High Oxygen Sensitivity of TiO2 Thin Films Deposited by ALD
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
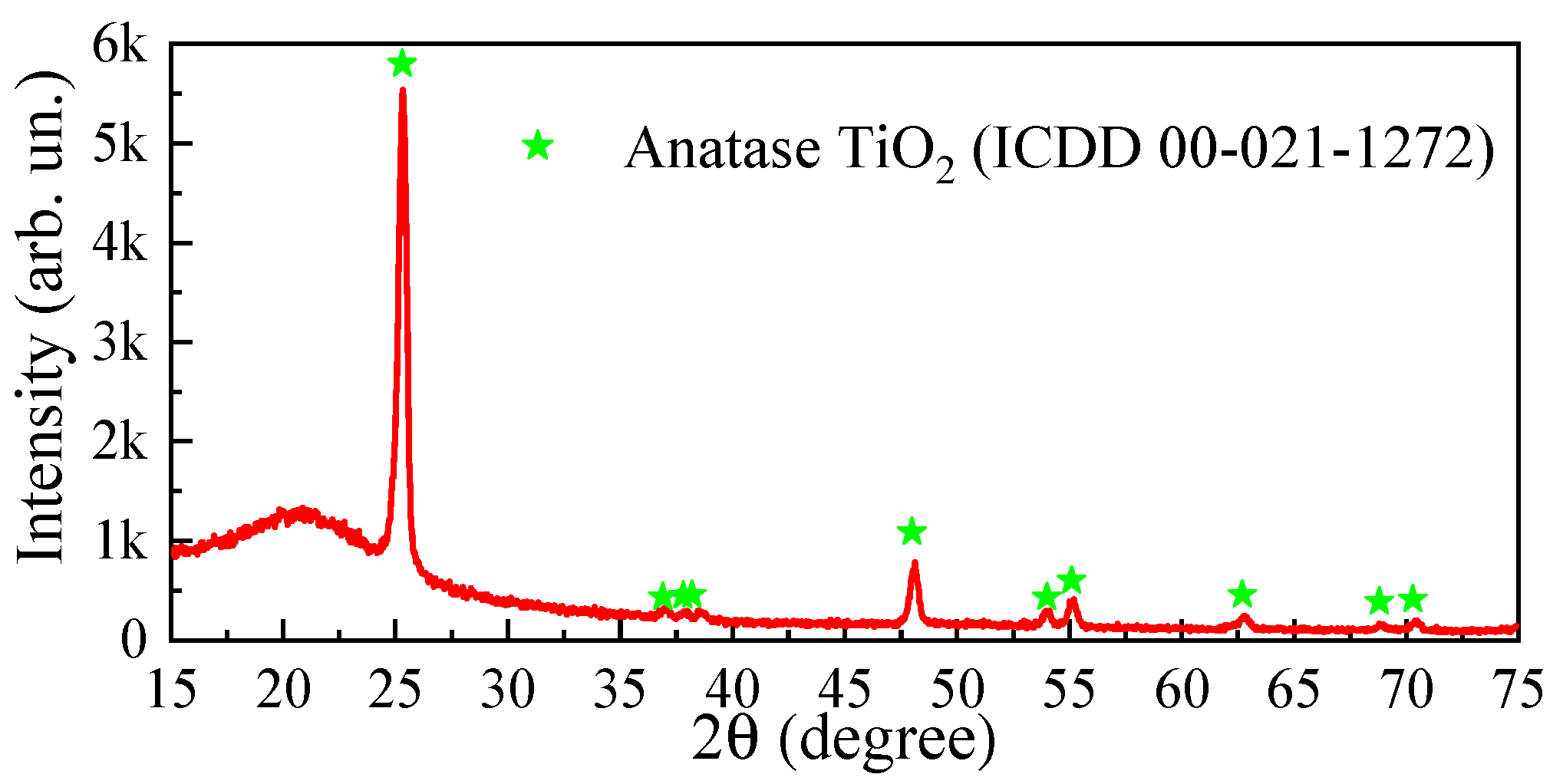
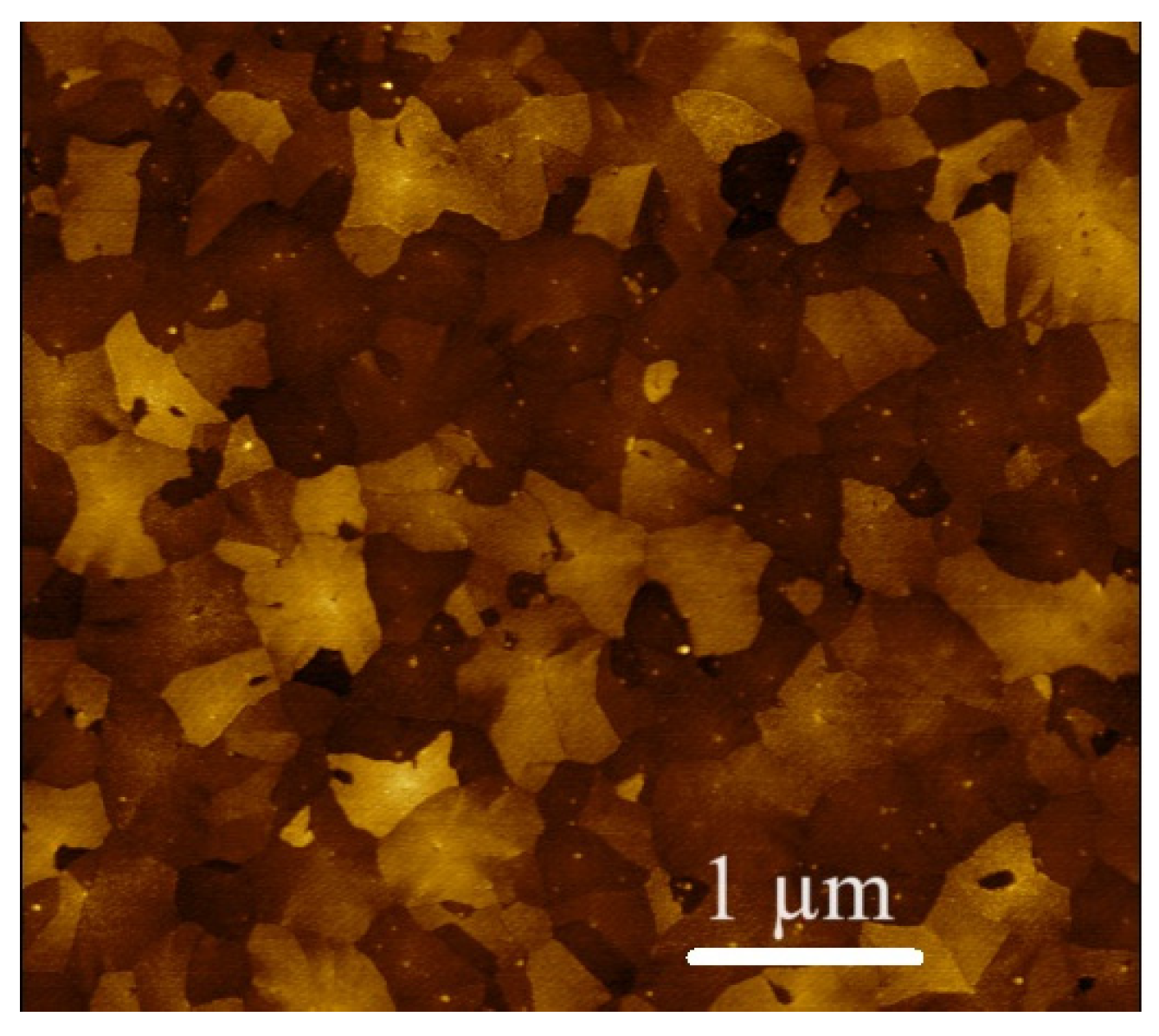
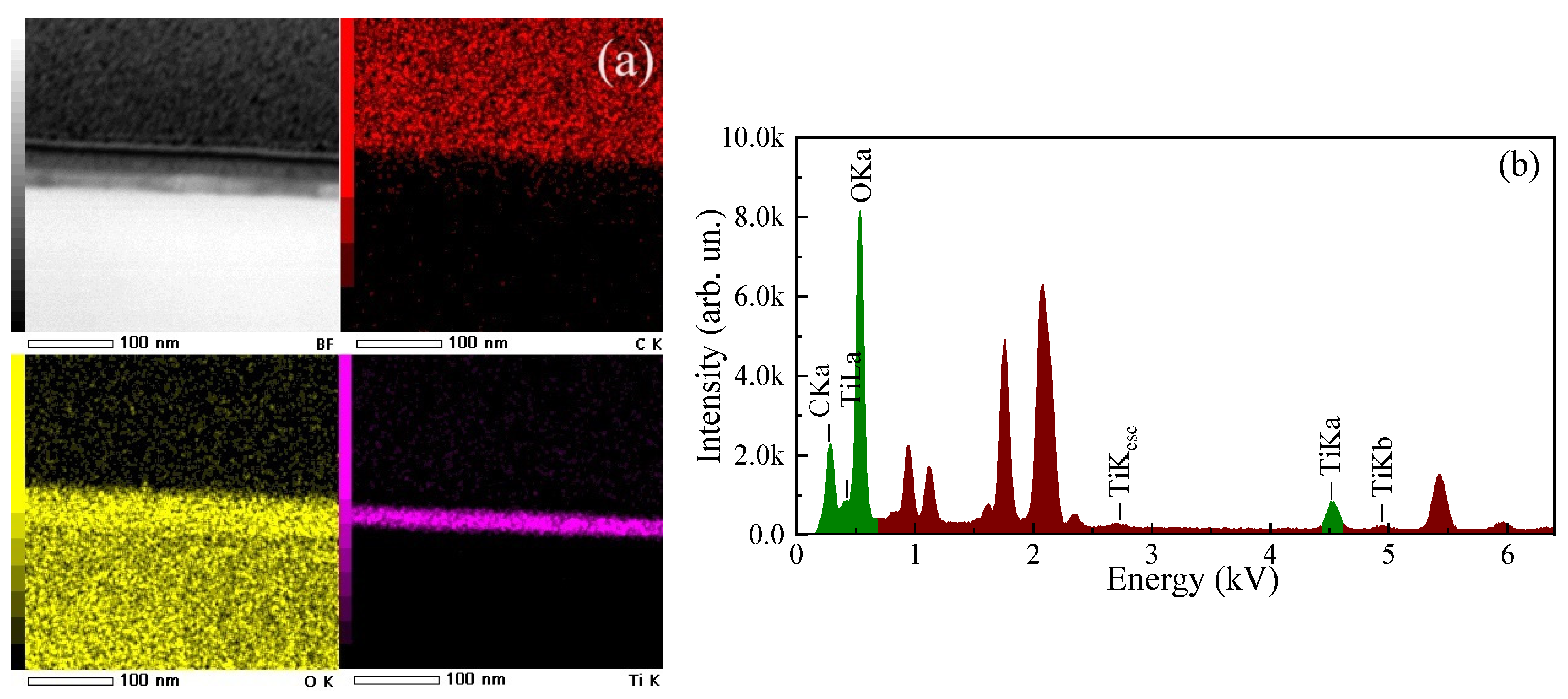
3.1. Structural Properties of the ALD-Deposited TiO2 Thin Films
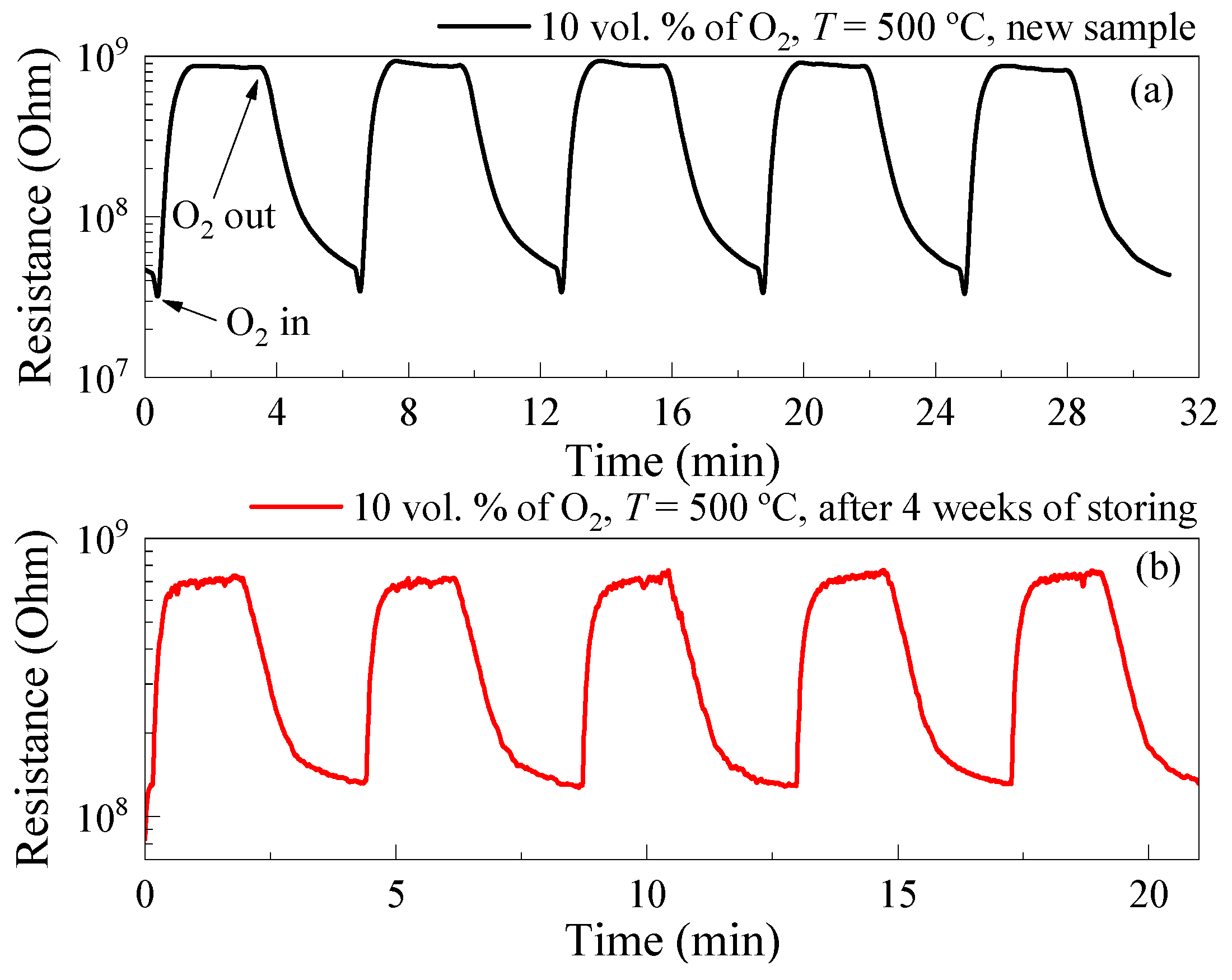
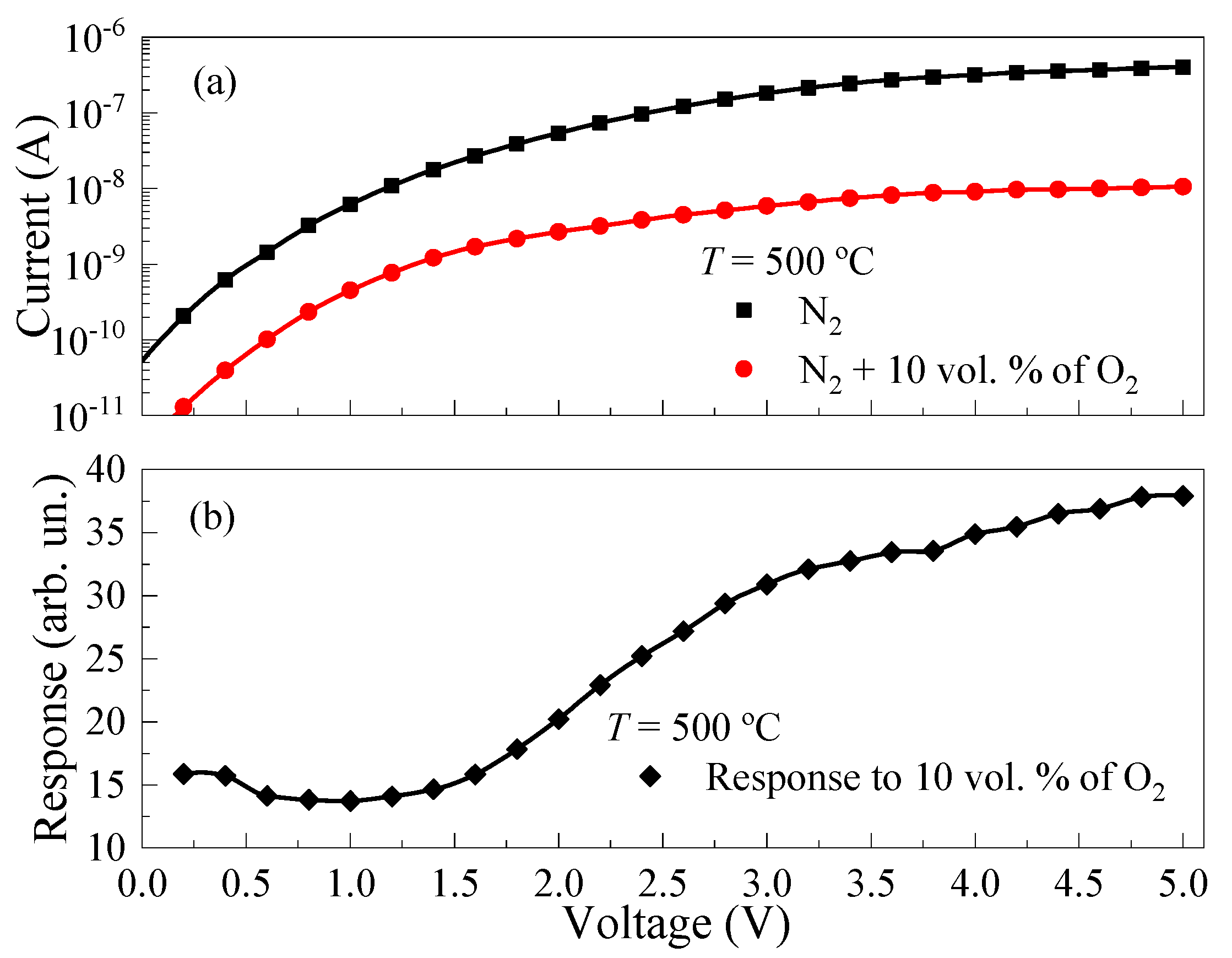
3.2. Gas-Sensetive Properties of the ALD-Deposited TiO2 Thin Films
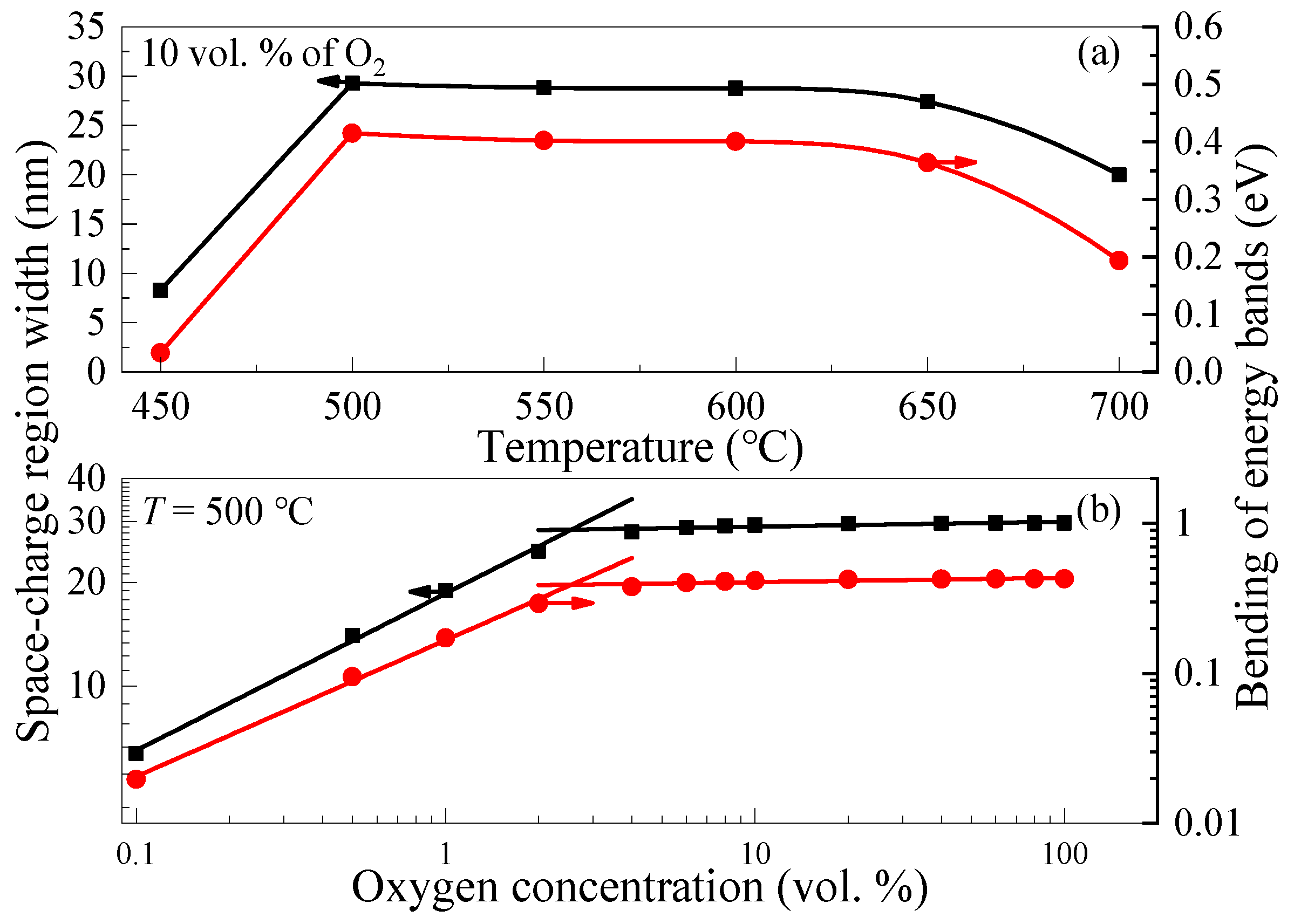
3.3. The Mechanism of the Sensory Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, X.-H.; Wang, X.-F.; Liu, Q.-G. Research on oxygen sensor for metallurgical process. J. Iron Steel Res. Int. 2001, 8, 60–62. [Google Scholar]
- Lampe, U.; Fleischer, M.; Meixner, H. Lambda measurement with Ga2O3. Sens. Actuators B Chem. 1994, 17, 187–196. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Jyothilal, H.; Shukla, G.; Walia, S.; Bharath, S.P.; Angappane, S. UV assisted room temperature oxygen sensors using titanium dioxide nanostructures. Mater. Res. Bull. 2021, 140, 111324. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Liu, B.; Chen, Y.; Lu, Y.; Wang, F.; Zhang, L. UV-induced desorption of oxygen at the TiO2 surface for highly sensitive room temperature O2 sensing. J. Alloys Compd. 2019, 793, 583–589. [Google Scholar] [CrossRef]
- Gan, L.; Wu, C.; Tan, Y.; Chi, B.; Pu, J.; Jian, L. Oxygen sensing performance of Nb-doped TiO2 thin film with porous structure. J. Alloys Compd. 2014, 585, 729–733. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Smirnov, N.B.; Shchemerov, I.V.; Yakimov, E.B.; Pearton, S.J.; Ren, F.; Chernykh, A.V.; Gogova, D.; Kochkova, A.I. Electrical properties, deep trap and luminescence spectra in semi-insulating, Czochralski β-Ga2O3 (Mg). ECS J. Solid State Sci. Technol. 2019, 8, Q3019. [Google Scholar] [CrossRef]
- Dimitrova, Z.; Gogova, D. On the structure, stress and optical properties of CVD tungsten oxide films. Mater. Res. Bull. 2005, 40, 333–340. [Google Scholar] [CrossRef]
- Gogova, D.; Gesheva, K.; Kakanakova-Georgieva, A.; Surtchev, M. Investigation of the structure of tungsten oxide films obtained by chemical vapor deposition. Eur. Phys. J. Appl. Phys. 2000, 11, 167–174. [Google Scholar] [CrossRef]
