eco-friendly fabrication of nonenzymatic electrochemical sensor based on cobalt/polymelamine/nitrogen-doped graphitic-porous carbon nanohybrid material for glucose monitoring in human blood. Environ. Res. 2023, 115403, online ahead of print. [Google Scholar] [CrossRef]
- Yao, T.; Dong, G.; Qian, S.; Cui, Y.; Chen, X.; Tan, T.; Li, L. Persistent luminescence nanoparticles/hierarchical porous ZIF-8 nanohybrids for autoluminescence-free detection of dopamine. Sens. Actuators B Chem. 2022, 357, 131470. [Google Scholar] [CrossRef]
- Marques, M.P.C.; Szita, N. Bioprocess microfluidics: Applying microfluidic devices for bioprocessing. Curr. Opin. Chem. Eng. 2017, 18, 61–68. [Google Scholar] [CrossRef]
- Charmet, J.; Arosio, P.; Knowles, T.P.J. Microfluidics for Protein Biophysics. J. Mol. Biol. 2018, 430, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, Y.; Yu, Y.; Huang, Q.; Ji, W.; Li, J.; Zhao, Y. Hierarchically porous composite microparticles from microfluidics for controllable drug delivery. Nanoscale 2018, 10, 12595–12604. [Google Scholar] [CrossRef]
- Martins, J.P.; Liu, D.; Fontana, F.; Ferreira, M.P.A.; Correia, A.; Valentino, S.; Kemell, M.; Moslova, K.; Mäkilä, E.; Salonen, J.; et al. Microfluidic Nanoassembly of Bioengineered Chitosan-Modified FcRn-Targeted Porous Silicon Nanoparticles @ Hypromellose Acetate Succinate for Oral Delivery of Antidiabetic Peptides. ACS Appl. Mater. Interfaces 2018, 10, 44354–44367. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Shahbazi, M.; Mäkilä, E.; HerranzBlanco, B.; Salonen, J.; Hirvonen, J.; Santos, H.A. Fabrication of a Multifunctional Nano-in-micro Drug Delivery Platform by Microfluidic Templated Encapsulation of Porous Silicon in Polymer Matrix. Adv. Mater. 2014, 26, 4497–4503. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, J.; Shao, C.; Zhao, Y. Tofu-inspired microcarriers from droplet microfluidics for drug delivery. Sci. China Chem. 2019, 62, 87–94. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Chen, R.; Wulff, J.E.; Moffitt, M.G. Microfluidic Processing Approach to Controlling Drug Delivery Properties of Curcumin-Loaded Block Copolymer Nanoparticles. Mol. Pharm. 2018, 15, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Surawathanawises, K.; Wiedorn, V.; Cheng, X. Micropatterned macroporous structures in microfluidic devices for viral separation from whole blood. Analyst 2017, 142, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Mou, L.; Yang, M.; Zou, W.; Du, C.; Zhang, W.; Jiang, X. Highly efficient capture of circulating tumor cells with low background signals by using pyramidal microcavity array. Anal Chim Acta 2019, 1060, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Choudhury, A.D.; Yamanaka, Y.J.; Adalsteinsson, V.A.; Gierahn, T.M.; Williamson, C.A.; Lamb, C.R.; Taplin, M.E.; Nakabayashi, M.; Chabot, M.S.; et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr. Biol. 2014, 6, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Cui, D.; Liu, C.; Li, H.; Chen, J. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal. Chim. Acta 2007, 584, 237–243. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Meng, Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2010, 6, 733–746. [Google Scholar] [CrossRef]
- Su, Y.; Hu, X.; Kang, Y.; Zhang, C.; Cheng, Y.Y.; Jiao, Z.; Nie, Y.; Song, K. Curcumin nanoparticles combined with 3D printed bionic tumor models for breast cancer treatment. Biofabrication 2023, 15, 014105. [Google Scholar] [CrossRef]
- Kappes, M.; Friedrich, B.; Pfister, F.; Huber, C.; Friedrich, R.P.; Stein, R.; Braun, C.; Band, J.; Schreiber, E.; Alexiou, C.; et al. Superparamagnetic Iron Oxide Nanoparticles for Targeted Cell Seeding: Magnetic Patterning and Magnetic 3D Cell Culture. Adv. Funct. Mater. 2022, 32, 2203672. [Google Scholar] [CrossRef]
- Yu, J.Z.; Korkmaz, E.; Berg, M.I.; LeDuc, P.R.; Ozdoganlar, O.B. Biomimetic scaffolds with three-dimensional undulated microtopographies. Biomaterials 2017, 128, 109–120. [Google Scholar] [CrossRef]
