Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes
Abstract
:1. Introduction
2. The Interplay between Plants and Microbial Communities
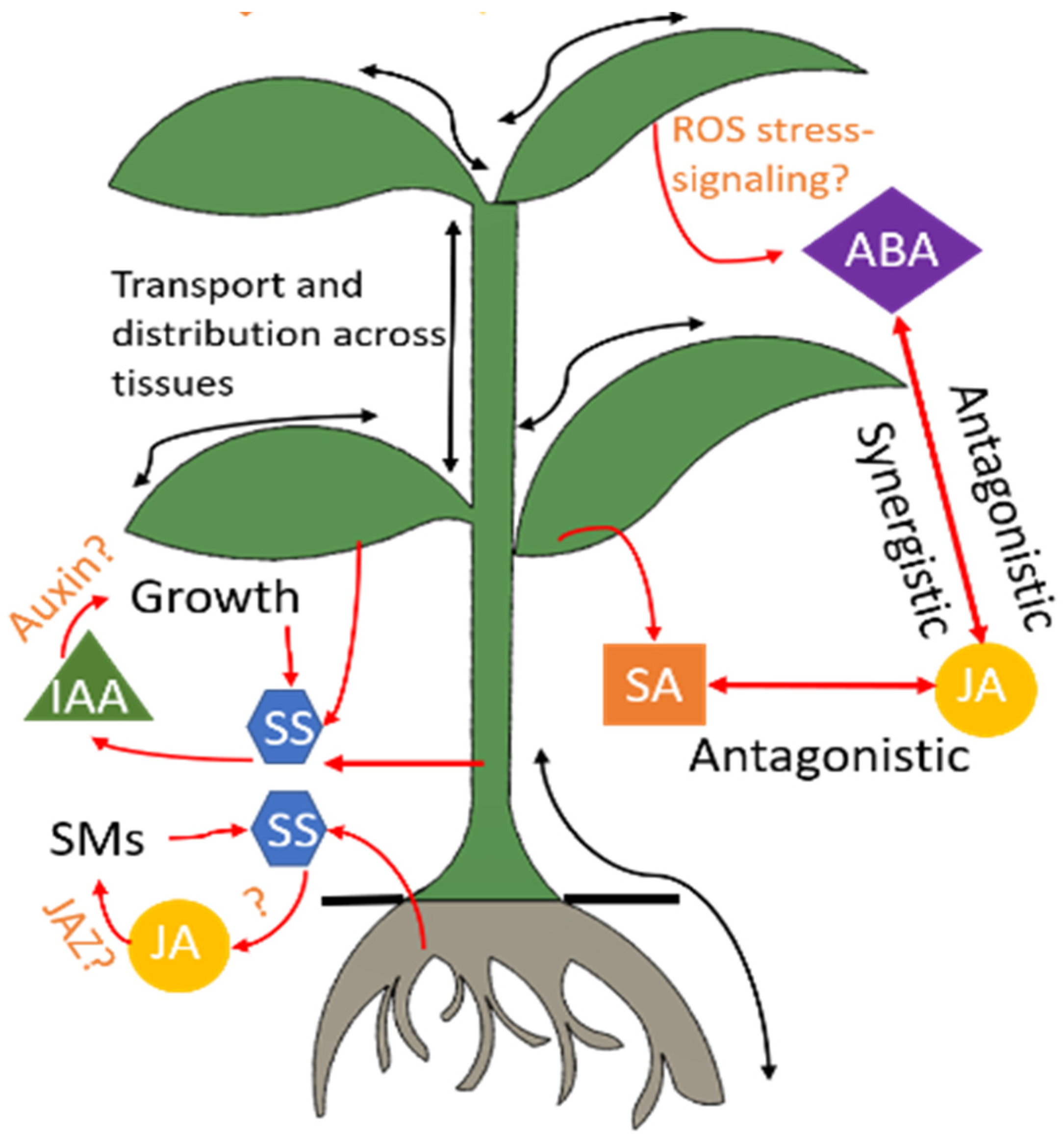
2.1. Phytohormone Mediated Plant-Microbe Mutualism
2.2. Role of Microorganisms in Plant Nutrition
2.3. Exudate Mediated Plant-Microbe Mutualism
2.4. Influence of Environment on Plant-Microbe Interactions
3. Existing Optical Sensing Technologies to Monitor Plant-Microbial Interactions
3.1. Localized Surface Plasmon Resonance Biosensors
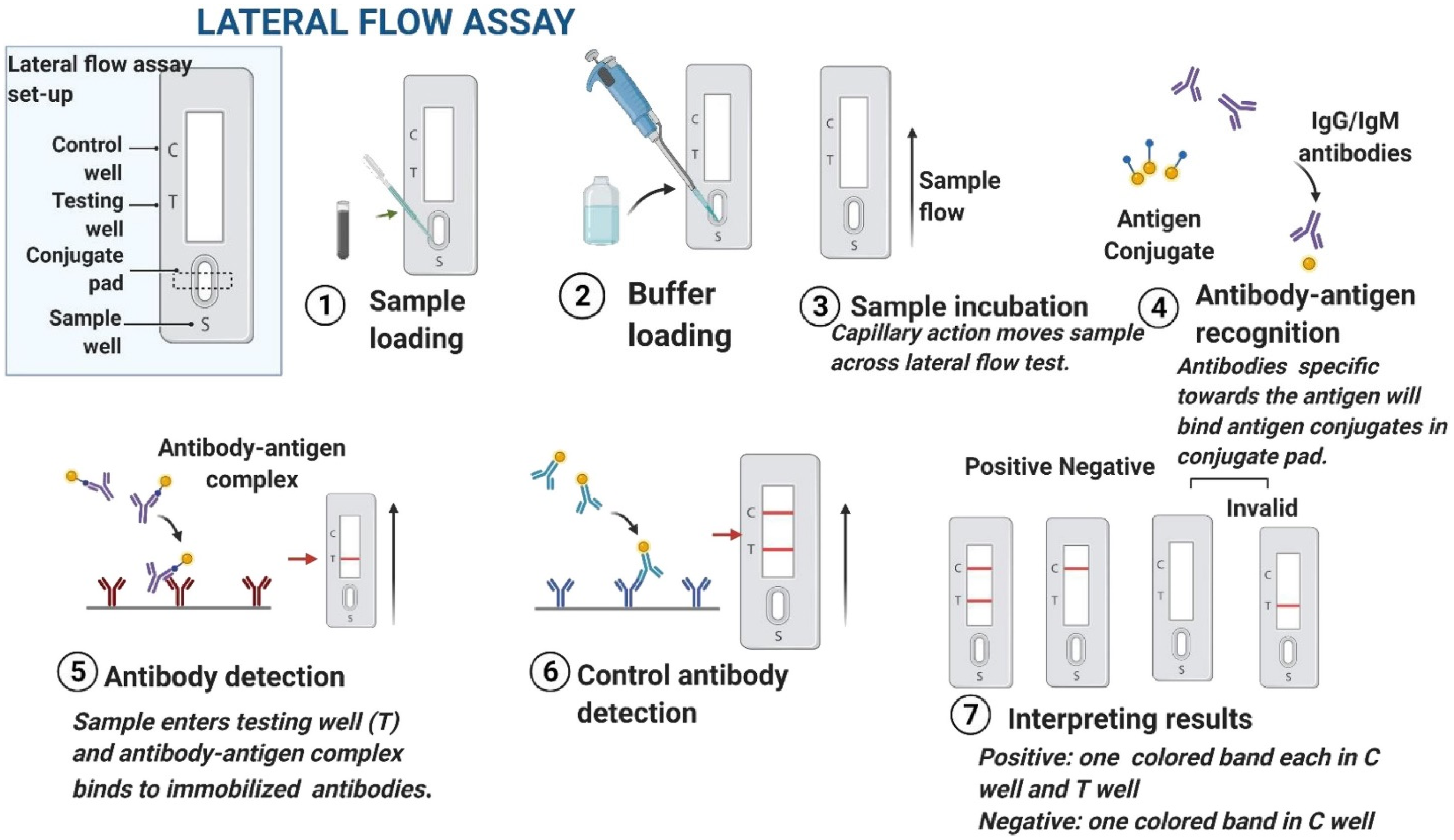
3.2. Lateral Flow Immunoassays
3.3. Lux Bioluminescent Biosensors
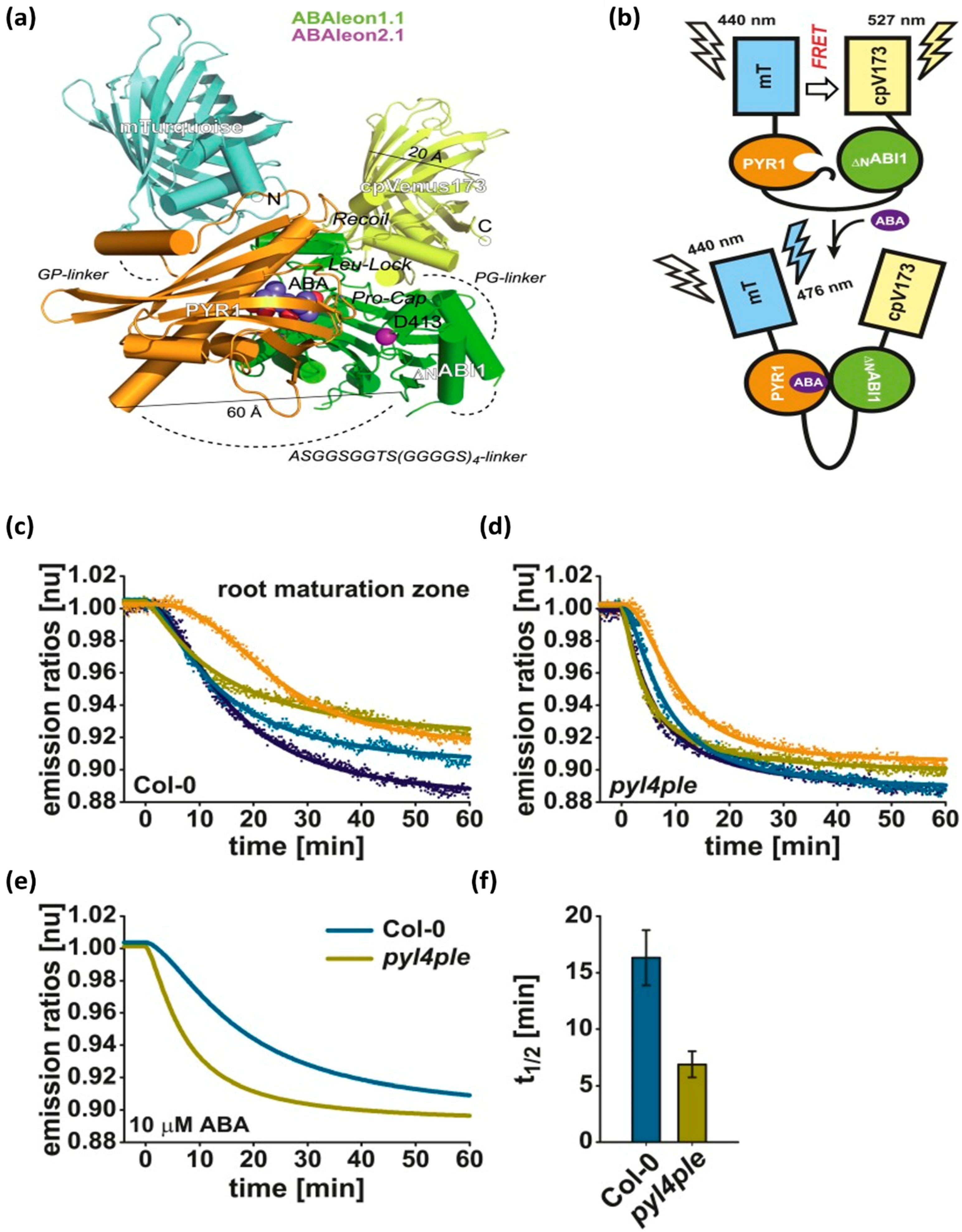
3.4. Fluorescence Resonance Energy Transfer Biosensors
3.5. Fluorometric Biosensors
| Ref. | Target | Sensor Configuration | Detection Technique | Sensitivity | Analyte Concentration Range/Limit of Detection (LOD) | Major Advantages and/or Limitations |
|---|---|---|---|---|---|---|
| [20] | Salicylic acid (SA) | Conjugate of gold nanoparticles and copper-based metal-organic framework | Fiber-tip LSPR | 0.0117% light reflection per μM | Conc. range: 100–1000 μM LOD: 37 μM | + in situ and real-time detection directly in plant sap + involves minimal damage to the plant + no need to extract plant samples + provides quantitative measurement + rapid detection in 1–2 min - the optical source and detector are bulky and not chip-scale, preventing scalability |
| [158] | cell wall protein of Pseudocercospora fijiensis | Gold-coated lateral flow assay immobilized with polyclonal antibody targeting a cell wall protein of P. fijiensis | LSPR | 0.0021 units of reflectance per ng mL−1 | Conc. range: 39.1 to 122 µg mL−1 LOD: 11.7 µg mL−1 | + reusable platform for routine monitoring + no matrix effects are observed during the sensor performance using real leaf banana extracts. - need to extract plant samples, which incurs a time delay between sample collection and analysis - destructive sample collection |
| [159] | Tomato yellow leaf curl virus (TYLCV) genome | Unmodified gold nanoparticles mixed with a complementary DNA probe | LSPR colorimetry | - | Conc. range: 0.75 to 200 ng/µL LOD: genome detection in 5 ng of the extracted DNA | + fast and sensitive detection, eliminating the need for sophisticated PCR amplification and detection equipment - extracted DNA sample goes through multiple steps: mixing with the designed probe, denaturing, annealing, and then cooling to room temperature followed by AuNPs addition. - lacks quantitative measurement - DNA sample needs to be extracted from infected leaves |
| [160] | Cucumber green mottle mosaic virus (CGMMV) RNA | Unmodified gold nanoparticles mixed with a species-specific probe | LSPR colorimetry | - | LOD: 30 pg/µL | + simple, low-cost, and visual detection + eliminates the need for sophisticated, expensive instrumentation + 100% specificity with good reproducibility - lacks quantitative measurement - RNA sample needs to be extracted from infected leaves and fruits |
| [165] | Single-stranded DNA (ssDNA) of the chili leaf curl virus (ChiLCV) | Amine-functionalized surface immobilized with gold nanoparticles and complementary ssDNA | ATR-LSPR | 0.833 a.u./(µg/mL) | Conc. range: 0.5 to 3.5 µg/mL LOD: 1 µg/mL | + provides quantitative measurement + the setup was capable to measure binding kinetics - the Kretschmann prism configuration resulted in a bulky and complex optical setup - sample needs to be extracted from infected plants |
| [166] | Begomovirus DNA | Functionalized gold nanoparticles | LSPR colorimetry | - | Conc. range: 1 ng/µL to 1 ag/µL LOD: 500 ag/µL | + the detection efficiency of LSPR assay (77.7%) was found to be better than PCR screening (49.4%) + able to detect begomoviruses infecting plants belonging to different genera + five different probes were designed to detect any differences in the detection limit or specificity among the probes - The DNA extraction procedure is lengthy and requires technical expertise |
| [174] | cis-jasmone, α-pinene, limonene, and γ-terpinene VOCs | Gold nanoparticles doped into molecularly imprinted sol-gel | LSPR | - | LOD: vapor flow rate of 0.3 L/min | + enhanced sensitivity through hot spot generation + detect plant VOCs in single and binary mixtures using a multichannel sensor configuration + sensing combined with a pattern recognition approach to establish plant VOC identification models. - additional setup is required to generate plant VOC vapor using the headspace method, which is not scalable for in-field monitoring - the sensor is not suitable for in-planta VOC detection |
| [175] | (E)-2-hexenal VOC emitted during Phytophthora infestans infection | sensor array comprised of cysteine-functionalized plasmonic nanoparticles and chemo-responsive organic dyes | LSPR | - | LOD: between 2.5 and 5 ppm | + smartphone-based handheld VOC fingerprinting platform + in-field monitoring + detects key plant volatiles at the ppm level within 1 min of reaction + early detection of tomato late blight 2 days after inoculation + a detection accuracy of ≥95% - lacks automation in monitoring. The user has to screen every plant manually with the handheld device |
| [178] | Ralstonia solanacearum | Gold nanoparticles functionalized with antibodies | LFIA | - | Conc. range: 101–108 cells/mL LOD: 3 × 104 cells/mL | + signal enhancement reduced the detection limit by 33 times + rapid detection in 3 min + quantitative assay - requires sample extraction, which is destructive and hinders real-time sensing - sensor was tested with artificially contaminated samples |
