The Impact of Side-Selective Laser Tailoring of Titania Nanotubes on Changes in Photoelectrocatalytic Activity
Abstract
1. Introduction
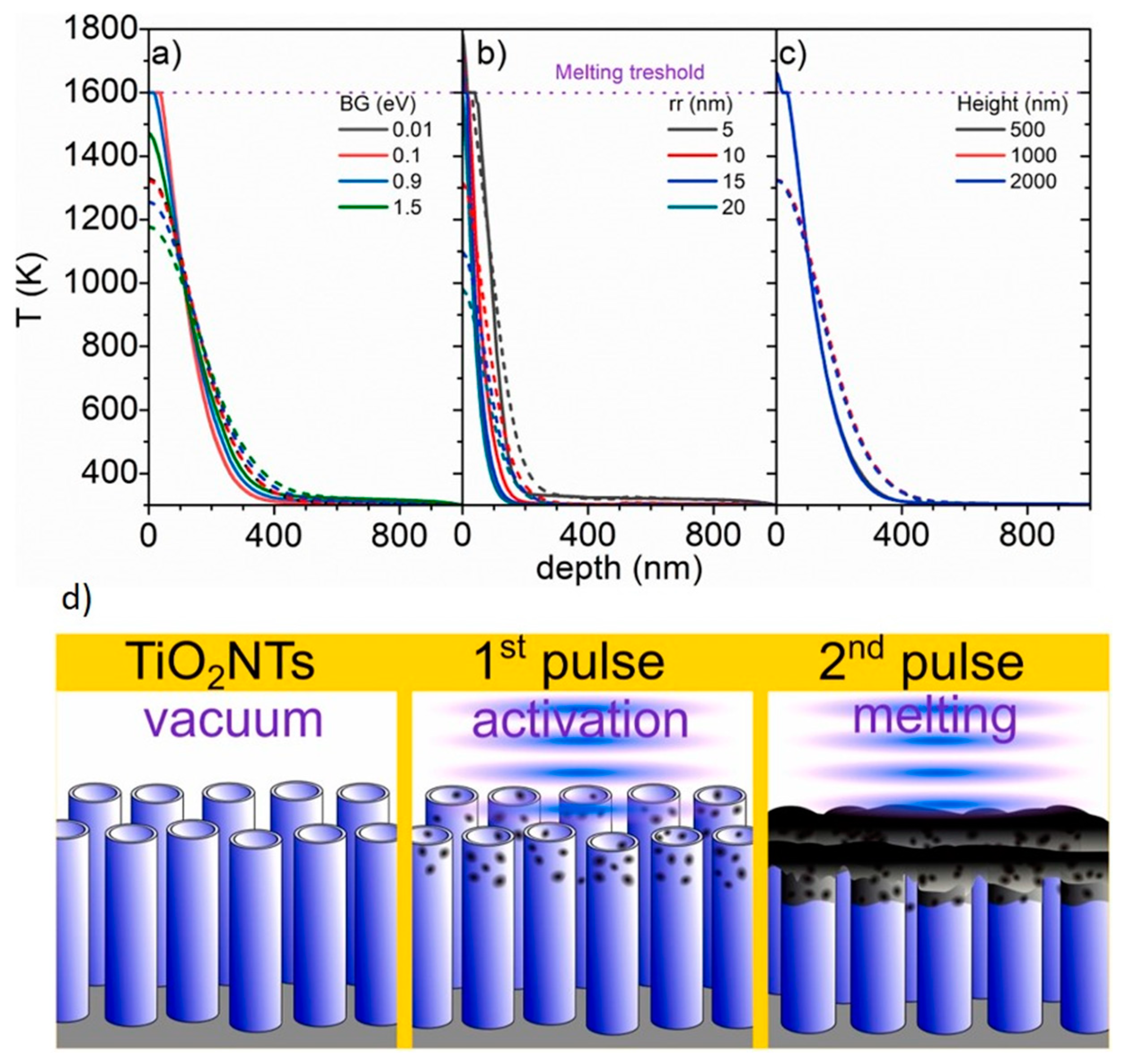
2. Theoretical Studies on the Effect of Laser Irradiation on Titania Nanotubes
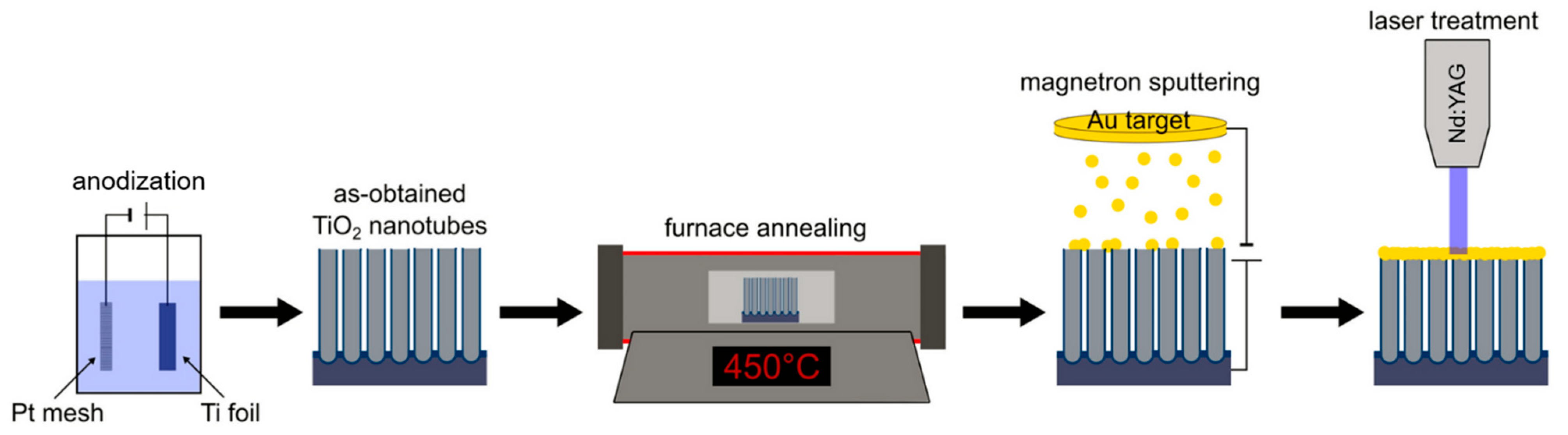
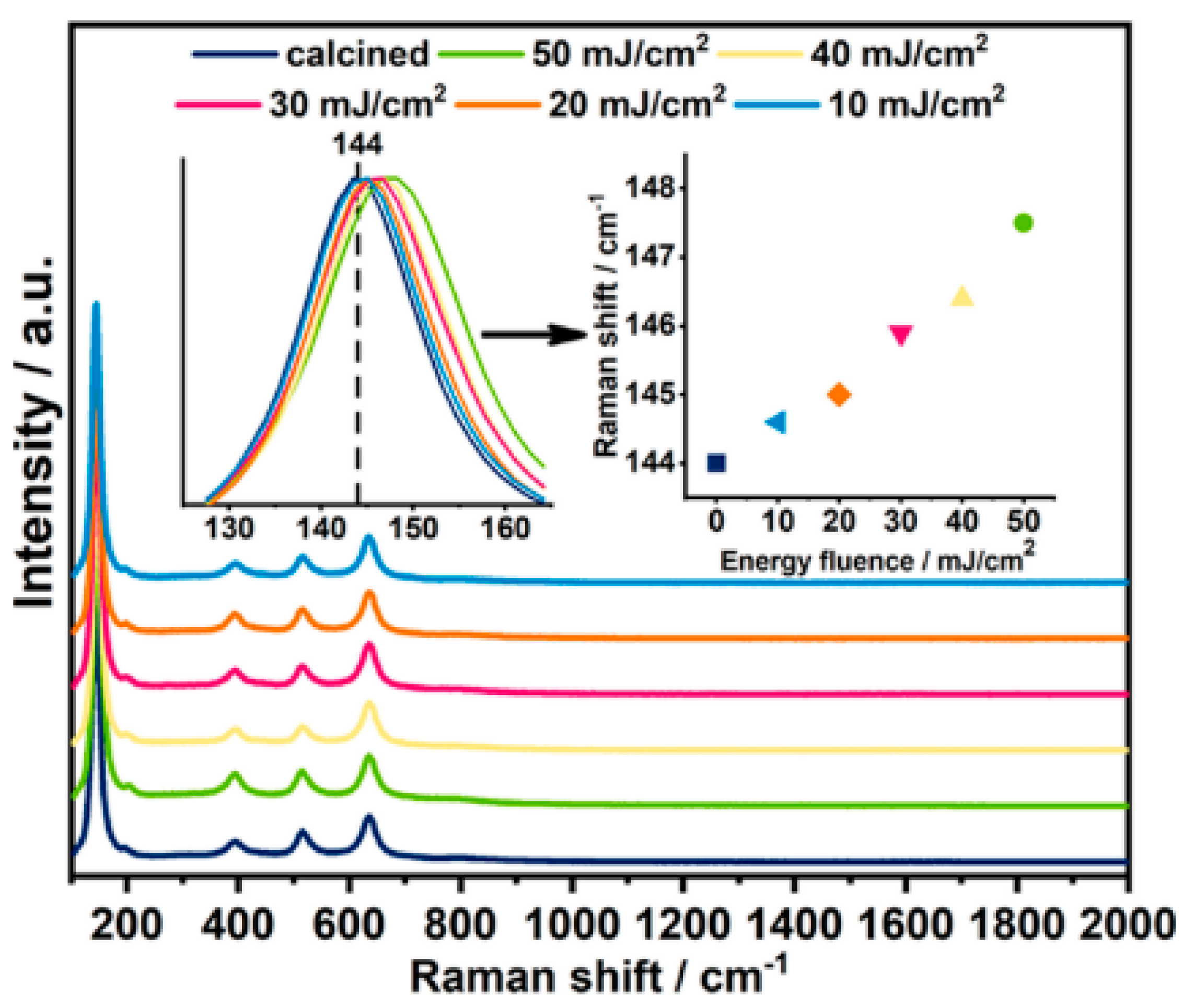
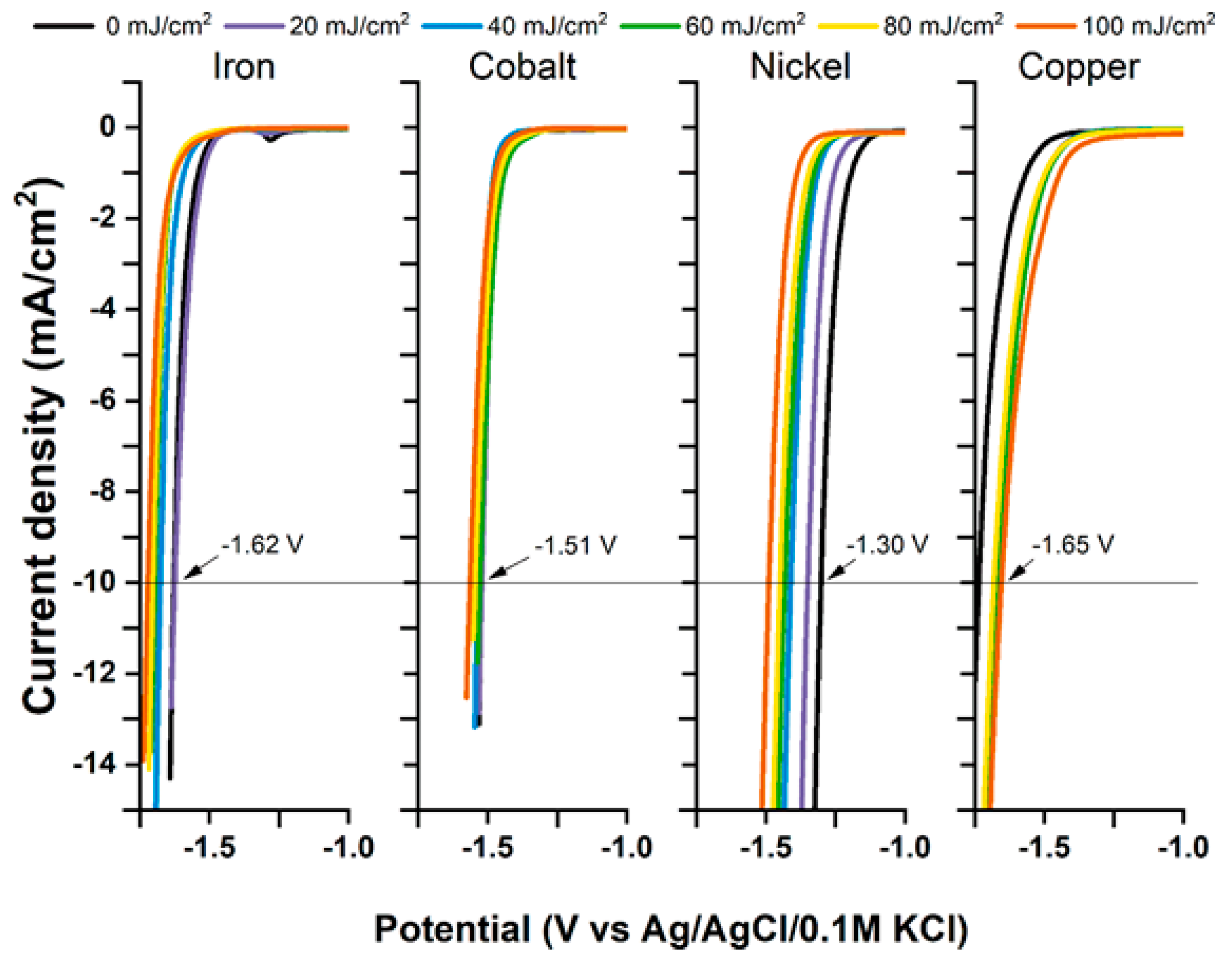
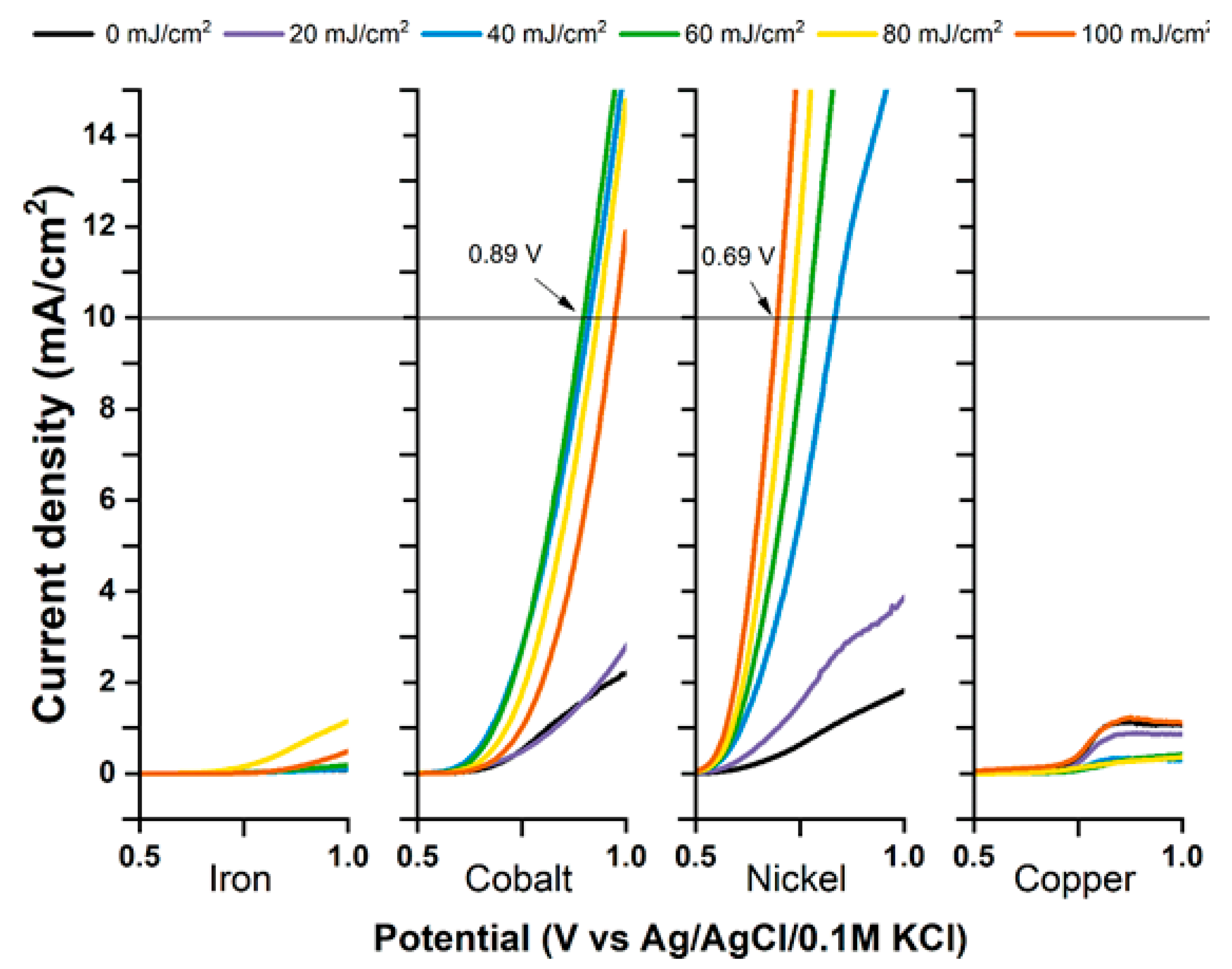
3. The Laser-Induced Phase Transition of Titania Nanotubes and Its Impact on the Photoelectrocatalytic Activity
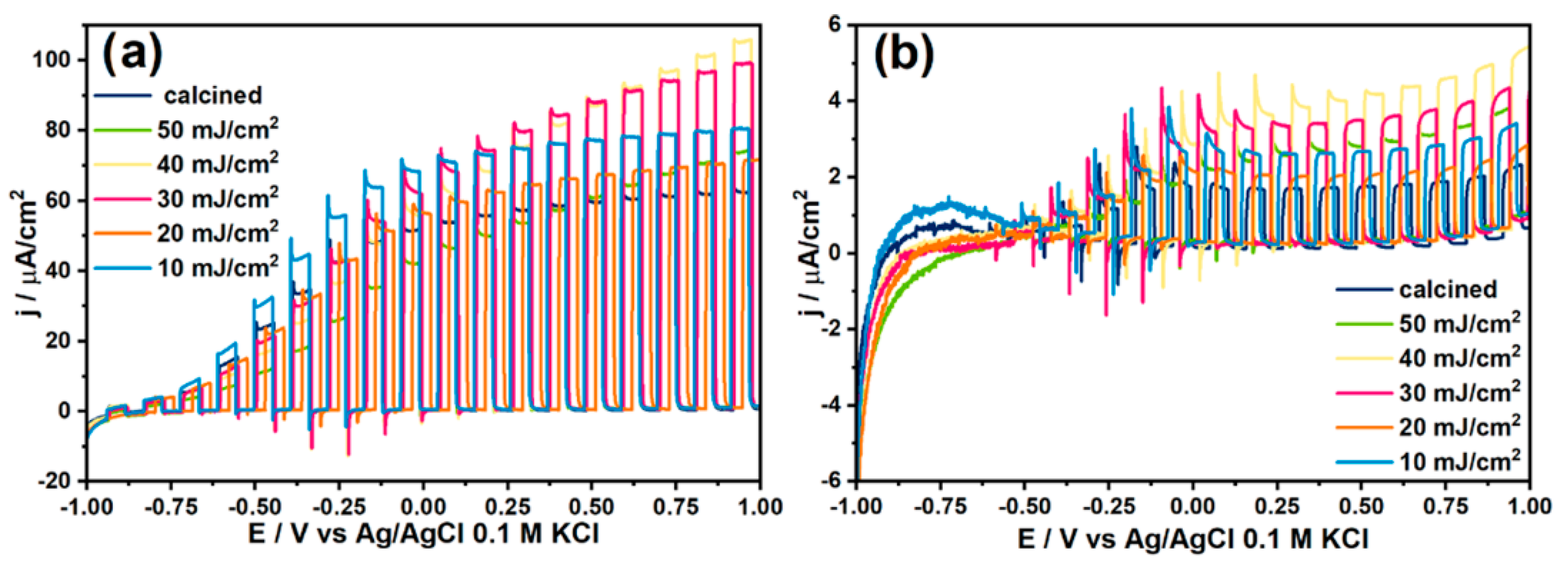
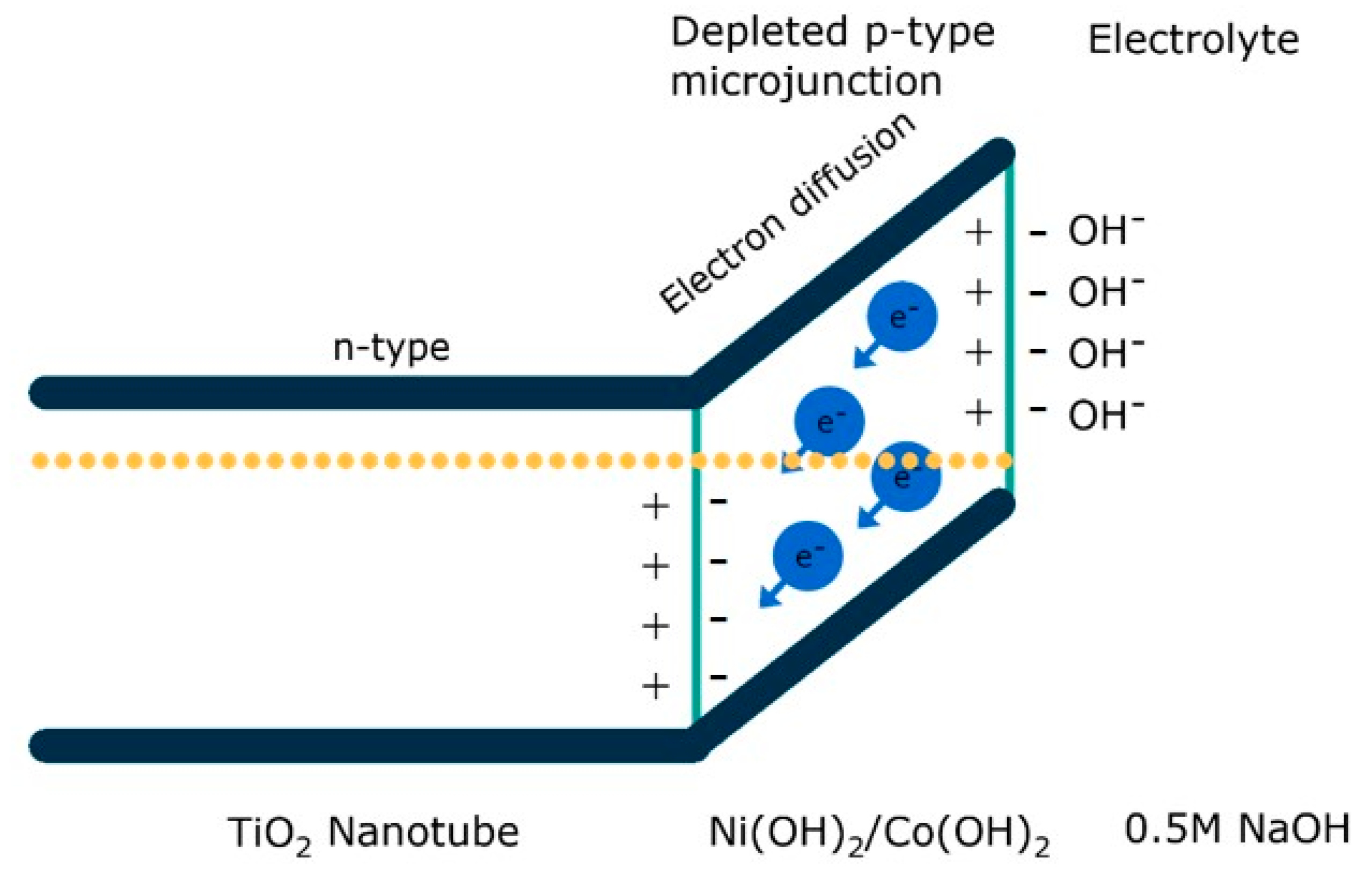
4. The Laser-Induced Morphological Transformation of Titania Nanotubes and Its Impact on Photoelectrocatalytic Activity
5. Conclusions
- A unique approach to TiO2 NTs laser processing was developed;
- The first FEM simulations regarding laser-treated TiO2 NTs were performed;
- Laser treatment as a highly selective method was successfully combined with the surface modifications of TiO2 NTs with transition metals;
- An in-depth discussion on the influence of the laser on the electrochemical properties of the materials was provided;
- A new way to selectively and accurately close nanotubes with a laser beam was developed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M. An electrochemical sensor for clozapine at ruthenium doped TiO2 nanoparticles modified electrode. Sens. Actuators B Chem. 2017, 247, 858–867. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P. Fabrication of a TiO2 and clay nanoparticle composite electrode as a sensor. Anal. Methods 2017, 9, 4387–4393. [Google Scholar] [CrossRef]
- Cesano, F.; Pellerej, D.; Scarano, D.; Ricchiardi, G.; Zecchina, A. Radially organized pillars in TiO2 and in TiO2/C microspheres: Synthesis, characterization and photocatalytic tests. J. Photochem. Photobiol. A Chem. 2012, 242, 51–58. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Panepinto, A.; Michiels, M.; Dürrschnabel, M.T.; Molina-Luna, L.; Bittencourt, C.; Cormier, P.-A.; Snyders, R. Synthesis of anatase (core)/rutile (shell) nanostructured TiO2 thin films by magnetron sputtering methods for dye-sensitized solar cell applications. ACS Appl. Energy Mater. 2020, 3, 759–767. [Google Scholar] [CrossRef]
