Pneumatic Microballoons for Active Control of the Vibration-Induced Flow
Abstract
:1. Introduction
2. Materials and Methods
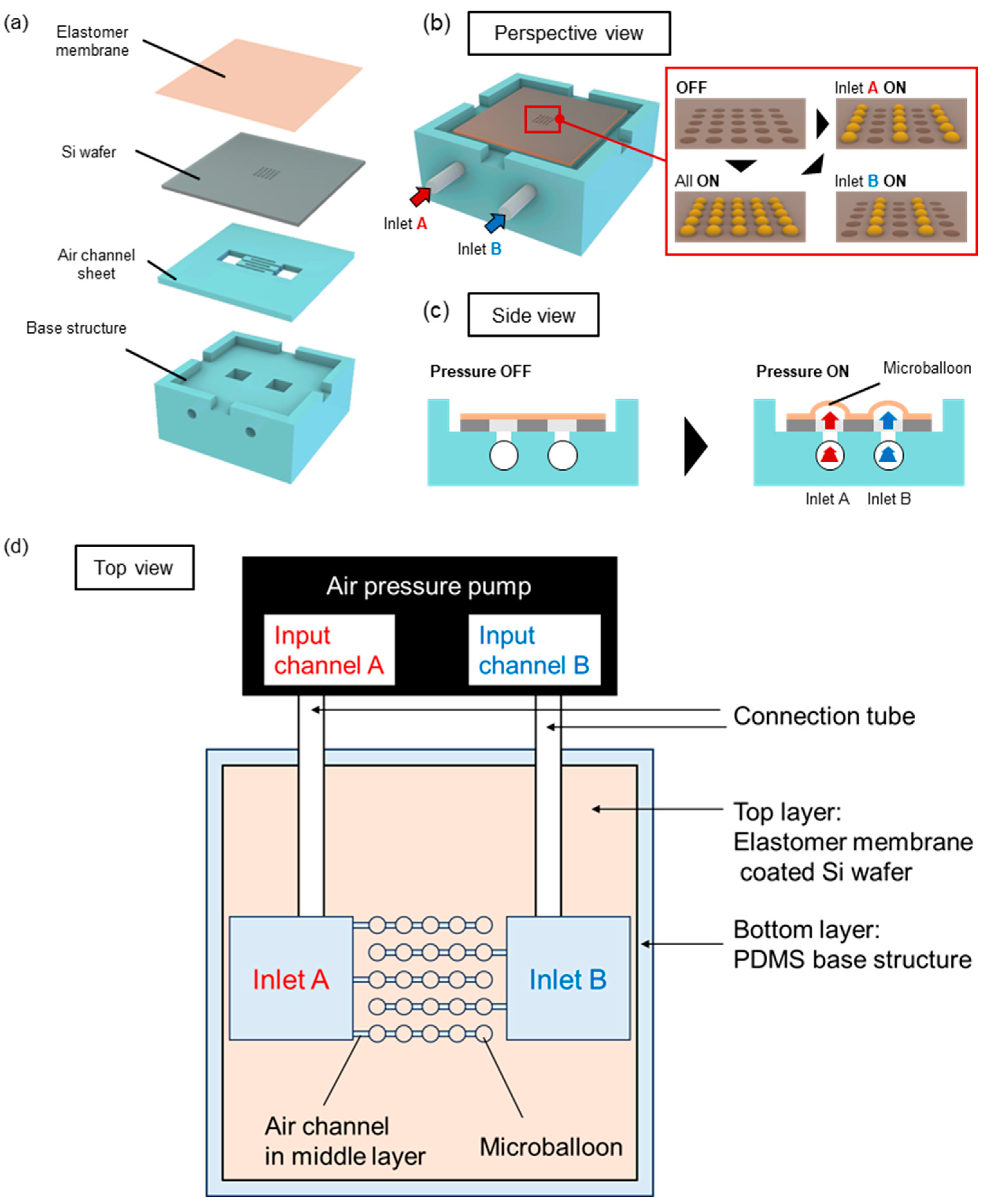
2.1. Structure and Driving Principles of the Microballoon Device
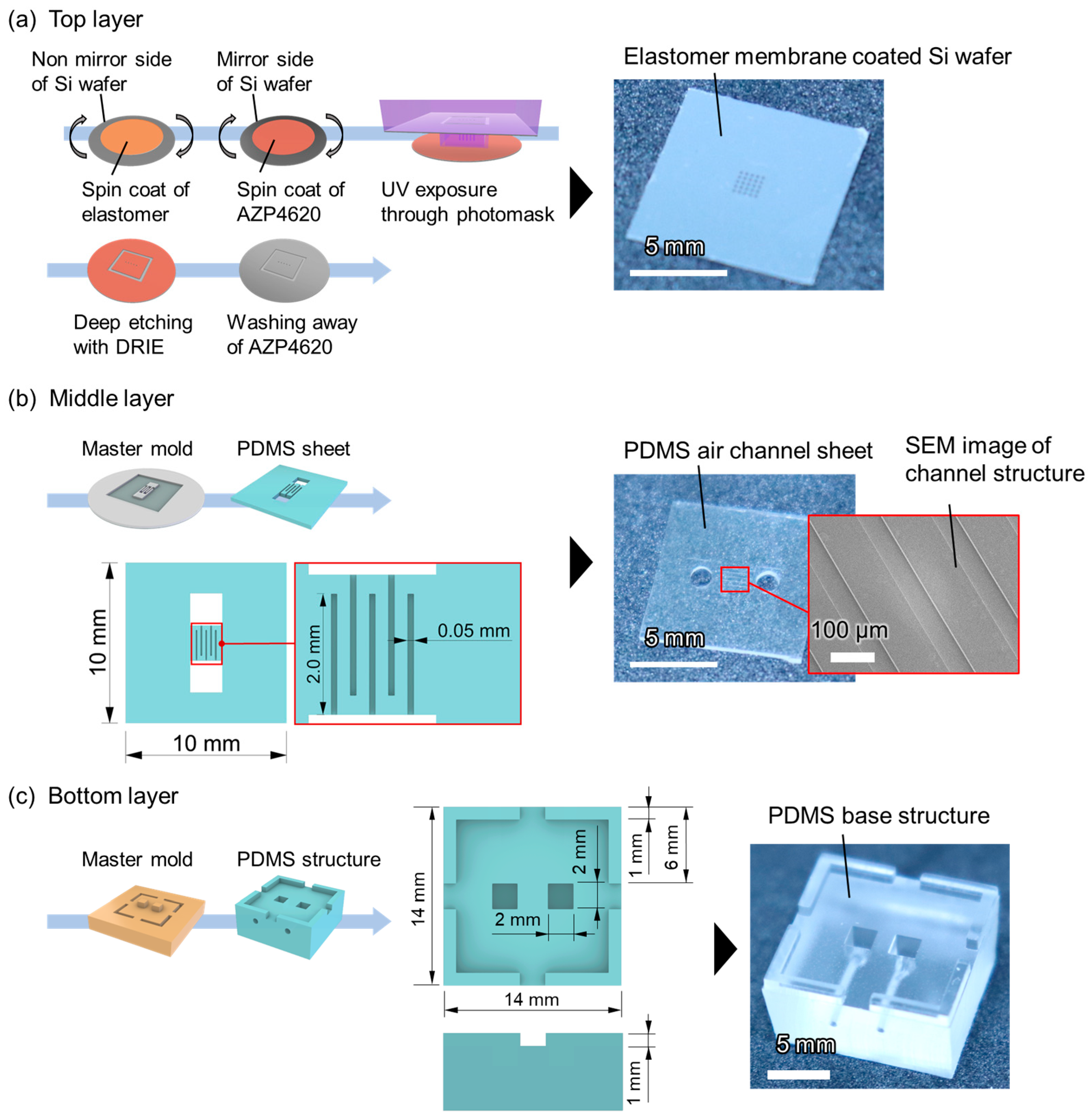
2.2. Fabrication Processes for Consisting Layers
2.3. Device Assembly
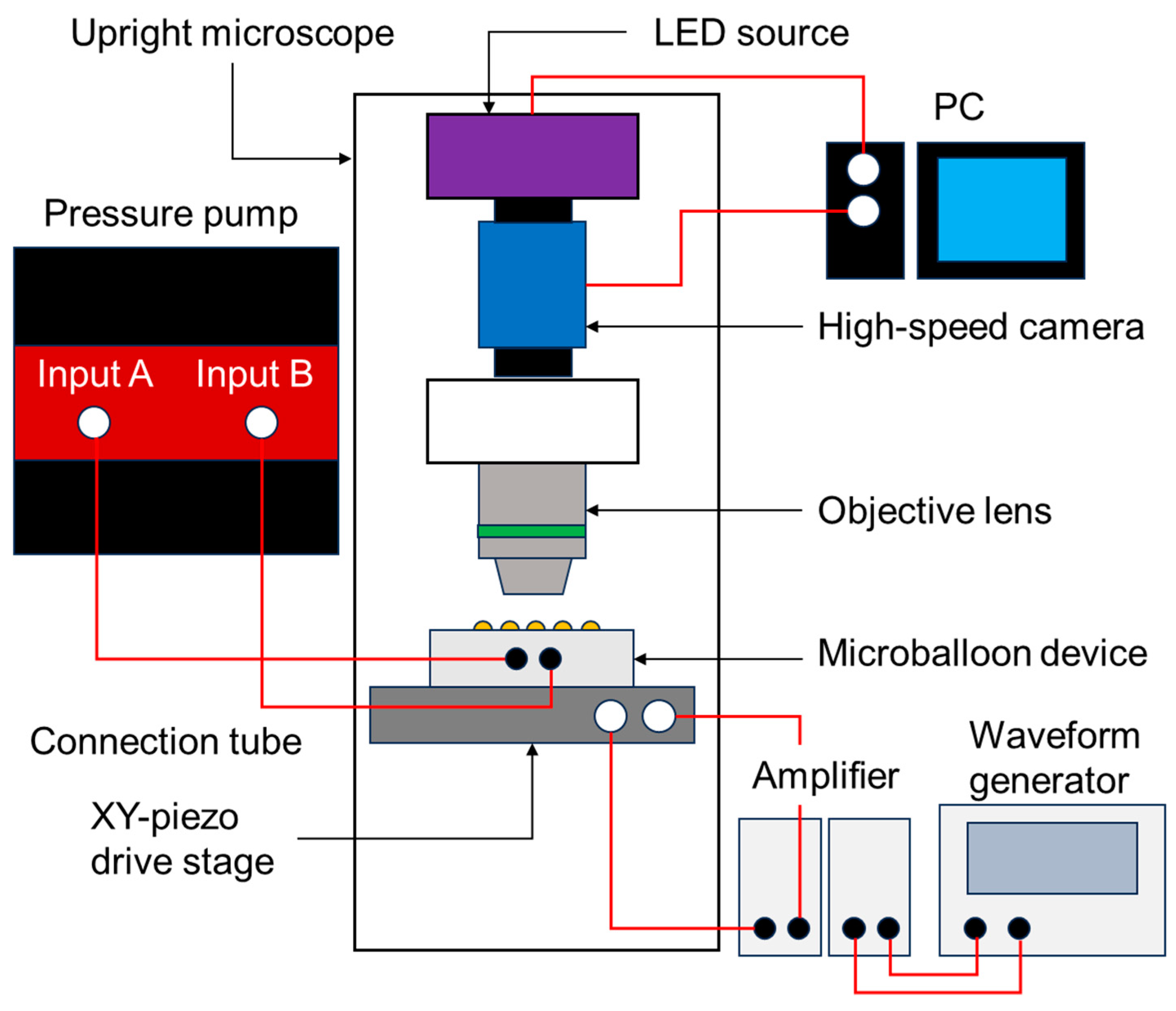
2.4. Actuation Test and Height Measurement of Balloon Actuators
2.5. Flow-Field Measurements of VIF with Particle Image Velocimetry
3. Results and Discussion
3.1. Characterization of the Balloon Actuator
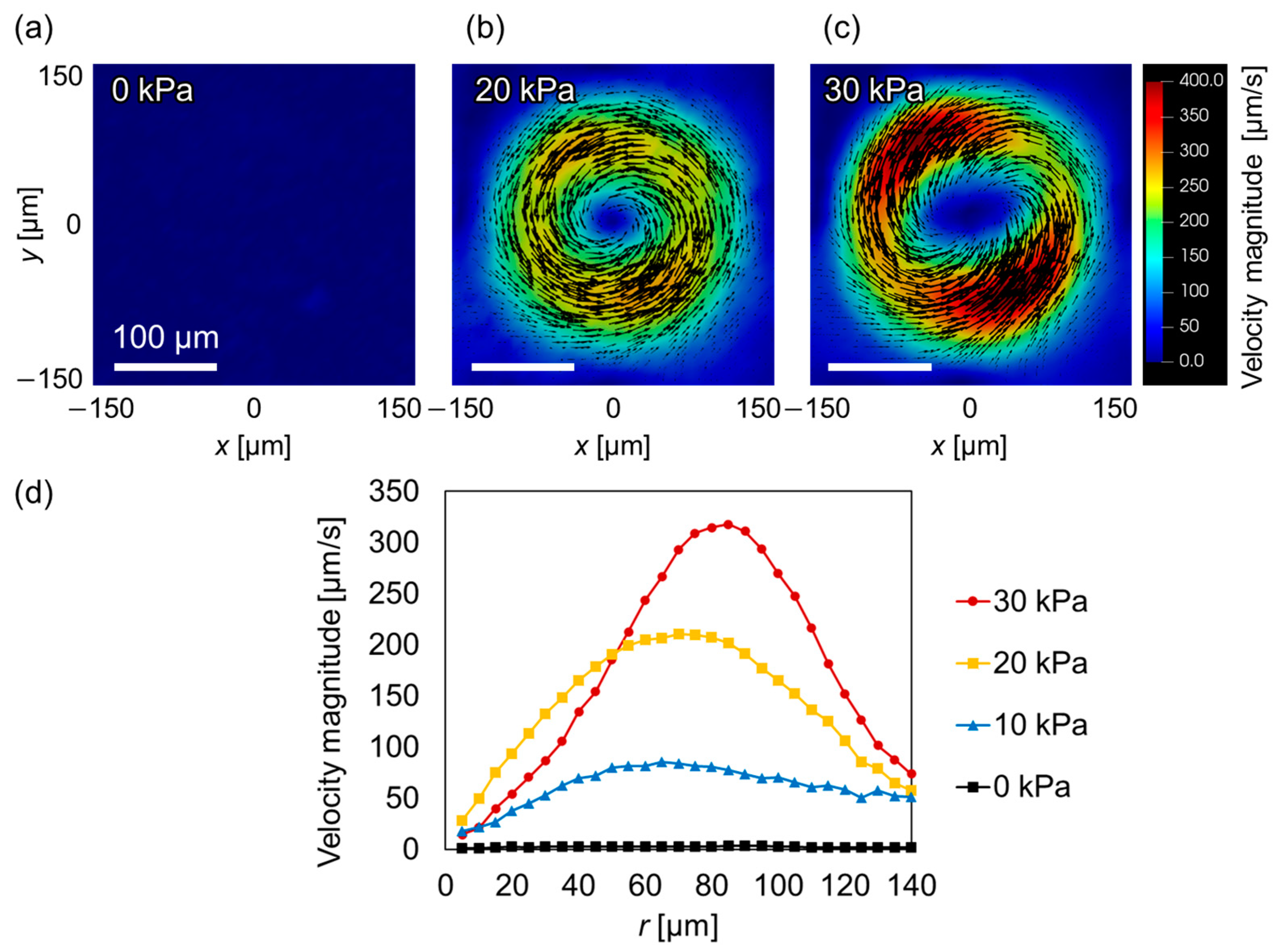
3.2. Induction of VIF around the Balloon Actuator
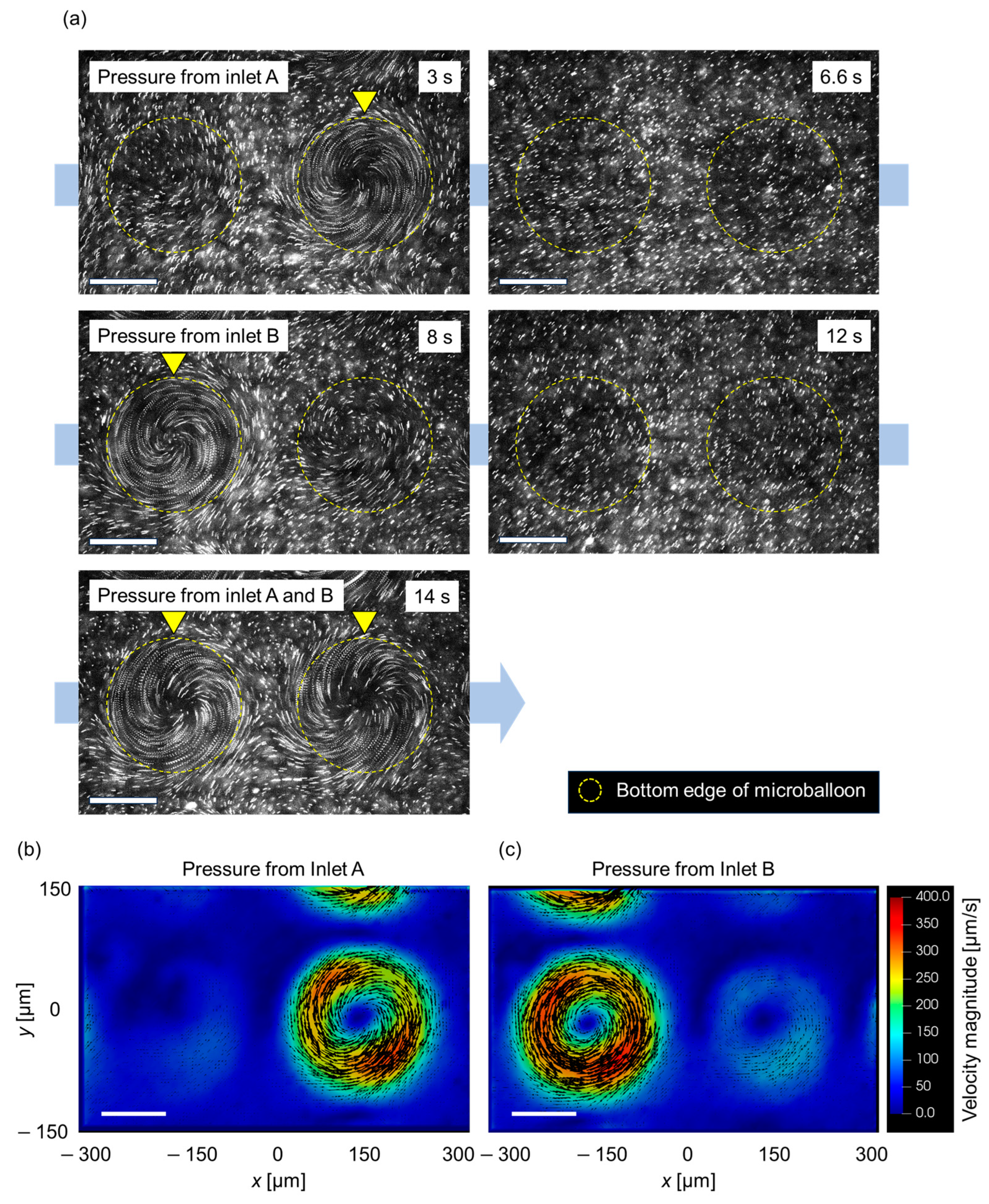
3.3. Spatial Control of Flow Patterns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, A. Micro total analysis systems. 1. introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.; Li, D. Dielectrophoresis in microfluidics technology. Electrophoresis 2011, 32, 2410–2427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ho, C.M.; Kasagi, N. A chaotic mixer for magnetic bead-based micro cell sorter. J. Microelectromech. Syst. 2004, 13, 779–790. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Sakuma, S.; Fukuhara, T.; Yokoyama, Y.; Arai, F. A single cell extraction chip using vibration-induced whirling flow and a thermo-responsive gel pattern. Micromachines 2014, 5, 681–696. [Google Scholar] [CrossRef]
- Hayakawa, T.; Sakuma, S.; Arai, F. On-chip 3D rotation of oocyte based on a vibration-induced local whirling flow. Microsyst. Nanoeng. 2015, 1, 15001. [Google Scholar] [CrossRef]
- Hayakawa, T.; Akita, Y.; Arai, F. Parallel trapping of single motile cells based on vibration-induced flow. Microfluid. Nanofluid. 2018, 22, 42. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.; Ai, Y. Submicron particle concentration and patterning with ultralow frequency acoustic vibration. Anal. Chem. 2020, 92, 12795–12800. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Ma, Z.; Yang, J.K.W.; Ai, Y. Acoustic vibration-induced actuation of multiple microrotors in microfluidics. Adv. Mater. Technol. 2020, 5, 2000323. [Google Scholar] [CrossRef]
- Lutz, B.R.; Chen, J.; Schwartz, D.T. Microscopic steady streaming eddies created around short cylinders in a channel: Flow visualization and stokes layer scaling. Phys. Fluids 2005, 17, 023601. [Google Scholar] [CrossRef]
- Lutz, B.R.; Chen, J.; Schwartz, D.T. Hydrodynamic tweezers: 1. Noncontact trapping of single cells using steady streaming microeddies. Anal. Chem. 2006, 78, 5429–5435. [Google Scholar] [CrossRef] [PubMed]
- Lieu, V.H.; House, T.A.; Schwartz, D.T. Hydrodynamic tweezers: Impact of design geometry on flow and microparticle trapping. Anal. Chem. 2012, 84, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- House, T.A.; Lieu, V.H.; Schwartz, D.T. A model for inertial particle trapping locations in hydrodynamic tweezers arrays. J. Micromech. Microeng. 2014, 24, 045019. [Google Scholar] [CrossRef]
- Kaneko, K.; Huang, Z.; Sato, T.; Ujikawa, N.; Hayakawa, T.; Hasegawa, Y.; Suzuki, H. Investigation of the vibration-induced local flow around a micro-pillar under various vibration conditions. Mech. Eng. J. 2023, 10, 22–223. [Google Scholar] [CrossRef]