- Gogova, D.; Iossifova, A.; Ivanova, T.; Dimitrova, Z.; Gesheva, K. Electrochromic behavior in CVD grown tungsten oxide films. J. Cryst. Growth 1999, 198–199, 1230–1234. [Google Scholar] [CrossRef]
- Kachel, K.; Korytov, M.; Gogova, D.; Galazka, Z.; Albrecht, M.; Zwierz, R.; Siche, D.; Golka, S.; Kwasniewski, A.; Schmidbauer, M.; et al. A new approach to free-standing GaN using β-Ga2O3 as a substrate. CrystEngComm 2012, 14, 8536–8540. [Google Scholar] [CrossRef]
- Stolze, M.; Gogova, D.; Thomas, L.-K. Analogy for the maximum obtainable colouration between electrochromic, gaschromic, and electrocolouration in DC-sputtered thin WO3−y films. Thin Solid Films 2005, 476, 185–189. [Google Scholar] [CrossRef]
- Nikolskaya, A.; Okulich, E.; Korolev, D.; Stepanov, A.; Nikolichev, D.; Mikhaylov, A.; Tetelbaum, D.; Almaev, A.; Bolzan, C.A.; Buaczik, A., Jr.; et al. Ion implantation in β-Ga2O3: Physics and technology. J. Vac. Sci. Technol. A 2021, 39, 030802. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Hashitha, M.M.; Sisman, O.; Comini, E. Metal oxide -based heterostructures for gas sensors. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Yakovlev, N.N.; Almaev, A.V.; Nikolaev, V.I.; Kushnarev, B.O.; Pechnikov, A.I.; Stepanov, S.I.; Chikiryaka, A.V.; Timashov, R.B.; Scheglov, M.P.; Butenko, P.N.; et al. Low-resistivity gas sensors based on the In2O3-Ga2O3 mixed compounds films. Mater. Today Commun. 2023, 34, 105241. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Ha, S.; Li, D.; Zhang, K.; Zhang, H.; Feng, J. Gallium oxide for gas sensor applications: A comprehensive review. Materials 2022, 15, 7339. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Gorobtsov, P.Y.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensing properties of nanostructured TiO2–xZrO2 thin films obtained by the sol–gel method. J. Sol.-Gel. Sci. Technol. 2019, 92, 415–426. [Google Scholar] [CrossRef]