- Nath, A.; Laha, S.S.; Khare, A. Synthesis of TiO2 nanoparticles via laser ablation at titanium-water interface. Integr. Ferroelectr. 2010, 121, 58–64. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, F.; Dai, Z.; Ma, D. Atomic layer deposition of TiO2 thin films on the inner walls of steel tubes increases anti-coking properties. ACS Omega 2020, 5, 32102–32111. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Mohd Kaus, N.H.; Jiang, Z.-T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef]
- Tomás-Gamasa, M.; Mascareñas, J.L. TiO2-based photocatalysis at the interface with biology and biomedicine. ChemBioChem 2020, 21, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Bukkitgar, S.D.; Shetti, N.P.; Kulkarni, R.M.; Halbhavi, S.B.; Wasim, M.; Mylar, M.; Durgi, P.S.; Chirmure, S.S. Electrochemical oxidation of nimesulide in aqueous acid solutions based on TiO2 nanostructure modified electrode as a sensor. J. Electroanal. Chem. 2016, 778, 103–109. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Aminabhavi, T.M.; Reddy, K.R. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchem. J. 2019, 150, 104124. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W. Pt nanoparticles confined in TiO2 nanotubes with enhanced catalytic performance for phenol hydrogenation by atomic layer deposition. Catal. Lett. 2022, 152, 1020–1028. [Google Scholar] [CrossRef]
- Farkhondehfal, M.A.; Hernández, S.; Rattalino, M.; Makkee, M.; Lamberti, A.; Chiodoni, A.; Bejtka, K.; Sacco, A.; Pirri, F.C.; Russo, N. Syngas production by electrocatalytic reduction of CO2 using Ag-decorated TiO2 nanotubes. Int. J. Hydrog. Energy 2020, 45, 26458–26471. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.K.; Soam, A.; Parida, S.; Sahajwalla, V.; Bhargava, P. In situ carbon-supported titanium dioxide (ICS-TiO2) as an electrode material for high performance supercapacitors. Nanoscale Adv. 2020, 2, 2376–2386. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surf. B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Kuchinad, G.T. Electrochemical oxidation of erythrosine at TiO2 nanoparticles modified gold electrode—An environmental application. J. Environ. Chem. Eng. 2017, 5, 2083–2089. [Google Scholar] [CrossRef]
- Rajakaruna, T.P.B.; Udawatte, C.P.; Chandrajith, R.; Rajapakse, R.M.G. Nonhazardous process for extracting pure titanium dioxide nanorods from geogenic ilmenite. ACS Omega. 2020, 5, 16176–16182. [Google Scholar] [CrossRef] [PubMed]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Biol. Tribol. Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Less known facts and findings about TiO2 nanotubes. Nanoscale 2020, 12, 8119–8132. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Liu, Z.; Misra, M. Dye-sensitized photovoltaic wires using highly ordered TiO2 nanotube arrays. ACS Nano 2010, 4, 2196–2200. [Google Scholar] [CrossRef]
- Meyerink, J.G.; Kota, D.; Wood, S.T.; Crawford, G.A. Transparent titanium dioxide nanotubes: Processing, characterization, and application in establishing cellular response mechanisms. Acta Biomater. 2018, 79, 364–374. [Google Scholar] [CrossRef]
- Xiang, C.; Sun, L.; Wang, Y.; Wang, G.; Zhao, X.; Zhang, S. Large-Scale, Uniform, and superhydrophobic titania nanotubes at the inner surface of 1000 mm long titanium tubes. J. Phys. Chem. C 2017, 121, 15448–15455. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Highly stable organic–inorganic junction composed of hydrogenated titania nanotubes infiltrated by a conducting polymer. RSC Adv. 2016, 6, 33101–33110. [Google Scholar] [CrossRef]
- Beketova, D.; Motola, M.; Sopha, H.; Michalicka, J.; Cicmancova, V.; Dvorak, F.; Hromadko, L.; Frumarova, B.; Stoica, M.; Macak, J.M. One-step decoration of TiO2 nanotubes with Fe3 O4 nanoparticles: Synthesis and photocatalytic and magnetic properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar] [CrossRef]