- Hayakawa, T.; Arai, F. On-chip micromanipulation method based on mode switching of vibration-induced asymmetric flow. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May 2017–3 June 2017; Volume 1, pp. 6631–6636. [Google Scholar] [CrossRef]
- Huang, P.H.; Xie, Y.; Ahmed, D.; Rufo, J.; Nama, N.; Chen, Y.; Chan, C.Y.; Huang, T.J. An acoustofluidic micromixer based on oscillating sidewall sharp-edges. Lab Chip 2013, 13, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Nama, N.; Huang, P.-H.; Huang, T.J.; Costanzo, F. Investigation of acoustic streaming patterns around oscillating sharp edges. Lab Chip 2014, 14, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Nama, N.; Huang, P.H.; Huang, T.J.; Costanzo, F. Investigation of micromixing by acoustically oscillated sharp edges. Biomicrofluidics 2016, 10, 024124. [Google Scholar] [CrossRef]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef]
- Liu, R.H.; Yang, J.; Pindera, M.Z.; Athavale, M.; Grodzinski, P. Bubble-induced acoustic micromixing. Lab Chip 2002, 2, 151–157. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Juluri, B.K.; Huang, T.J. A fast microfluidic mixer based on acoustically driven sidewall-trapped microbubbles. Microfluid. Nanofluid. 