| [179] | Potato leafroll virus | Sandwich of gold nanoparticles and silver enhancement | LFIA | - | Conc. Range: 0.1–100 ng/mL LOD: 0.2 ng/mL | + silver enhancement makes the assay 15 times more sensitive + up to 0.2 ng/mL of PLRV can be detected with the naked eye - leaves are crushed in a mortar for sample collection - specificity reduces for non-specific virus concentration >1000 ng/mL |
| [183] | Root exudates | Rhizobium leguminosarum bv. viciae strain 3841 | Lux | - | LOD: 0.001 mM for sugars and polyols, 0.01 mM for organic acids, and 0.001 mM for amino acids | + in-vivo spatial and temporal mapping of 376 molecules in pea root exudate + non-destructive sensing - no analysis is presented on the toxic effect of the lux-marked biosensors on the plant or soil - the stress response of the plant upon injection of foreign objects was also not analyzed |
| [185] | C-substrate availability | Pseudomonas fluorescens 10586 pUCD607) tagged with the lux CDABE | Lux | - | - | + determine the relationship between shoot nitrate concentration and root exudation in vivo - lacks information on sensitivity, detection limit, and stability of the sensor |
| [194] | ABA | FRET reporter termed ABAleon | FRET | - | Conc. range: 0.8–50 μM LOD: ~0.8 μM | + direct visualization of ABA concentration changes and distribution - binding to the reporter may reduce the amount of ABA that is available to perform its role as a hormone |
| [196] | IAA | FRET reporter termed AuxSens | FRET | 0.8 (FRET ratio)/(10−2–10−4) M IAA | LOD: ~1 μM | + quantitative in-vivo visualization of auxin distribution in plants - further analysis of the stability and toxicity of the reporter is needed |
| [197] | Glucose | A compound of horse radish peroxidase, glucose oxidase, and Ampliflu Red | Fluorescence | - | LOD: down to 7 ng min−1 root−1 was shown | + detects spatial variability of glucose released from plant roots - the roots were placed separately on a gel |
| [198] | Galactosides | A strain of the bacterium Sinorhizobium meliloti containing a gfp gene fused to the melA promoter | Fluorescence | - | - | + non-destructive method to examine rhizosphere soil chemical composition - lacks information on sensitivity, detection limit, and stability of the sensor |
| [199] | H2O2 profile | DNA functionalized SWCNT | Fluorescence | - | Conc. Range: 10−8–10−1 M | + species-independent nanosensor probe + a simulation model explains the differences in H2O2 wave velocity across species - requires a non-portable optical setup comprising a laser, camera lens, and filter wheel |
4. Other Biosensors
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dazzo, F.B.; Ganter, S. Rhizosphere. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 335–349. ISBN 978-0-12-373944-5. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Mantelin, S. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2003, 55, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharan, B.; Nehra, V. Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sci. Med. Res. 2011, 2011, 31. [Google Scholar]
- Joseph, B.; Ranjan Patra, R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2012, 1, 141–152. [Google Scholar] [CrossRef]
- Wei, F.; Feng, H.; Zhang, D.; Feng, Z.; Zhao, L.; Zhang, Y.; Deakin, G.; Peng, J.; Zhu, H.; Xu, X. Composition of Rhizosphere Microbial Communities Associated With Healthy and Verticillium Wilt Diseased Cotton Plants. Front. Microbiol. 2021, 12, 618169. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Yu, S.; Jiang, Z.; Liu, S.; Wu, Y.; Huang, X. Rhizosphere Microbial Community Structure Is Selected by Habitat but Not Plant Species in Two Tropical Seagrass Beds. Front. Microbiol. 2020, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Monitoring Biotic Stress in Plants Due to Pathogens and Pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Bhar, A.; Chakraborty, A.; Roy, A. Plant Responses to Biotic Stress: Old Memories Matter. Plants Basel Switz. 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Knief, C. Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front. Plant Sci. 2014, 5, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelake, R.M.; Pramanik, D.; Kim, J.-Y. Exploration of Plant-Microbe Interactions for Sustainable Agriculture in CRISPR Era. Microorganisms 2019, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.W.; Ha, D.; Lee, J.; Lee, S.K.; Kim, T. Review of Micro/Nanotechnologies for Microbial Biosensors. Front. Bioeng. Biotechnol. 2015, 3, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levak, V.; Lukan, T.; Gruden, K.; Coll, A. Biosensors: A Sneak Peek into Plant Cell’s Immunity. Life 2021, 11, 209. [Google Scholar] [CrossRef]
- Zolti, A.; Green, S.J.; Sela, N.; Hadar, Y.; Minz, D. The microbiome as a biosensor: Functional profiles elucidate hidden stress in hosts. Microbiome 2020, 8, 71. [Google Scholar] [CrossRef]
- Galvan, C.; Montiel, R.; Lorenz, K.; Carter, J.; Hossain, N.I.; Tabassum, S. A microneedle-based Leaf Patch with IoT Integration for Real-time Monitoring of Salinity Stress in Plants. In Proceedings of the 2022 IEEE 15th Dallas Circuit And System Conference (DCAS), Dallas, TX, USA, 17–19 June 2022; pp. 1–2. [Google Scholar]
- Tabassum, S. Real-time quantification of salicylic acid with a fiber optic sensor functionalized by gold nanoparticles-copper metal organic conjugate coating. In Sensing for Agriculture and Food Quality and Safety XIV; SPIE Defense + Commercial Sensing: Orlando, FL, USA, 2022; Volume 12120, pp. 52–59. [Google Scholar] [CrossRef]