- Hasanzadeh Kafshgari, M.; Kah, D.; Mazare, A.; Nguyen, N.T.; Distaso, M.; Peukert, W.; Goldmann, W.H.; Schmuki, P.; Fabry, B. Anodic titanium dioxide nanotubes for magnetically guided therapeutic delivery. Sci. Rep. 2019, 9, 13439. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; Garcia-Rey, S.; Ojeda, E.; Basabe-Desmonts, L.; Benito-Lopez, F. TiO2 nanotubes alginate hydrogel scaffold for rapid sensing of sweat biomarkers: Lactate and glucose. ACS Appl. Mater. Interfaces 2021, 13, 37734–37745. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Yan, J.; Qu, L.; Zhao, B.; Yin, J.; Cui, T.; Jiang, L. Femtosecond laser induced phase transformation of TiO2 with exposed reactive facets for improved photoelectrochemistry performance. ACS Appl. Mater. Interfaces 2020, 12, 41250–41258. [Google Scholar] [CrossRef] [PubMed]
- Bjelajac, A.; Petrović, R.; Stan, G.E.; Socol, G.; Mihailescu, A.; Mihailescu, I.N.; Veltruska, K.; Matolin, V.; Siketić, Z.; Provatas, G.; et al. C-doped TiO2 nanotubes with pulsed laser deposited Bi2O3 films for photovoltaic application. Ceram. Int. 2022, 48, 4649–4657. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Zhang, X.; Fan, Z.; Wang, K.; Ma, P.; Liu, J.; Su, M. UV photoelectric properties of aligned TiO2 nanotubes with different wall thickness. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114467. [Google Scholar] [CrossRef]
- Xu, Y.; Zangari, G. TiO2 nanotubes architectures for solar energy conversion. Coatings 2021, 11, 931. [Google Scholar] [CrossRef]
- Alheshibri, M.; Elsayed, K.; Haladu, S.A.; Musa Magami, S.; Al Baroot, A.; Ercan, İ.; Ercan, F.; Manda, A.A.; Çevik, E.; Kayed, T.S.; et al. Synthesis of Ag nanoparticles-decorated on CNTs/TiO2 nanocomposite as efficient photocatalysts via nanosecond pulsed laser ablation. Opt. Laser Technol. 2022, 155, 108443. [Google Scholar] [CrossRef]