2009, 7, 727–731. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Shi, J.; Juluri, B.K.; Huang, T.J. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 2009, 9, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jalikop, S.V.; Hilgenfeldt, S. Size-sensitive sorting of microparticles through control of flow geometry. Appl. Phys. Lett. 2011, 99, 034101. [Google Scholar] [CrossRef]
- Wang, C.; Jalikop, S.V.; Hilgenfeldt, S. Efficient manipulation of microparticles in bubble streaming flows. Biomicrofluidics 2012, 6, 012801. [Google Scholar] [CrossRef]
- Chung, S.K.; Cho, S.K. On-chip manipulation of objects using mobile oscillating bubbles. J. Micromech. Microeng. 2008, 18, 125024. [Google Scholar] [CrossRef]
- Lv, H.; Jiang, C.; Hou, H.; Zhou, Z.; Deng, J.; Ma, B.; Yuan, W. Flexible balloon actuators for active flow control. Microsys. Technol. 2012, 18, 267–275. [Google Scholar] [CrossRef]
- Mosadegh, B.; Mazzeo, A.D.; Shepherd, R.F.; Morin, S.A.; Gupta, U.; Sani, I.Z.; Lai, D.; Takayama, S.; Whitesides, G.M. Control of soft machines using actuators operated by a braille display. Lab Chip 2014, 14, 189–199. [Google Scholar] [CrossRef]
- Hu, S.; Cao, X.; Reddyhoff, T.; Ding, X.; Shi, X.; Dini, D.; de Mello, A.J.; Peng, Z.; Wang, Z. Pneumatic programmable superrepellent surfaces. Droplet 2022, 1, 48–55. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Grover, W.H.; Ivester, R.H.C.; Jensen, E.C.; Mathies, R.A. Development and multiplexed control of latching pneumatic valves using microfluidic logical structures. Lab Chip 2006, 6, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lee, G. Bin pneumatically driven peristaltic micropumps utilizing serpentine-shape channels. J. Micromechanics Microengineering 2006, 16, 341–348. [Google Scholar] [CrossRef]
- Aref, H. Stirring by chaotic advection. J. Fluid Mech. 1984, 143, 341–348. [Google Scholar] [CrossRef]
- Aref, H.; Blake, J.R.; Budišić, M.; Cardoso, S.S.S.; Cartwright, J.H.E.; Clercx, H.J.H.; El Omari, K.; Feudel, U.; Golestanian, R.; Gouillart, E.; et al. Frontiers of chaotic advection. Rev. Mod. Phys. 2017, 89, 025007. [Google Scholar] [CrossRef]