- Ishtiaque Hossain, N.; Tabassum, S. Stem-FIT: A Microneedle-based Multi-parametric Sensor for In Situ Monitoring of Salicylic Acid and pH Levels in Live Plants. In Proceedings of the 2022 IEEE 17th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Taoyuan, Taiwan, 14–17 April 2022; pp. 312–316. [Google Scholar]
- Hossain, N.I.; Noushin, T.; Tabassum, S. Leaf-FIT: A Wearable Leaf Sensor for In-Situ and Real-Time Monitoring of Plant Phytohormones. In Proceedings of the 2021 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2021; pp. 1–4. [Google Scholar]
- Hossain, N.I.; Tabassum, S. Fruit-FIT: Drone Interfaced Multiplexed Sensor Suite to Determine the Fruit Ripeness. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2022; pp. 1–4. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Song, C.; Zhu, F.; Carrión, V.J.; Cordovez, V. Beyond Plant Microbiome Composition: Exploiting Microbial Functions and Plant Traits via Integrated Approaches. Front. Bioeng. Biotechnol. 2020, 8, 896. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán, P.; Tortella, G.; Viscardi, S.; Barra, P.J.; Carrion, V.J.; de la Luz Mora, M.; Pozo, M.J. Microbial Community Composition in Take-All Suppressive Soils. Front. Microbiol. 2018, 9, 2198. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.-H.; Doty, S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016, 6, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuťáková, E.; Herben, T.; Münzbergová, Z. Heterospecific plant–soil feedback and its relationship to plant traits, species relatedness, and co-occurrence in natural communities. Oecologia 2018, 187, 679–688. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Huang, J.; Reichelt, M.; Chowdhury, S.; Hammerbacher, A.; Hartmann, H. Increasing carbon availability stimulates growth and secondary metabolites via modulation of phytohormones in winter wheat. J. Exp. Bot. 2017, 68, 1251–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Harper, C.P. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2018, 69, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; John, R.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci. Rep. 2018, 8, 2831. [Google Scholar] [CrossRef] [Green Version]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Janda, T.; Szalai, G. Abscisic Acid May Alter the Salicylic Acid-Related Abiotic Stress Response in Maize: Abscisic Acid- and Salicylic Acid-Related Responses. J. Agron. Crop Sci. 2011, 197, 368–377. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.-B.; Liu, Y.-Q.; Chen, D.-Y.; Chen, F.-Y.; Fang, X.; Hong, G.-J.; Wang, L.-J.; Wang, J.-W.; Chen, X.-Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol. Plant. 2012, 34, 97–106. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhakem, A. Salicylic acid ameliorates salinity tolerance in maize by regulation of phytohormones and osmolytes. Plant Soil Environ. 2020, 66, 533–541. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Akter, A.; Tahjib-Ul-Arif, M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy 2021, 11, 790. [Google Scholar] [CrossRef]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging Players in Controlling Temperature Stress Tolerance. Front. Plant Sci. 2016, 6, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhang, C.; Zhang, G.; Fu, W.; Feng, B.; Chen, T.; Peng, S.; Tao, L.; Fu, G. Abscisic Acid Negatively Modulates Heat Tolerance in Rolled Leaf Rice by Increasing Leaf Temperature and Regulating Energy Homeostasis. Rice 2020, 13, 18. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [Green Version]
- Yarzábal, L.A.; Chica, E.J. Chapter 3—Role of Rhizobacterial Secondary Metabolites in Crop Protection Against Agricultural Pests and Diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–53. ISBN 978-0-444-63504-4. [Google Scholar]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Galbally, I.E.; Kirstine, W. The Production of Methanol by Flowering Plants and the Global Cycle of Methanol. J. Atmos. Chem. 2002, 43, 195–229. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Shallcross, D.E. Modelling terrestrial biogenic isoprene fluxes and their potential impact on global chemical species using a coupled LSM–CTM model. Atmos. Environ. 2000, 34, 2909–2925. [Google Scholar] [CrossRef]
- Tiku, A.R. Antimicrobial Compounds and Their Role in Plant Defense. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 283–307. ISBN 978-981-10-7371-7. [Google Scholar]
- Bartel, B. AUXIN BIOSYNTHESIS. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Meuwly, P.; Pilet, P.E. Local treatment with indole-3-acetic acid induces differential growth responses in Zea mays L. roots. Planta 1991, 185, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Suzuki, T.; Fujita, T.; Masuda, M.; Shimizu, S. Occurrence of enzymes involved in biosynthesis of indole-3-acetic acid from indole-3-acetonitrile in plant-associated bacteria, Agrobacterium and Rhizobium. Proc. Natl. Acad. Sci. USA 1995, 92, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Koga, J.; Adachi, T.; Hidaka, H. Purification and characterization of indolepyruvate decarboxylase. A novel enzyme for indole-3-acetic acid biosynthesis in Enterobacter cloacae. J. Biol. Chem. 1992, 267, 15823–15828. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Fry, S.C. Cellulases, hemicelluloses and auxin-stimulated growth: A possible relationship. Physiol. Plant. 1989, 75, 532–536. [Google Scholar] [CrossRef]