- Lu, C.; Huang, Y.; Huang, J.; Chang, C.; Wu, S. A Macroporous TiO2 oxygen sensor fabricated using anodic aluminium oxide as an etching mask. Sensors 2010, 10, 670–683. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Wang, J.; Sun, Q.; Zhao, Y. A micro oxygen sensor based on a nano sol-gel TiO2 thin film. Sensors 2014, 14, 16423–16433. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Q.; Yao, Y.; Li, Y.; Wang, J.; Chen, L. A micro sensor based on TiO2 nanorod arrays for the detection of oxygen at room temperature. Ceram. Int. 2016, 42, 8565–8571. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Blackman, C.S.; Carmalt, C.J.; Stanoiu, A.; Di Maggio, F.; Covington, J.A. The effect of film thickness on the gas sensing properties of ultra-thin TiO2 films deposited by atomic layer deposition. Sensors 2018, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; George, S.M. Thickness dependence of sensor response for CO gas sensing by tin oxide films grown using atomic layer deposition. Sens. Actuators B Chem. 2008, 135, 152–160. [Google Scholar] [CrossRef]
- Marichy, C.; Donato, N.; Latino, M.; Willinger, M.G.; Tessonnier, J.-P.; Neri, G.; Pinna, N. Gas sensing properties and p-type response of ALD TiO2 coated carbon nanotubes. Nanotechnology 2015, 26, 024004. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, A.J.; Varpula, A.; Utriainen, M.; Natarajan, G.; Cameron, D.C.; Novikov, S.; Airaksinen, V.-M.; Sinkkonen, J.; Franssila, S. Atomic layer deposition of tin dioxide sensing film in microhotplate gas sensors. Sens. Actuators B Chem. 2010, 148, 227–232. [Google Scholar] [CrossRef]
- Rosental, A.; Tarre, A.; Gerst, A.; Sundqvist, J.; Hårsta, A.; Aidla, A.; Aarik, J.; Sammelselg, V.; Uustare, T. Gas sensing properties of epitaxial SnO2 thin films prepared by atomic layer deposition. Sens. Actuators B Chem. 2003, 93, 552–555. [Google Scholar] [CrossRef]
- Wei, Z.; Akbari, M.K.; Hai, Z.; Ramachandran, R.K.; Detavernier, C.; Verpoort, F.; Kats, E.; Xu, H.; Hu, J.; Zhuiykov, S. Ultra-thin sub-10 nm Ga2O3-WO3 heterostructures developed by atomic layer deposition for sensitive and selective C2H5OH detection on ppm level. Sens. Actuators B Chem. 2019, 287, 147–156. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, W.-S.; Lee, S.B.; Hong, S.-H. Gas sensing properties in epitaxial SnO2 films grown on TiO2 single crystals with various orientations. Sens. Actuators B Chem. 2010, 147, 653–659. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mirzaei, A.; Kim, J.-H.; Kim, J.-Y.; Nasriddinov, A.F.; Rumyantseva, M.N.; Kim, H.W.; Kim, S.S. Gas-sensing behaviors of TiO2-layer-modified SnO2 quantum dots in self-heating mode and effects of the TiO2 layer. Sens. Actuators B Chem. 2020, 310, 127870. [Google Scholar] [CrossRef]