- Kaneko, K.; Osawa, T.; Kametani, Y.; Hayakawa, T.; Hasegawa, Y.; Suzuki, H. Numerical and experimental analyses of three-dimensional unsteady flow around a micro-pillar subjected to rotational vibration. Micromachines 2018, 9, 668–685. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, T.; Kaneko, K.; Hayakawa, T.; Suzuki, H. Pneumatic Microballoons for Active Control of the Vibration-Induced Flow. Micromachines 2023, 14, 2010. https://doi.org/10.3390/mi14112010
Sato T, Kaneko K, Hayakawa T, Suzuki H. Pneumatic Microballoons for Active Control of the Vibration-Induced Flow. Micromachines. 2023; 14(11):2010. https://doi.org/10.3390/mi14112010
Chicago/Turabian StyleSato, Taku, Kanji Kaneko, Takeshi Hayakawa, and Hiroaki Suzuki. 2023. "Pneumatic Microballoons for Active Control of the Vibration-Induced Flow" Micromachines 14, no. 11: 2010. https://doi.org/10.3390/mi14112010
APA StyleSato, T., Kaneko, K., Hayakawa, T., & Suzuki, H. (2023). Pneumatic Microballoons for Active Control of the Vibration-Induced Flow. Micromachines, 14(11), 2010. https://doi.org/10.3390/mi14112010