- Gutierrez, C.K.; Matsui, G.Y.; Lincoln, D.E.; Lovell, C.R. Production of the Phytohormone Indole-3-Acetic Acid by Estuarine Species of the Genus Vibrio. Appl. Environ. Microbiol. 2009, 75, 2253–2258. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Gimenez-Ibanez, S.; Chini, A.; Solano, R. How Microbes Twist Jasmonate Signaling around Their Little Fingers. Plants 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollah, M.I.; Choi, H.W.; Yeam, I.; Lee, J.M.; Kim, Y. Salicylic Acid, a Plant Hormone, Suppresses Phytophagous Insect Immune Response by Interrupting HMG-Like DSP1. Front. Physiol. 2021, 12, 744272. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.F. Systemic acquired resistance induced by localized virus infections in plants. Virology 1961, 14, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, S.; Van Wees, S.C.M.; Pieterse, C.M.J. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.K.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Basso, V.; Veneault-Fourrey, C. Role of Jasmonates in Beneficial Microbe-Root Interactions. Methods Mol. Biol. Clifton NJ 2020, 2085, 43–67. [Google Scholar] [CrossRef]
- Díaz, J.; ten Have, A.; van Kan, J.A.L. The Role of Ethylene and Wound Signaling in Resistance of Tomato to Botrytis cinerea. Plant Physiol. 2002, 129, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Thomma, B.P.H.J.; Eggermont, K.; Tierens, K.F.M.-J.; Broekaert, W.F. Requirement of Functional Ethylene-Insensitive 2 Gene for Efficient Resistance of Arabidopsis to Infection by Botrytis cinerea. Plant Physiol. 1999, 121, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef]
- Solano, R.; Gimenez-Ibanez, S. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Front. Plant Sci. 2013, 4, 72. [Google Scholar]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Freeman An Overview of Plant Defenses against Pathogens and Herbivores. Plant Health Instr. 2008. [CrossRef] [Green Version]
- Mothershead, K.; Marquis, R.J. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology 2000, 81, 30–40. [Google Scholar] [CrossRef]
- Shimotori, Y.; Hoshi, M.; Okabe, H.; Miyakoshi, T.; Kanamoto, T.; Nakashima, H. Synthesis, odour characteristics and antibacterial activities of the stereoisomeric forms of whisky lactone and its thiono analogues: Biological activities of the stereosimeric forms of whisky lactone and thiono analogues. Flavour Fragr. J. 2017, 32, 29–35. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. cis-Jasmone Elicits Aphid-Induced Stress Signalling in Potatoes. J. Chem. Ecol. 2017, 43, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Bison, J.V.; Cardoso-Gustavson, P.; de Moraes, R.M.; da Silva Pedrosa, G.; Cruz, L.S.; Freschi, L.; de Souza, S.R. Volatile organic compounds and nitric oxide as responses of a Brazilian tropical species to ozone: The emission profile of young and mature leaves. Environ. Sci. Pollut. Res. Int. 2018, 25, 3840–3848. [Google Scholar] [CrossRef]
- Graham, K.K.; Brown, S.; Clarke, S.; Röse, U.S.R.; Starks, P.T. The European wool-carder bee (Anthidium manicatum) eavesdrops on plant volatile organic compounds (VOCs) during trichome collection. Behav. Process. 2017, 144, 5–12. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant-Microbe Interact. MPMI 2013, 26, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting Plant Volatile Organic Compounds (VOCs) in Agriculture to Improve Sustainable Defense Strategies and Productivity of Crops. Front. Plant Sci. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Yuzikhin, O.S.; Gogoleva, N.E.; Shaposhnikov, A.I.; Konnova, T.A.; Osipova, E.V.; Syrova, D.S.; Ermakova, E.A.; Shevchenko, V.P.; Nagaev, I.Y.; Shevchenko, K.V.; et al. Rhizosphere Bacterium Rhodococcus sp. P1Y Metabolizes Abscisic Acid to Form Dehydrovomifoliol. Biomolecules 2021, 11, 345. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Weger, L.A.; van der Vlugt, C.I.; Wijfjes, A.H.; Bakker, P.A.; Schippers, B.; Lugtenberg, B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J. Bacteriol. 1987, 169, 2769–2773. [Google Scholar] [CrossRef] [Green Version]
- Guerre, P. Ergot Alkaloids Produced by Endophytic Fungi of the Genus Epichloë. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Kalia, V.C.; Gong, C.; Patel, S.K.S.; Lee, J.-K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef]
- Schwelm, A.; Badstöber, J.; Bulman, S.; Desoignies, N.; Etemadi, M.; Falloon, R.E.; Gachon, C.M.M.; Legreve, A.; Lukeš, J.; Merz, U.; et al. Not in your usual Top 10: Protists that infect plants and algae. Mol. Plant Pathol. 2017, 19, 1029–1044. [Google Scholar] [CrossRef] [Green Version]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and Function of the Bacterial Root Microbiota in Wild and Domesticated Barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [Green Version]
- Beneduzi, A.; Peres, D.; Vargas, L.K.; Bodanese-Zanettini, M.H.; Passaglia, L.M.P. Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing bacilli isolated from rice fields in South Brazil. Appl. Soil Ecol. 2008, 39, 311–320. [Google Scholar] [CrossRef]
- Govindarajan, M.; Balandreau, J.; Kwon, S.-W.; Weon, H.-Y.; Lakshminarasimhan, C. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb. Ecol. 2008, 55, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Chen, S.C.; Chen, Y.S.; Lin, H.M.; Chen, Y.L. Detection of Burkholderia pseudomallei in rice fields with PCR-based technique. Folia Microbiol. 2003, 48, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Interactions of Gramineous Plants with Azoarcus spp. and Other Diazotrophs: Identification, Localization, and Perspectives to Study their Function. Crit. Rev. Plant Sci. 1998, 17, 29–54. [Google Scholar] [CrossRef]