- Sharma, S.; Sidhartha, P.N.; Chappanda, K.N. Influence of laser and alkali treatment on an Ag/TiO2 nanotube based dopamine sensor. Nanotechnology 2022, 33, 015502. [Google Scholar] [CrossRef]
- Favet, T.; Keller, V.; Cottineau, T.; El Khakani, M.A. Enhanced visible-light-photoconversion efficiency of TiO2 nanotubes decorated by pulsed laser deposited CoNi nanoparticles. Int. J. Hydrogen Energy 2019, 44, 28656–28667. [Google Scholar] [CrossRef]
- Sopha, H.; Mirza, I.; Turčičova, H.; Pavlinak, D.; Michalicka, J.; Krbal, M.; Rodriguez-Pereira, J.; Hromadko, L.; Novák, O.; Mužík, J.; et al. Laser-induced crystallization of anodic TiO2 nanotube layers. RSC Adv. 2020, 10, 22137–22145. [Google Scholar] [CrossRef]
- Xu, Y.; Melia, M.A.; Tsui, L.; Fitz-Gerald, J.M.; Zangari, G. Laser-induced surface modification at anatase TiO2 nanotube array photoanodes for photoelectrochemical water oxidation. J. Phys. Chem. C 2017, 121, 17121–17128. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Jiang, L.; Ran, P.; Wang, H.; Chen, X.; Xu, C.; Tian, M.; Wang, S.; Zhang, J.; et al. Femtosecond laser mediated fabrication of micro/nanostructured TiO2-x photoelectrodes: Hierarchical nanotubes array with oxygen vacancies and their photocatalysis properties. Appl. Catal. B Environ. 2020, 277, 119231. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Sawczak, M.; Karczewski, J.; Ryl, J.; Cenian, A. Ordered titania nanotubes layer selectively annealed by laser beam for high contrast electrochromic switching. Thin Solid Film 2018, 659, 48–56. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Karczewski, J.; Kupracz, P.; Grochowska, K.; Coy, E.; Mazikowski, A.; Ryl, J.; Siuzdak, K. Formation of the hollow nanopillar arrays through the laser-induced transformation of TiO2 nanotubes. Sci. Rep. 2020, 10, 20235. [Google Scholar] [CrossRef]
- Enachi, M.; Stevens-Kalceff, M.A.; Sarua, A.; Ursaki, V.; Tiginyanu, I. Design of titania nanotube structures by focused laser beam direct writing. J. Appl. Phys. 2013, 114, 234302. [Google Scholar] [CrossRef]