- Xu, H.; Akbari, M.K.; Wei, Z.; Hu, J.; Verpoort, F.; Zhuiykov, S. Plasma-induced sub-10 nm Au-SnO2-In2O3 heterostructures fabricated by atomic layer deposition for highly sensitive ethanol detection on ppm level. Appl. Surf. Sci. 2021, 563, 150400. [Google Scholar] [CrossRef]
- Eadi, S.B.; Shin, H.J.; Song, K.W.; Choi, H.W.; Kim, S.H.; Lee, H.D. Development of ultrasensitive indium oxide layer with high response to NO2 gas in indium gallium zinc oxide stack structure using atomic layer deposition. Mater. Lett. 2021, 297, 129943. [Google Scholar] [CrossRef]
- Lou, C.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Atomic layer deposition of ZnO on SnO2 nanospheres for enhanced formaldehyde detection. Sens. Actuators B Chem. 2021, 329, 129218. [Google Scholar] [CrossRef]
- Lou, C.; Huang, Q.; Li, Z.; Lei, G.; Liu, X.; Zhang, J. Fe2O3-sensitized SnO2 nanosheets via atomic layer deposition for sensitive formaldehyde detection. Sens. Actuators B Chem. 2021, 345, 130429. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Chang, S.-J.; Wang, P.-H.; Hsueh, T.-J. A TSV-structured room temperature p-Type TiO2 nitric oxide gas sensor. Appl. Sci. 2022, 12, 9946. [Google Scholar] [CrossRef]
- Kuo, C.-G.; Chen, J.-H.; Chao, Y.-C.; Chen, P.-L. Fabrication of a P3HT-ZnO nanowires gas sensor detecting ammonia gas. Sensors 2018, 18, 37. [Google Scholar] [CrossRef]
- Bukauskas, V.; Olekas, A.; Senulien, D.; Strazdien, V.; Šetkus, A.; Kaciulis, S.; Pandolfi, L. Effect of thickness of ultra-thin tin oxide film based gas sensors. Lith. J. Phys. 2007, 47, 475–483. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Bodneva, V.L.; Trakhtenberg, L.I. Sensor effect theory for the detection of reducing gases. Rus. J. Phys. Chem. A 2012, 86, 1281–1287. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gromov, V.F.; Gerasimov, G.N.; Spiridonova, E.Y.; Erofeeva, A.R.; Kurmangaleev, K.S.; Polunin, K.S.; Ilegbusi, O.J.; Trakhtenberg, L.I. Structure, conductivity, and sensor properties of nanosized ZnO-In2O3 composites: Influence of synthesis method. Micromachines 2023, 14, 1685. [Google Scholar] [CrossRef]