- Li, Q.; Hatakeyama, M.; Kitaoka, T. Bioadaptive Porous 3D Scaffolds Comprising Cellulose and Chitosan Nanofibers Constructed by Pickering Emulsion Templating. Adv. Funct. Mater. 2022, 32, 2200249. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Chen, Y.; Song, S.; Huang, X.; Yang, Y.; Li, Y.; Tong, Y.; Xie, Y.; Li, J.; et al. Construction of Exosome SORL1 Detection Platform Based on 3D Porous Microfluidic Chip and its Application in Early Diagnosis of Colorectal Cancer. Small 2023, e2207381, online ahead of print. [Google Scholar] [CrossRef]


| Material | Advantages | Limitations | Fabrication Technique | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|---|
| PDMS | Easy fabrication, high flexibility, and thermal stability | Poor long-term stability | Soft lithography | High-resolution, facile fabrication | Difficulty in fabrication over large areas | [112,113] |
| Sacrificial template | Low-cost, size adjustable | Difficulty in removing templates, uneven pore distribution may exist | [35,36,114] | |||
| Track etching | Allows the formation of uniform pore sizes and controlled pore densities | Time consuming | [115] | |||
| Gas foaming technique | Facile and eco-friendly fabrication procedures | Poor control of pore size and porosity | [116] | |||
| Laser micromachining | Simple, fast and low cast, high precision | The principle of interaction between the material and the laser is not entirely clear | [117,118] | |||
| 3D printing | Desirable pore size and porosity | Relatively high cost, low fabrication efficiency | [36,119] | |||
| PMMA | Low processing cost, good mechanical properties, | Poor biocompatibility | Hot embossing | Facile, low cast | Requires high temperature and pressure conditions | [86,120] |
| Direct laser writing | Short cycle time of production | Limited resolution | [121,122] | |||
| Injection molding | Fast, high production efficiency | High cost of mold equipment | [123] | |||
| Paper | Cost-effective, simple, disposable, and portable | Channel size is not easy to control and not standardized, Auto-fluorescence interference | Photolithography | High resolution | Difficulty in fabrication process, time consuming, high cost | [1,124] |
| Printing | Low cast, simple operation procedures | Low resolution | [100,125] | |||
| Etching | Low cast | Low resolution and complexity, difficult to fabricate high-density microchannel networks | [126,127] | |||
| Embossing | Complex microfluidic networks can be prepared | The preparation process is complicated | [100,128] | |||
| Hydrogels | Biocompatibility, mechanical tenability | Complex preparation process | Templated-assisted | Control of the porous properties, morphology, and structure | Time consuming, complex process of template leaching | [129] |
| Freeze drying | Suitable for almost any material | High energy consumption, inability to precisely control porosity | [130,131] | |||
| 3D printing | Rapid and can produce complex, three-dimensional structures. | Limited resolution | [132,133] | |||
| Textile | Excellent biocompatibility | Non-standardized, not easy to mass produce | Spinning | Facile fabrication | Difficult to precise modeling | [134,135] |
| Electrospinning | Can prepare nano-scale microfiber | Inability to precisely control fiber diameter | [20] |
| Ref. and Years | Materials | Fabrication Methods | Detection Methods | Target and Sample Matrices | Detection Limit |
|---|---|---|---|---|---|
| [170] Patarajarin et al., 2022 | Paper | Wax printing | Antigen test | SARS-CoV-2 (Saliva) | 1 fg/μL |
| [184] Hong et al., 2022 | Hybrid Janus Membrane | Roller-assisted liquid printing. | Electrochemical | Glucose and lactate (sweat). | 0.15 μL |
| [185] Li et al., 2022 | Hydrogel paper | Self-assembled | Electrochemical | Glucose (sweat) | 10.3 μM |
| [186] Mogera et al., 2022 | Paper | Cutting | Surface-enhanced Raman spectroscopy (SERS) | Uric acid (sweat) | 1 μM |