- Menendez, E.; Garcia-Fraile, P. Plant probiotic bacteria: Solutions to feed the world. AIMS Microbiol. 2017, 3, 502–524. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy Cycle: An Oxidative Process in Plants for Nutrient Extraction from Symbiotic Microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- el Zahar Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Tadych, M.; White, J.F. Endophytic Microbes☆. In Encyclopedia of Microbiolog, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 123–136. ISBN 978-0-12-811737-8. [Google Scholar]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Islam, S.U.; Hassan Dar, T.U. Role of Micronutrients in Secondary Metabolism of Plants. In Plant Micronutrients: Deficiency and Toxicity Management; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 311–329. ISBN 978-3-030-49856-6. [Google Scholar]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. Camb. Engl. 2003 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.E.; Kleiner, M.; Garrell, A.-K. Elucidating Plant-Microbe-Environment Interactions Through Omics-Enabled Metabolic Modelling Using Synthetic Communities. Front. Plant Sci. 2022, 13, 910377. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Horsfall, J.G.; Dlmond, A.E. Plant Pathology: An Advanced Treatise; Academic Press: New York, NY, USA, 1960; Volume II, pp. 1–715, ASIN B004YTRFI8. [Google Scholar]
- Peng, Y.; van Wersch, R.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant-Microbe Interact. MPMI 2018, 31, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, S.; Sureshkumar, S.; Lempe, J.; Weigel, D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2006, 2, e106. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, D.; Liu, C.; Zhao, J.; Liu, J.; Liu, N.; Tang, D.; Hu, Y. TCP transcription factors interact with ZED1-related kinases as components of the temperature-regulated immunity. Plant Cell Environ. 2019, 42, 2045–2056. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, W.; Hua, J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Z.; Bao, Z.; Yang, L.; Wu, D.; Shu, X.; Hua, J. MOS1 functions closely with TCP transcription factors to modulate immunity and cell cycle in Arabidopsis. Plant J. Cell Mol. Biol. 2018, 93, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Halane, M.K.; Kim, S.H.; Gassmann, W. Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 2011, 334, 1405–1408. [Google Scholar] [CrossRef]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual impact of elevated temperature on plant defence and bacterial virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.-F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans Strain PsJN on Tomato (Lycopersicon esculentum L.) Under High Temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miotto-Vilanova, L.; Jacquard, C.; Courteaux, B.; Wortham, L.; Michel, J.; Clément, C.; Barka, E.A.; Sanchez, L. Burkholderia phytofirmans PsJN Confers Grapevine Resistance against Botrytis cinerea via a Direct Antimicrobial Effect Combined with a Better Resource Mobilization. Front. Plant Sci. 2016, 7, 1236. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.-J.; Shimono, M.; Sugano, S.; Kojima, M.; Yazawa, K.; Yoshida, R.; Inoue, H.; Hayashi, N.; Sakakibara, H.; Takatsuji, H. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol. Plant-Microbe Interact. MPMI 2010, 23, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Clode, P.L.; Kilburn, M.R.; Stockdale, E.A.; Murphy, D.V. Competition between plant and bacterial cells at the microscale regulates the dynamics of nitrogen acquisition in wheat (Triticum aestivum). New Phytol. 2013, 200, 796–807. [Google Scholar] [CrossRef] [Green Version]
- Wiedenfeld, B.; Enciso, J. Sugarcane Responses to Irrigation and Nitrogen in Semiarid South Texas. Agron. J. 2008, 100, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat. Plants 2020, 6, 800–808. [Google Scholar] [CrossRef]
- Tsikou, D.; Yan, Z.; Holt, D.B.; Abel, N.B.; Reid, D.E.; Madsen, L.H.; Bhasin, H.; Sexauer, M.; Stougaard, J.; Markmann, K. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 2018, 362, 233–236. [Google Scholar] [CrossRef]
- Okuma, N.; Soyano, T.; Suzaki, T.; Kawaguchi, M. MIR2111-5 locus and shoot-accumulated mature miR2111 systemically enhance nodulation depending on HAR1 in Lotus japonicus. Nat. Commun. 2020, 11, 5192. [Google Scholar] [CrossRef]
- Van der Ent, S.; Verhagen, B.W.M.; Van Doorn, R.; Bakker, D.; Verlaan, M.G.; Pel, M.J.C.; Joosten, R.G.; Proveniers, M.C.G.; Van Loon, L.C.; Ton, J.; et al. MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol. 2008, 146, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamioudis, C.; Hanson, J.; Pieterse, C.M.J. β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 2014, 204, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Nobori, T.; Velásquez, A.C.; Wu, J.; Kvitko, B.H.; Kremer, J.M.; Wang, Y.; He, S.Y.; Tsuda, K. Transcriptome landscape of a bacterial pathogen under plant immunity. Proc. Natl. Acad. Sci. USA 2018, 115, E3055–E3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantigoso, H.A.; He, Y.; DiLegge, M.J.; Vivanco, J.M. Methods for Root Exudate Collection and Analysis. In The Plant Microbiome: Methods and Protocols; Carvalhais, L.C., Dennis, P.G., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 291–303. ISBN 978-1-07-161040-4. [Google Scholar]