- Almaev, A.V.; Nikolaev, V.I.; Yakovlev, N.N.; Butenko, P.N.; Stepanov, S.I.; Pechnikov, A.I.; Scheglov, M.P.; Chernikov, E.V. Hydrogen sensors based on Pt/α-Ga2O3:Sn/Pt structures. Sens. Actuators B Chem. 2022, 364, 131904. [Google Scholar] [CrossRef]
- Bulyarskiy, S.; Koiva, D.; Gusarov, S.; Latipov, E.; Rudakov, G.; Svetukhin, V. Crystallization of amorphous titanium oxide films upon annealing in an oxygen atmosphere. Mater. Sci. Eng. B 2022, 283, 115802. [Google Scholar] [CrossRef]
- Maksimova, N.K.; Almaev, A.V.; Sevastyanov, E.Y.; Potekaev, A.I.; Chernikov, E.V.; Sergeychenko, N.V.; Korusenko, P.M.; Nesov, S.N. Effect of additives Ag and rare-earth elements Y and Sc on the properties of hydrogen sensors based on thin SnO2 films during long-term testing. Coatings 2019, 9, 423. [Google Scholar] [CrossRef]
- Meixner, H.; Lampe, U.; Gerblinger, J.; Fleischer, M. Chemosensors for motor management systems of the future. Fresenius’ J. Anal. Chem. 1994, 348, 536–541. [Google Scholar] [CrossRef]
- Baranzahi, A.; Spetz, A.L.; Glavmo, M.; Carlsson, C.; Nytomt, J.; Salomonsson, P.; Jobson, E.; Häggendal, B.; Mårtensson, P.; Lundström, I. Response of metal-oxide-silicon carbide sensors to simulated and real exhaust gases. Sens. Actuators B Chem. 1997, 43, 52–59. [Google Scholar] [CrossRef]
- Gaman, V.I. Physical principles of operation of oxidizing gas sensors based on metal oxide semiconductors. Rus. Phys. J. 2012, 54, 1364–1371. [Google Scholar] [CrossRef]
- Simion, C.E.; Schipani, F.; Papadogianni, A.; Stanoiu, A.; Budde, M.; Oprea, A.; Weimar, U.; Bierwagen, O.; Barsan, N. Conductance model for single-crystalline/compact metal oxide gas-sensing layers in the nondegenerate limit: Example of epitaxial SnO2(101). ACS Sens. 2019, 4, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Gaman, V.I.; Almaev, A.V.; Sevast’yanov, E.Y.; Maksimova, N.K. Influence of water vapors and hydrogen on the energy band bending in the SnO2 microcrystals of polycrystalline tin dioxide films. Rus. Phys. J. 2015, 58, 179–187. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Novikov, V.V.; Kushnarev, B.O.; Yakovlev, N.N.; Chuprakova, E.V.; Oleinik, V.L.; Lozinskaya, A.D.; Gogova, D.S. Impact of Cr2O3 additives on the gas-sensitive properties of β-Ga2O3 thin films to oxygen, hydrogen, carbon monoxide, and toluene vapors. J. Vac. Sci. Technol. A 2021, 39, 023405. [Google Scholar] [CrossRef]
- Simakov, V.V.; Yakusheva, O.V.; Grebennikov, A.I.; Kisin, V.V. Current-voltage characteristics of thin-film gas sensor structures based on tin dioxide. Tech. Phys. Lett. 2005, 31, 339. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Kushnarev, B.O.; Kopyev, V.V.; Novikov, V.A.; Zinoviev, M.M.; Yudin, N.N.; Podzivalov, S.N.; Erzakova, N.N.; Chikiryaka, A.V.; et al. Gas sensitivity of IBSD deposited TiO2 Thin Films. Coatings 2022, 12, 1565. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, Y.; Hwang, S.; Kim, J.K. Highly-sensitive H2 sensor operating at room temperature using Pt/TiO2 nanoscale Schottky contacts. Sens. Actuators B Chem. 2017, 241, 985–992. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Kirner, U.K.; Geiger, J.F.; Göpel, W. Schottky-barrier and conductivity gas sensors based upon Pd/SnO2 and Pt/TiO2. Sens. Actuators B Chem. 1991, 4, 87–94. [Google Scholar] [CrossRef]
- Ebisuzaki, Y.; Kass, W.J.; O’Keeffe, M. Solubility and diffusion of hydrogen and deuterium in platinum. J. Chem. Phys. 1968, 49, 3329–3332. [Google Scholar] [CrossRef]
- Ishikawa, T.; McLellan, R.B. The diffusivity of hydrogen in the noble metals at low temperature. Acta Metall. 1985, 33, 1979–1985. [Google Scholar] [CrossRef]
- Ferrin, P.; Kandoi, S.; Nilekar, A.; Mavrikakis, M. Hydrogen adsorption, absorption and diffusion on and in transition metal surfaces: A DFT study. Surf. Sci. 2012, 606, 679–689. [Google Scholar] [CrossRef]
- Barth, J. Transport of adsorbates at metal surfaces: From thermal migration to hot precursors. Surf. Sci. Rep. 2000, 40, 75–149. [Google Scholar] [CrossRef]
- Schmiedl, R.; Demuth, V.; Lahnor, P.; Godehardt, H.; Bodschwinna, Y.; Harder, C.; Hammer, L.; Strunk, H.; Schulz, M.; Heinz, K. Oxygen diffusion through thin Pt films on Si(100). Appl. Phys. A 1996, 62, 223–230. [Google Scholar] [CrossRef]