- Grochowska, K.; Nedyalkov, N.; Karczewski, J.; Haryński, Ł.; Śliwiński, G.; Siuzdak, K. Anodic titania nanotubes decorated with gold nanoparticles produced by laser-induced dewetting of thin metallic films. Sci. Rep. 2020, 10, 20506. [Google Scholar] [CrossRef] [PubMed]
- Kupracz, P.; Grochowska, K.; Wawrzyniak, J.; Siuzdak, K. The interaction of the pulsed laser irradiation with titania nanotubes—Theoretical studies on the thermal effect. Int. J. Therm. Sci. 2021, 162, 106800. [Google Scholar] [CrossRef]
- Khenner, M.; Yadavali, S.; Kalyanaraman, R. Formation of organized nanostructures from unstable bilayers of thin metallic liquids. Phys. Fluids 2011, 23, 122105. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Siuzdak, K.; Haryński, Ł.; Wawrzyniak, J.; Grochowska, K. Review on robust laser light interaction with titania—Patterning, crystallisation and ablation processes. Prog. Solid State Chem. 2020, 62, 100297. [Google Scholar] [CrossRef]
- Denisov, N.; Qin, S.; Cha, G.; Yoo, J.; Schmuki, P. Photoelectrochemical properties of “increasingly dark” TiO2 nanotube arrays. J. Electroanal. Chem. 2020, 872, 114098. [Google Scholar] [CrossRef]
- Guisbiers, G.; Van Overschelde, O.; Wautelet, M. Theoretical investigation of size and shape effects on the melting temperature and energy bandgap of TiO2 nanostructures. Appl. Phys. Lett. 2008, 92, 103121. [Google Scholar] [CrossRef]
- Likodimos, V.; Stergiopoulos, T.; Falaras, P.; Kunze, J.; Schmuki, P. Phase composition, size, orientation, and antenna effects of self-assembled anodized titania nanotube arrays: A polarized micro-Raman investigation. J. Phys. Chem. C 2008, 112, 12687–12696. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Karczewski, J.; Ryl, J.; Siuzdak, K. Scalable route toward superior photoresponse of UV-laser-treated TiO2 nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.M.; Taveira, L.