- Dundek, P.; Holík, L.; Rohlík, T.; Hromádko, L.; Vranová, V.; Rejšek, K.; Formánek, P. Methods of plant root exudates analysis: A review. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 59, 241. [Google Scholar] [CrossRef] [Green Version]
- Escolà Casas, M.; Matamoros, V. Analytical challenges and solutions for performing metabolomic analysis of root exudates. Trends Environ. Anal. Chem. 2021, 31, e00130. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview From Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2020, 11, 609155. [Google Scholar] [CrossRef]
- del Cerro, J.; Cruz Ulloa, C.; Barrientos, A.; de León Rivas, J. Unmanned Aerial Vehicles in Agriculture: A Survey. Agronomy 2021, 11, 203. [Google Scholar] [CrossRef]
- Folnović, T. Crop Data Delivered from the Air. AGRIVI. 2017. Available online: https://www.agrivi.com/blog/crop-data-delivered-from-the-air-with-unmanned-aerial-vehicle-uav/ (accessed on 29 December 2022).
- Zhang, Z.; Boubin, J.; Stewart, C.; Khanal, S. Whole-Field Reinforcement Learning: A Fully Autonomous Aerial Scouting Method for Precision Agriculture. Sensors 2020, 20, 6585. [Google Scholar] [CrossRef]
- Noirot-Gros, M.-F.; Shinde, S.V.; Akins, C.; Johnson, J.L.; Zerbs, S.; Wilton, R.; Kemner, K.M.; Noirot, P.; Babnigg, G. Functional Imaging of Microbial Interactions With Tree Roots Using a Microfluidics Setup. Front. Plant Sci. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawy, T. Capturing microbial interactions. Nat. Methods 2017, 14, 35. [Google Scholar] [CrossRef]
- Imam, J.; Singh, P.K.; Shukla, P. Plant Microbe Interactions in Post Genomic Era: Perspectives and Applications. Front. Microbiol. 2016, 7, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.A.; Ford, R. Biosensor Technologies for Early Detection and Quantification of Plant Pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2023, 7, 7–25. [Google Scholar] [CrossRef]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.J.; Bohlmeyer, D.; Hemmer, P.; Kolomiets, M.V.; Yuan, J.S.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393–3396. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Huang, C.H.; Singh, G.P.; Park, B.S.; Chua, N.-H.; Ram, R.J. Portable Raman leaf-clip sensor for rapid detection of plant stress. Sci. Rep. 2020, 10, 20206. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Marques, J.P.R.; de Almeida, E.; Dos Reis, A.R.; Pereira de Carvalho, H.W. X-ray fluorescence spectroscopy (XRF) applied to plant science: Challenges towards in vivo analysis of plants. Met. Integr. Biometal Sci. 2020, 12, 183–192. [Google Scholar] [CrossRef]
- Singh, A.; Mai, D.; Kumar, A.; Steyn, A.J.C. Dissecting virulence pathways of Mycobacterium tuberculosis through protein-protein association. Proc. Natl. Acad. Sci. USA 2006, 103, 11346–11351. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Domínguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martínez-Júlvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP+ Reductase for the Interaction with NADP+. Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef]
- Marcuello, C.; de Miguel, R.; Lostao, A. Molecular Recognition of Proteins through Quantitative Force Maps at Single Molecule Level. Biomolecules 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.; Nam, K.; Park, I.S.; Son, M.; Ko, H.; Lee, S.; Yoon, D.S.; Chang, W.-J.; Lee, S.Y.; et al. Automated Dielectrophoretic Tweezers-Based Force Spectroscopy System in a Microfluidic Device. Sensors 2017, 17, 2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Arora, K. Trends in nano-inspired biosensors for plants. Mater. Sci. Energy Technol. 2020, 3, 255–273. [Google Scholar] [CrossRef]
- Ortiz-Tena, J.G.; Rühmann, B.; Sieber, V. Colorimetric Determination of Sulfate via an Enzyme Cascade for High-Throughput Detection of Sulfatase Activity. Anal. Chem. 2018, 90, 2526–2533. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Sánchez-Álvarez, A.; Islas-Flores, I.; Canto-Canche, B.; Carrillo-Pech, M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M. Early Detection of the Fungal Banana Black Sigatoka Pathogen Pseudocercospora fijiensis by an SPR Immunosensor Method. Sensors 2019, 19, 465. [Google Scholar] [CrossRef] [Green Version]
- Razmi, A.; Golestanipour, A.; Nikkhah, M.; Bagheri, A.; Shamsbakhsh, M.; Malekzadeh-Shafaroudi, S. Localized surface plasmon resonance biosensing of tomato yellow leaf curl virus. J. Virol. Methods 2019, 267, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Xia, X.; Yang, C.; Huang, J.; Wan, S. Colorimetric detection of Cucumber green mottle mosaic virus using unmodified gold nanoparticles as colorimetric probes. J. Virol. Methods 2017, 243, 113–119. [Google Scholar] [CrossRef]
- Ekgasit, S.; Thammacharoen, C.; Knoll, W. Surface Plasmon Resonance Spectroscopy Based on Evanescent Field Treatment. Anal. Chem. 2004, 76, 561–568. [Google Scholar] [CrossRef]
- Kurihara, K.; Suzuki, K. Theoretical Understanding of an Absorption-Based Surface Plasmon Resonance Sensor Based on Kretchmann’s Theory. Anal. Chem. 2002, 74, 696–701. [Google Scholar] [CrossRef]
- Lambert, A.S.; Valiulis, S.N.; Malinick, A.S.; Tanabe, I.; Cheng, Q. Plasmonic Biosensing with Aluminum Thin Films under the Kretschmann Configuration. Anal. Chem. 2020, 92, 8654–8659. [Google Scholar] [CrossRef]