- Velho, L.; Bartlett, R. Diffusivity and solubility of oxygen in platinum and Pt-Ni alloys. Metall. Trans. 1972, 3, 65–72. [Google Scholar] [CrossRef]
- Castañeda, L.; López-Suárez, A.; Tiburcio-Silver, A. Influence of colloidal silver nanoparticles on the novel flower-like titanium dioxide oxygen sensor performances. J. Nanosci. Nanotechnol. 2010, 10, 1343–1348. [Google Scholar] [CrossRef]
- Galatsis, K.; Li, Y.X.; Wlodarski, W.; Comini, E.; Faglia, G.; Sberveglieri, G. Semiconductor MoO3–TiO2 thin film gas sensors. Sens. Actuators B Chem. 2001, 77, 472–477. [Google Scholar] [CrossRef]
- Jiao, M.; Zhao, X.; He, X.; Wang, G.; Zhang, W.; Rong, Q.; Nguyen, D. High-performance MEMS oxygen sensors based on Au/TiO2 films. Chemosensors 2023, 11, 476. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Pullithadathil, B. Highly Sensitive, Temperature-independent oxygen gas sensor based on anatase TiO2 nanoparticle grafted, 2D mixed valent VOx nanoflakelets. ACS Sens. 2018, 3, 1811–1821. [Google Scholar] [CrossRef]
- Engelkamp, B.; Schierbaum, K. Oxygen sensing of Pt/PEO-TiO2 in humid atmospheres at moderate temperatures. Sensors 2021, 21, 2558. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Li, F.; Liu, G.; Chen, Z.G.; Wang, D.W.; Fang, H.T.; Lu, G.Q.; Jiang, Z.H.; Cheng, H.M. Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 2008, 19, 405504. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Chen, L.; Yao, Y.; Sun, Q.; Qunming, Z. Integrated microoxygen sensor based on nanostructured TiO2 thin films. Micro Nano Lett. 2015, 10, 597–602. [Google Scholar] [CrossRef]





| Material | d (nm) | Gas | cg (ppm) | T (°C) | S (arb. un.) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 | 50 | NH3 | 100 | 350 | 4.24 | [21] |
| CNT/TiO2 | 10 (TiO2) | NO2 | 8 | 150 | ~10 | [23] |
| SnO2 | 17.5 | C2H5OH | 500 | 300 | 1.64 | [24] |
| SnO2 | 10 | CO | 104 | 450 | ~21 | [25] |
| WO3 | 6.5 | C2H5OH | 100 | 275 | ~14 | [26] |
| Ga2O3 | 1.5 | ~1.4 | ||||
| Ga2O3/WO3 | 1.5/6.5 | ~3.5 | ||||
| SnO2 | 90 | H2 | 1000 | 400 | ~380 | [27] |
| TiO2/SnO2 QDs | 30 (TiO2) | CO | 1 | 300 | 1.8 | [28] |
| SnO2 | 4.4 | C2H5OH | 200 | 400 | 20 | [29] |