| [191] Li et al., 2021 | Paper | Printing | Electrochemical | Glucose and lactate (sweat) | 17.05 μM |
| [192] Bagheri et al., 2021 | Paper | Wax printing | Electrochemical | Copper ions (sweat and serum) | 3 ppb |
| [193] Fiore et al., 2023 | Paper | Waxing printing | Electrochemical | Cortisol (sweat) | 101 mM |
| [194] Weng et al., 2022 | Paper | Screen-printing | Electrochemical | Cortisol (sweat) | 0.1 nM |
| [195] Singh et al., 2022 | Paper | Cutting | Electrochemical | Glucose (sweat) | 0.5 μM |
| [196] Fabiani et al., 2022 | Paper | Wax printing | Electrochemical | SARS-CoV-2 (saliva) | 0.1 ug/mL |
| [197] Moon et al., 2022 | PVA-based hydrogel | Sacrificial template | Electrochemical | βHydroxybutyrate (sweat) | 62 μM |
| [198] Gunatilake et al., 2021 | Nanotubes alginate hydrogel | Freeze-drying | Colorimetric | Glucose (sweat) | 0.8 mM |
| [199] Guzman et al., 2020 | Hydrogel | Sacrificial template | Colorimetric | Lipocalin-1 (tear) | 1 ng/mL |
| [200] Xu et al., 2021 | PEDOT:PSS hydrogel | Sacrificial template | Electrochemical | Uric acid (sweat) | 1.2 μM |
| [201] Siripongpreda et al., 2021 | Hydrogel | Matrix deposition | Colorimetric | Glucose (sweat) | 25 μM |
| [202] Yeung et al., 2022 | Graphene | Chemical vapor deposition | Electrochemical | Na+ (sweat) | 10 mM |
| [203] Yoon et al., 2020 | Graphene | Laser-induced | Electrochemical | Glucose (sweat) | 300 nM |
| [204] Wang et al., 2021 | Hydrogels | Cross-linking | Strain sensor | NaCl (sweat) | 0.15 μL |
| [205] Saha et al., 2021 | Paper | Cutting | Colorimetry | Lactate (sweat) | 20 mM |
| [47] Baretta et al., 2023 | Hydrogel | Template | Electrochemical | Glucose (serum) | 1 mM |
| [206] Liu et al., 2021 | PDMS | Template | Electrochemical | Cortisol (sweat) | 0.3 fg/mL |
| [207] Li et al., 2023 | Graphene | Hydrothermal | Electrochemical | Glucose (sweat) | 2.45 μM |
| [208] Xuan et al., 2018 | Graphene | Laser-induced | Electrochemical | Glucose (sweat) | 5 μM |
| [209] Kil et al., 2022 | Graphene inks | Printing | Electrochemical | Na+ (sweat) | 9.1 × 10−7 M |
| [210] Liu et al., 2021 | PEN and SFNFs | Hybridization material strategy | Electrochemical | Glucose (sweat) | 2 mM |
| [211] Xu et al., 2021 | Reduced graphene oxide | Electrostatic self-assembly | Electrochemical | Glucose (sweat) | 3.7 μM |
| [212] Poletti et al., 2021 | Graphene oxide | Chemical functionalization | Electrochemical | Glucose and lactate (sweat) | 32/68 nM |
| [173] Chakraborty et al., 2020 | CuO | Hydrothermal synthesis | Electrochemical | Enzyme-less glucose (saliva) | 0.41 μM |
| [213] Park et al., 2022 | Platinum nanozyme-hydrogel composite | Photopolymerization | Colorimetry | Glucose (serum) | 3.9 μM |
| [214] Elancheziyan et al., 2023 | Co-PM-NDGPC/SPE | Single-step electrodeposition | Electrochemical | Glucose (blood) | 7.9 μM |
| [215] Yao et al., 2022 | ZGC PLNPs | Self-assembly | Fluorescence analysis | Dopamine (serum) | 0.001 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Guo, X.; Sun, X.; Zhang, S.; Wu, J.; Yu, H.; Zhang, T.; Cheng, W.; Shi, Y.; Pan, L. Porous Structural Microfluidic Device for Biomedical Diagnosis: A Review. Micromachines 2023, 14, 547. https://doi.org/10.3390/mi14030547
Chen L, Guo X, Sun X, Zhang S, Wu J, Yu H, Zhang T, Cheng W, Shi Y, Pan L. Porous Structural Microfluidic Device for Biomedical Diagnosis: A Review. Micromachines. 2023; 14(3):547. https://doi.org/10.3390/mi14030547
Chicago/Turabian StyleChen, Luyao, Xin Guo, Xidi Sun, Shuming Zhang, Jing Wu, Huiwen Yu, Tongju Zhang, Wen Cheng, Yi Shi, and Lijia Pan. 2023. "Porous Structural Microfluidic Device for Biomedical Diagnosis: A Review" Micromachines 14, no. 3: 547. https://doi.org/10.3390/mi14030547
APA StyleChen, L., Guo, X., Sun, X., Zhang, S., Wu, J., Yu, H., Zhang, T., Cheng, W., Shi, Y., & Pan, L. (2023). Porous Structural Microfluidic Device for Biomedical Diagnosis: A Review. Micromachines, 14(3), 547. https://doi.org/10.3390/mi14030547