- Zaera, F. Probing Liquid/Solid Interfaces at the Molecular Level. Chem. Rev. 2012, 112, 2920–2986. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Agarwal, D.K.; Mandal, B.; Rao, V.R.; Kundu, T. Detection of the Chilli Leaf Curl Virus Using an Attenuated Total Reflection-Mediated Localized Surface-Plasmon-Resonance-Based Optical Platform. ACS Omega 2021, 6, 17413–17423. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Arun, V. Detection of Begomovirus in chilli and tomato plants using functionalized gold nanoparticles. Sci. Rep. 2021, 11, 14203. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Nguyen, H.A.; Zhang, J.; Tabassum, S.; Cao, H. Towards Multiplexed and Multimodal Biosensor Platforms in Real-Time Monitoring of Metabolic Disorders. Sensors 2022, 22, 5200. [Google Scholar] [CrossRef]
- Tabassum, S.; Kumar, D.P.; Kumar, R. Copper Complex-Coated Nanopatterned Fiber-Tip Guided Mode Resonance Device for Selective Detection of Ethylene. IEEE Sens. J. 2021, 21, 17420–17429. [Google Scholar] [CrossRef]
- Tabassum, S.; Kumar, R.; Dong, L. Nanopatterned Optical Fiber Tip for Guided Mode Resonance and Application to Gas Sensing. IEEE Sens. J. 2017, 17, 7262–7272. [Google Scholar] [CrossRef]
- Kumar, R.; Tabassum, S.; Dong, L. Nano-Patterning Methods Including: (1) Patterning Of Nanophotonic Structures at Optical Fiber Tip for Refractive Index Sensing and (2) Plasmonic Crystal Incorporating Graphene Oxide Gas Sensor for Detection of Volatile Organic Compounds. U.S. Patent 10,725,373, 8 September 2020. [Google Scholar]
- Tabassum, S.; Aryal, S. Real-time quantification of CD63 with anti-CD63 functionalized plasmonic fiber optic probe. In Optical Fibers and Sensors for Medical Diagnostics, Treatment and Environmental Applications XXII; SPIE BIOS: San Francisco, CA, USA, 2022; Volume 11953, pp. 18–22. [Google Scholar]
- Tabassum, S.; Kumar, R. Advances in Fiber-Optic Technology for Point-of-Care Diagnosis and In Vivo Biosensing. Adv. Mater. Technol. 2020, 5, 1900792. [Google Scholar] [CrossRef]
- Tabassum, S.; Wang, Y.; Qu, J.; Wang, Q.; Oren, S.; Weber, R.J.; Lu, M.; Kumar, R.; Dong, L. Patterning of nanophotonic structures at optical fiber tip for refractive index sensing. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Shang, L.; Liu, C.; Chen, B.; Hayashi, K. Plant Biomarker Recognition by Molecular Imprinting Based Localized Surface Plasmon Resonance Sensor Array: Performance Improvement by Enhanced Hotspot of Au Nanostructure. ACS Sens. 2018, 3, 1531–1538. [Google Scholar] [CrossRef]
- Li, Z.; Paul, R.; Ba Tis, T.; Saville, A.C.; Hansel, J.C.; Yu, T.; Ristaino, J.B.; Wei, Q. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 2019, 5, 856–866. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. A review of recent advances in plant-pathogen detection systems. Heliyon 2022, 8, e11855. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Razo, S.C.; Panferova, N.A.; Panferov, V.G.; Safenkova, I.V.; Drenova, N.V.; Varitsev, Y.A.; Zherdev, A.V.; Pakina, E.N.; Dzantiev, B.B. Enlargement of Gold Nanoparticles for Sensitive Immunochromatographic Diagnostics of Potato Brown Rot. Sensors 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panferov, V.G.; Safenkova, I.V.; Byzova, N.A.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Silver-enhanced lateral flow immunoassay for highly-sensitive detection of potato leafroll virus. Food Agric. Immunol. 2018, 29, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, V.K.; East, A.K.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Adaptation of Rhizobium leguminosarum to pea, alfalfa and sugar beet rhizospheres investigated by comparative transcriptomics. Genome Biol. 2011, 12, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotova, V.Y.; Manukhov, I.V.; Zavilgelskii, G.B. Lux-biosensors for detection of SOS-response, heat shock, and oxidative stress. Appl. Biochem. Microbiol. 2010, 46, 781–788. [Google Scholar] [CrossRef]
- Zavil’gel’skii, G.B.; Kotova, V.Y.; Mironov, A.S. Lux Biosensors for Antibiotic Detection: The Contribution from Reactive Oxygen Species to the Bactericidal Activity of Antibiotics. Russ. J. Phys. Chem. B 2015, 9, 7. [Google Scholar] [CrossRef]
- Pini, F.; East, A.K.; Appia-Ayme, C.; Tomek, J.; Karunakaran, R.; Mendoza-Suárez, M.; Edwards, A.; Terpolilli, J.J.; Roworth, J.; Downie, J.A.; et al. Bacterial Biosensors for in Vivo Spatiotemporal Mapping of Root Secretion. Plant Physiol. 2017, 174, 1289–1306. [Google Scholar] [CrossRef] [Green Version]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Darwent, M.J.; Paterson, E.; McDonald, A.J.S.; Tomos, A.D. Biosensor reporting of root exudation from Hordeum vulgare in relation to shoot nitrate concentration. J. Exp. Bot. 2003, 54, 325–334. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Lee, B.-R.; Jin, Y.L.; Avice, J.-C.; Cliquet, J.-B.; Ourry, A.; Kim, T.-H. Increased proline loading to phloem and its effects on nitrogen uptake and assimilation in water-stressed white clover (Trifolium repens). New Phytol. 2009, 182, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Hildreth, S.; Helm, R.F.; Scharf, B.E. Sinorhizobium meliloti chemoreceptor McpU mediates chemotaxis toward host plant exudates through direct proline sensing. Appl. Environ. Microbiol. 2014, 80, 3404–3415. [Google Scholar] [CrossRef] [Green Version]