| In2O3/SnO2 | 4/4.4 | 350 | 37 | |||
| IGZO | 150 | NO2 | 100 | 200 | 5154 | [30] |
| ZnO/SnO2 NSs | 24 (ZnO) | HCHO | 20 | 200 | 38.2 | [31] |
| Fe2O3/SnO2 NShs | 20 cycles | HCHO | 20 | 220 | 4.57 | [32] |
| p-TiO2 | 70 | NO | 10 | RT | 1.244 | [33] |
| P3HT/ZnO NWs | - | NH3 | 5 | RT | 1.35 | [34] |
| Material | Methods | d (nm) | cox (vol. %) | T (°C) | Sox (arb. un.) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 | RFMS | 50 | 0.6 | 500 | 1.14 | [18] |
| TiO2 | DCMS | 60 | 10 | RT | 76 | [5] |
| TiO2 | IBSD | 130 | 40 | 750 | 7.64 | [48] |
| TiO2 | sol-gel | - | 2 | 700 | 6.5 | [6] |
| Nb (6%):TiO2 | - | 73.2 | ||||
| TiO2 | sol-gel | - | 1 | 400 | 4.4 | [17] |
| TiO2 + ZrO2 (10 mol. %) | - | 5 | ||||
| TiO2 | RFMS | 30 | 10 | RT + UV | ~5.5 | [4] |
| Cr-TiO2/TiO2 | GLAD + EBE | - | ~9 | |||
| TiO2 NRA | AVO | - | 8 | RT | ~1.9 | [20] |
| TiO2 | USP | - | 0.1 | 300 | 5 | [57] |
| TiO2-Ag NPs | - | 9 | ||||
| Pd:TiO2 | sol-gel | ~110 | 1→20 | 240 | 1.27 | [19] |
| TiO2 | sol-gel | - | 0.1 | 420 | 28 | [58] |
| TiO2 + MoO3 (25 at. %) | 370 | 30 | ||||
| Au (6 nm)/TiO2 | RFMS | 300 | 5 | 400 | 61.3 | [59] |
| VOx/TiO2 NFs | TD + HM | - | 0.01 | 500 | 1.32 | [60] |
| Pt/TiO2 | PEO | - | 10 | RT | 2 | [61] |
| TiO2 NTs | AO | - | 4 | 100 | 160 | [62] |
| TiO2 | sol-gel | - | 4 | 252 | 1.16 | [63] |
| TiO2 | ALD | 23 | 0.1 | 500 | 1.27 | This work |
| 1 | 2.70 | |||||
| 2 | 5.55 | |||||
| 10 | 41.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaev, A.V.; Yakovlev, N.N.; Almaev, D.A.; Verkholetov, M.G.; Rudakov, G.A.; Litvinova, K.I. High Oxygen Sensitivity of TiO2 Thin Films Deposited by ALD. Micromachines 2023, 14, 1875. https://doi.org/10.3390/mi14101875
Almaev AV, Yakovlev NN, Almaev DA, Verkholetov MG, Rudakov GA, Litvinova KI. High Oxygen Sensitivity of TiO2 Thin Films Deposited by ALD. Micromachines. 2023; 14(10):1875. https://doi.org/10.3390/mi14101875
Chicago/Turabian StyleAlmaev, Aleksei V., Nikita N. Yakovlev, Dmitry A. Almaev, Maksim G. Verkholetov, Grigory A. Rudakov, and Kristina I. Litvinova. 2023. "High Oxygen Sensitivity of TiO2 Thin Films Deposited by ALD" Micromachines 14, no. 10: 1875. https://doi.org/10.3390/mi14101875