- Rubia, M.I.; Ramachandran, V.K.; Arrese-Igor, C.; Larrainzar, E.; Poole, P.S. A novel biosensor to monitor proline in pea root exudates and nodules under osmotic stress and recovery. Plant Soil 2020, 452, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Sekar, R.B.; Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaper, T.; Lager, I.; Looger, L.L.; Chermak, D.; Frommer, W.B. Fluorescence resonance energy transfer sensors for quantitative monitoring of pentose and disaccharide accumulation in bacteria. Biotechnol. Biofuels 2008, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Bourdès, A.; Rudder, S.; East, A.K.; Poole, P.S. Mining the Sinorhizobium meliloti transportome to develop FRET biosensors for sugars, dicarboxylates and cyclic polyols. PLoS ONE 2012, 7, e43578. [Google Scholar] [CrossRef] [Green Version]
- Sadanandom, A.; Napier, R.M. Biosensors in plants. Curr. Opin. Plant Biol. 2010, 13, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Waadt, R.; Hitomi, K.; Nishimura, N.; Hitomi, C.; Adams, S.R.; Getzoff, E.D.; Schroeder, J.I. FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 2014, 3, e01739. [Google Scholar] [CrossRef]
- Jones, A.M.; Danielson, J.A.; Manojkumar, S.N.; Lanquar, V.; Grossmann, G.; Frommer, W.B. Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. eLife 2014, 3, e01741. [Google Scholar] [CrossRef]
- Herud-Sikimić, O.; Stiel, A.C.; Kolb, M.; Shanmugaratnam, S.; Berendzen, K.W.; Feldhaus, C.; Höcker, B.; Jürgens, G. A biosensor for the direct visualization of auxin. Nature 2021, 592, 768–772. [Google Scholar] [CrossRef]
- Voothuluru, P.; Braun, D.M.; Boyer, J.S. An in Vivo Imaging Assay Detects Spatial Variability in Glucose Release from Plant Roots. Plant Physiol. 2018, 178, 1002–1010. [Google Scholar] [CrossRef]
- Bringhurst, R.M.; Cardon, Z.G.; Gage, D.J. Galactosides in the rhizosphere: Utilization by Sinorhizobium meliloti and development of a biosensor. Proc. Natl. Acad. Sci. USA 2001, 98, 4540–4545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, T.T.S.; Koman, V.B.; Silmore, K.S.; Seo, J.S.; Gordiichuk, P.; Kwak, S.-Y.; Park, M.; Ang, M.C.-Y.; Khong, D.T.; Lee, M.A.; et al. Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat. Plants 2020, 6, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xu, X.; Cheng, Y.; Tan, Y.; Xiao, L.; Tang, D.; Jiang, H.; Qin, X.; Wang, H. Chemical pre-reduction and electro-reduction guided preparation of a porous graphene bionanocomposite for indole-3-acetic acid detection. Nanoscale 2019, 11, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Teig-Sussholz, O.; Schuster, S.; Avni, A.; Shacham-Diamand, Y. Integrated electrochemical Chip-on-Plant functional sensor for monitoring gene expression under stress. Biosens. Bioelectron. 2018, 117, 493–500. [Google Scholar] [CrossRef]
- Sun, L.-J.; Xie, Y.; Yan, Y.-F.; Yang, H.; Gu, H.-Y.; Bao, N. Paper-based analytical devices for direct electrochemical detection of free IAA and SA in plant samples with the weight of several milligrams. Sens. Actuators B Chem. 2017, 247, 336–342. [Google Scholar] [CrossRef]
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical Biosensors for Pathogen Detection: An Updated Review. Biosensors 2022, 12, 927. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wu, H.; Wee, E.J.H.; Trau, M.; Wang, Y.; Botella, J.R. Specific and Sensitive Isothermal Electrochemical Biosensor for Plant Pathogen DNA Detection with Colloidal Gold Nanoparticles as Probes. Sci. Rep. 2017, 7, 38896. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Gong, B.; Jiang, N.; Li, Y.; Wu, Y. Electrochemical biosensor for cytokinins based on the CHASE domain of Arabidopsis histidine kinases 4. Bioelectrochemistry 2021, 141, 107872. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.I.; Tabassum, S. Leaf-mounted microneedle-based multisensory platform for multiplexed monitoring of phytohormones in live plants. In Proceedings of the Hilton Head Workshop on Solid-State Sensors, Actuators & Microsystems, Hilton Head Island, SC, USA, 5–7 June 2022; p. 48. [Google Scholar]
- Kumar, R.; Kumar, P. Future Microbial Applications for Bioenergy Production: A Perspective. Front. Microbiol. 2017, 8, 450. [Google Scholar] [CrossRef]



| Microbial Strains | Phytohormone/Root Exudate | Host Plant/Source | Reference |
|---|---|---|---|
| Pseudomonas fluorescens CHA0, WCS374, WCS417, Pf4–92, P. aeruginosa 7NSK2, Serratia marcescens, P. fluorescens Pf4–92 | SA | Tobacco, potato, wheat, cucumber, barley, and chickpeas | [73] |
| Ralstonia solanacearum | Ethylene | Banana | [77] |
| P. fluorescens SPB2145, PCL1751, Stenotrophomonas rhizophila e-p10 | IAA | Cucumber plant | [90] |
| Bradyrhizobium sp. Azospirillum sp. Bacillus pumilus and Bacillus licheniformis | Gibberellin | Phaseolus lunatus, Alnus glutinosa, and L. Pinus pinea plants | [91] |
| Azospirillum lipoferum, Arthrobacter koreensis, Achromobacter xylosoxidans, Bacillus licheniformis, Bacillus pumilus, and Brevibacterium halotolerans | ABA | helianthus annuus and rhizobacteria (PGPR) | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neelam, A.; Tabassum, S. Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes. Micromachines 2023, 14, 195. https://doi.org/10.3390/mi14010195
Neelam A, Tabassum S. Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes. Micromachines. 2023; 14(1):195. https://doi.org/10.3390/mi14010195
Chicago/Turabian StyleNeelam, Asia, and Shawana Tabassum. 2023. "Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes" Micromachines 14, no. 1: 195. https://doi.org/10.3390/mi14010195
APA StyleNeelam, A., & Tabassum, S. (2023). Optical Sensing Technologies to Elucidate the Interplay between Plant and Microbes. Micromachines, 14(1), 195. https://doi.org/10.3390/mi14010195