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Karczewski, J.; Kupracz, P.; Grochowska, K.; Załęski, K.; Pshyk, O.; Coy, E.; Bartmański, M.; Szkodo, M.; Siuzdak, K. Laser-assisted modification of titanium dioxide nanotubes in a tilted mode as surface modification and patterning strategy. Appl. Surf. Sci. 2020, 508, 145143. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Kupracz, P.; Karczewski, J.; Coy, E.; Siuzdak, K. The in-depth studies of pulsed UV laser-modified TiO2 nanotubes: The influence of geometry, crystallinity, and processing parameters. Nanomaterials 2020, 10, 430. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Van Thang, N.; Wang, C.; Leu, J. Structural and morphological transformations of TiO2 nanotube arrays induced by excimer laser treatment. Thin Solid Film. 2012, 520, 3593–3599. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Xiang, Q.; Li, Y.; Liu, M.; Liao, Y. Transition-Metal-Ion (Fe, Co, Cr, Mn, Etc.) Doping of TiO2 nanotubes: A general approach. Inorg. Chem. 2019, 58, 12511–12515. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.; Román-Martínez, M. TiO2 modification with transition metallic species (Cr, Co, Ni, and Cu) for photocatalytic abatement of acetic acid in liquid phase and propene in gas phase. Materials 2018, 12, 40. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Šuligoj, A.; Arčon, I.; Mazaj, M.; Dražić, G.; Arčon, D.; Cool, P.; Štangar, U.L.; Tušar, N.N. Surface modified titanium dioxide using transition metals: Nickel as a winning transition metal for solar light photocatalysis. J. Mater. Chem. A 2018, 6, 9882–9892. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Karczewski, J.; Coy, E.; Iatsunskyi, I.; Ryl, J.; Gazda, M.; Grochowska, K.; Siuzdak, K. Spectacular oxygen evolution reaction enhancement through laser processing of the nickel-decorated titania nanotubes. Adv. Mater. Interfaces 2021, 8, 2001420. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Karczewski, J.; Coy, E.; Ryl, J.; Grochowska, K.; Siuzdak, K. Nanostructure of the laser-modified transition metal nanocomposites for water splitting. Nanotechnology 2022, 33, 205401. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. p-type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef]
- Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-type material for CO sensing in humid air. Sensors 2017, 17, 2216. [Google Scholar] [CrossRef] [PubMed]
- Coronado, J.M.; Hernández-Alonso, M.D. The keys of success: TiO2 as a benchmark photocatalyst. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; pp. 85–101. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ichimura, M. Electrochemical deposition of Cu-doped p-type iron oxide thin films. Semicond. Sci. Technol. 2018, 33, 105006. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, S.; Yang, X.; Qiu, M.; Chen, Z.; Yu, Y. p-type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties. Electrochim. Acta 2011, 56, 2735–2739. [Google Scholar] [CrossRef]
- Kang-Wen, Q.; Xi, C.; Zhang, Y.; Zhang, R.; Li, Z.; Sheng, G.-R.; Liu, H.; Dong, C.-K.; Chen, Y.-J.; Du, X.-W. Laser-induced oxygen vacancies in FeCo2O4 nanoparticles for boosting oxygen evolution and reduction. Chem. Commun. 2019, 55, 8579–8582. [Google Scholar] [CrossRef]
- Linnik, O.; Chorna, N.; Smirnova, N.; Eremenko, A.; Korduban, O.; Stefan, N.; Ristoscu, C.; Socol, G.; Miroiu, M.; Mihailescu, I.N. Pulsed laser-deposited TiO2-based films: Synthesis, electronic structure and photocatalytic activity. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Wawrzyniak, J.; Banach-Kopeć, A.; Cieślik, B.M.; Jurak, K.; Karczewski, J.; Tylingo, R.; Siuzdak, K.; Zieliński, A. Antibacterial properties of laser-encapsulated titanium oxide nanotubes decorated with nanosilver and covered with chitosan/Eudragit polymers. Biomater. Adv. 2022, 138, 212950. [Google Scholar] [CrossRef]

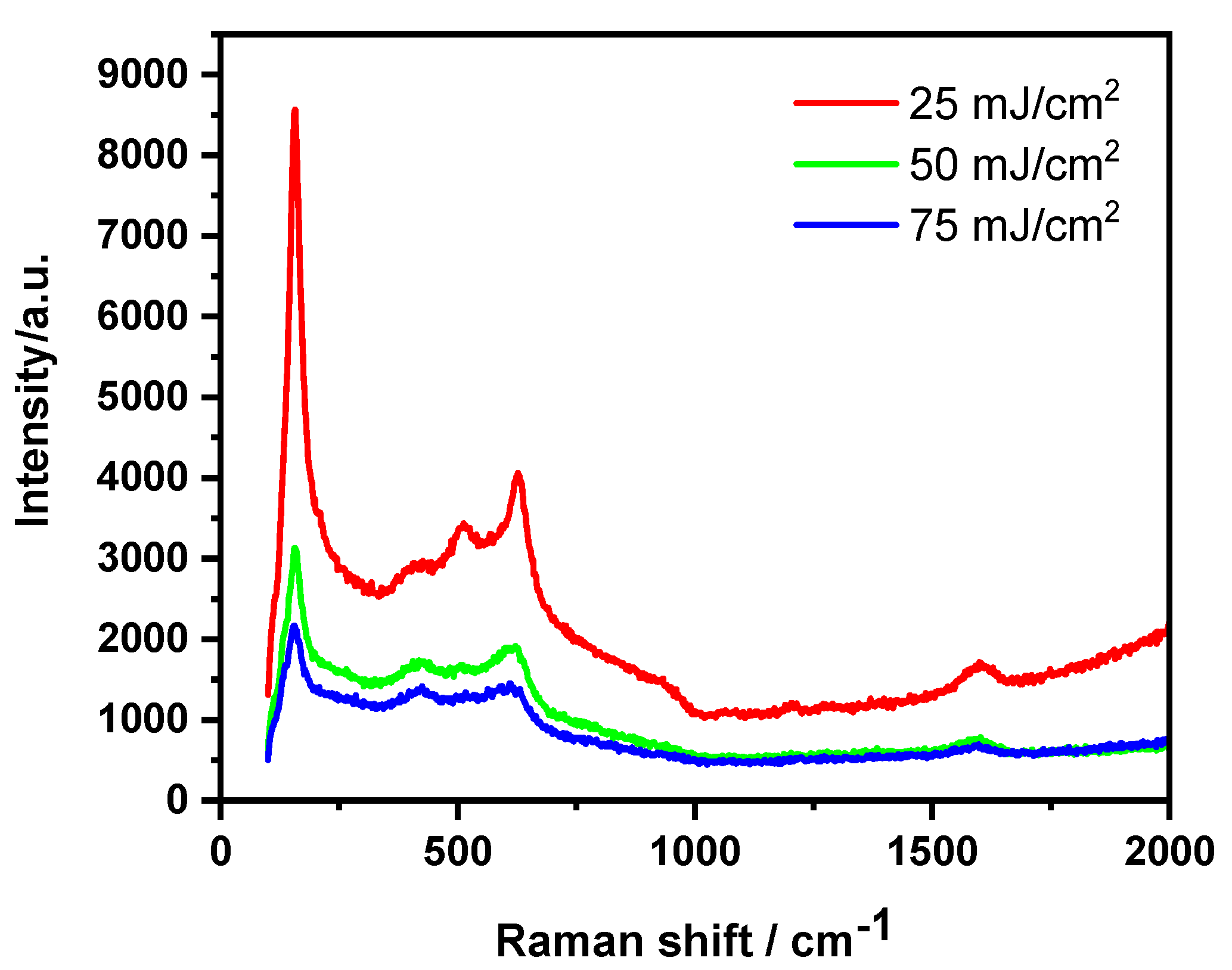
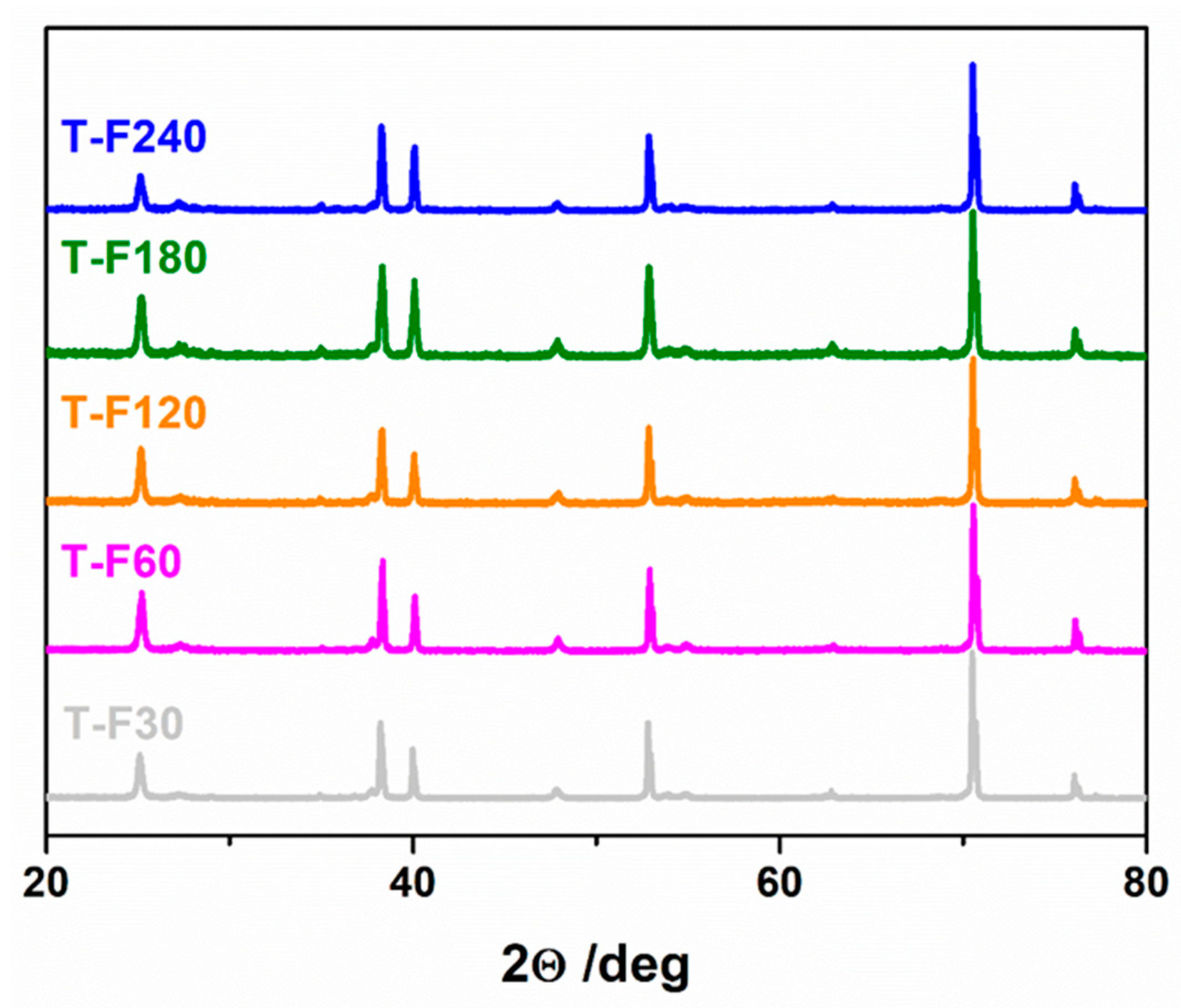
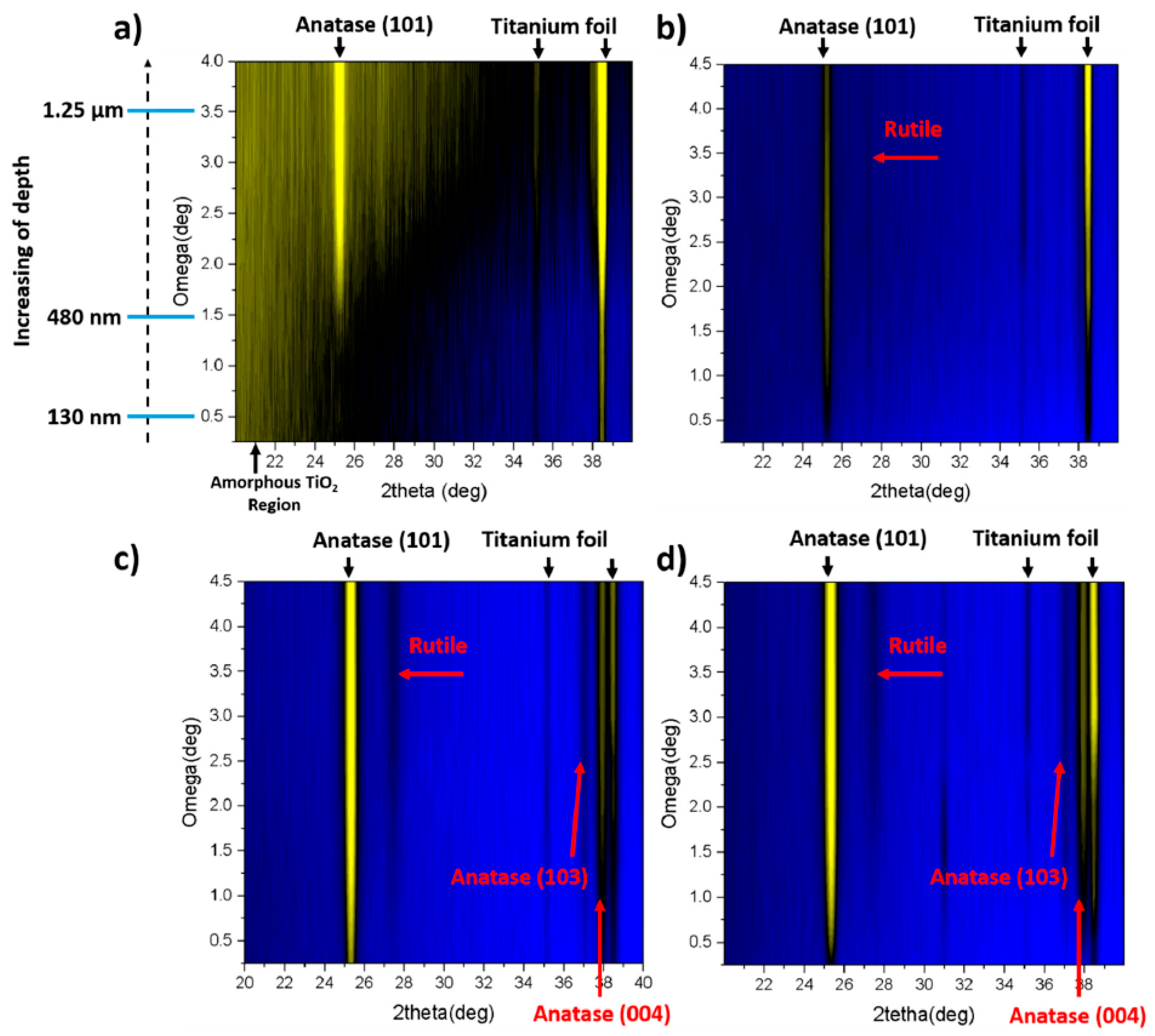
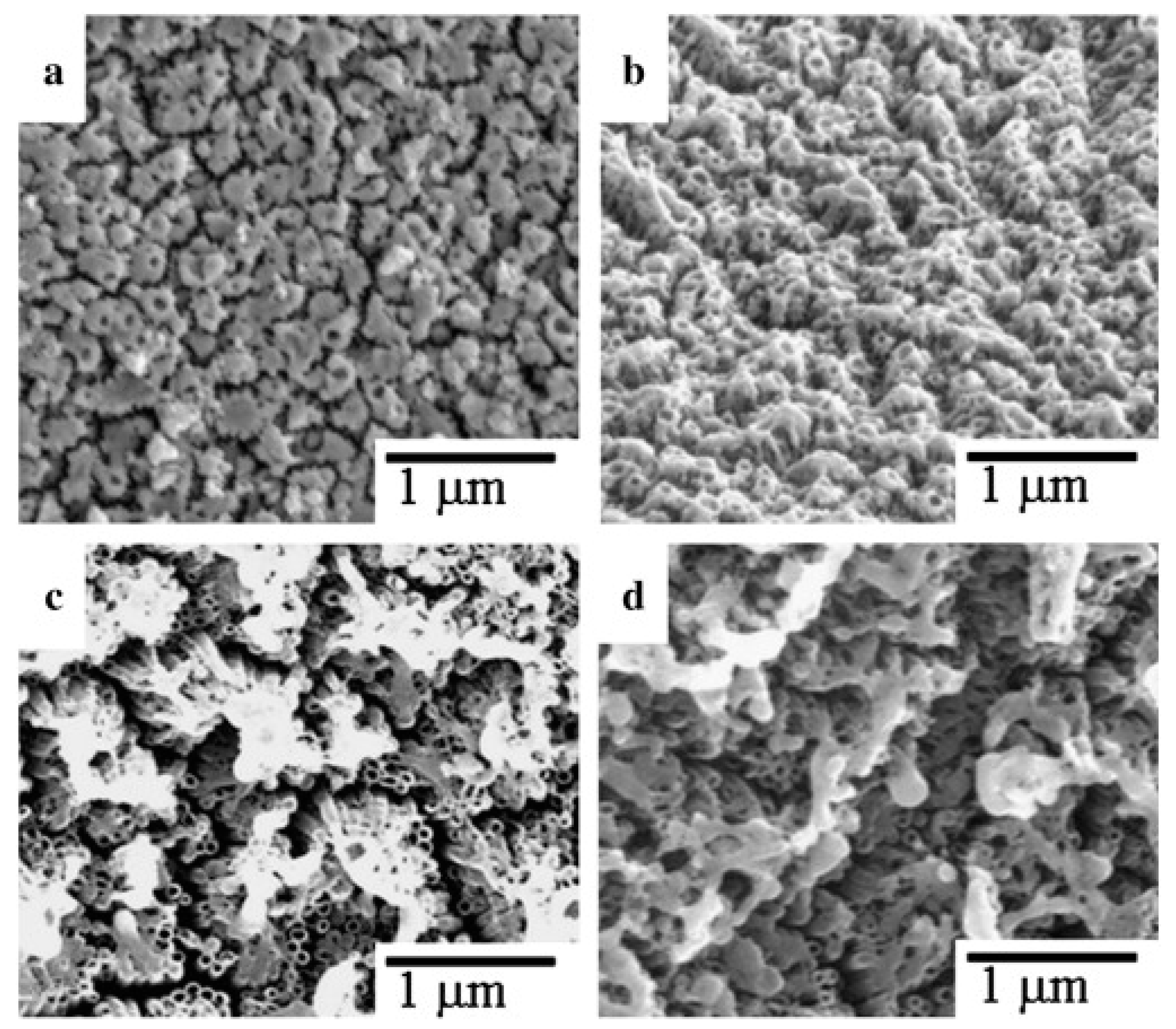
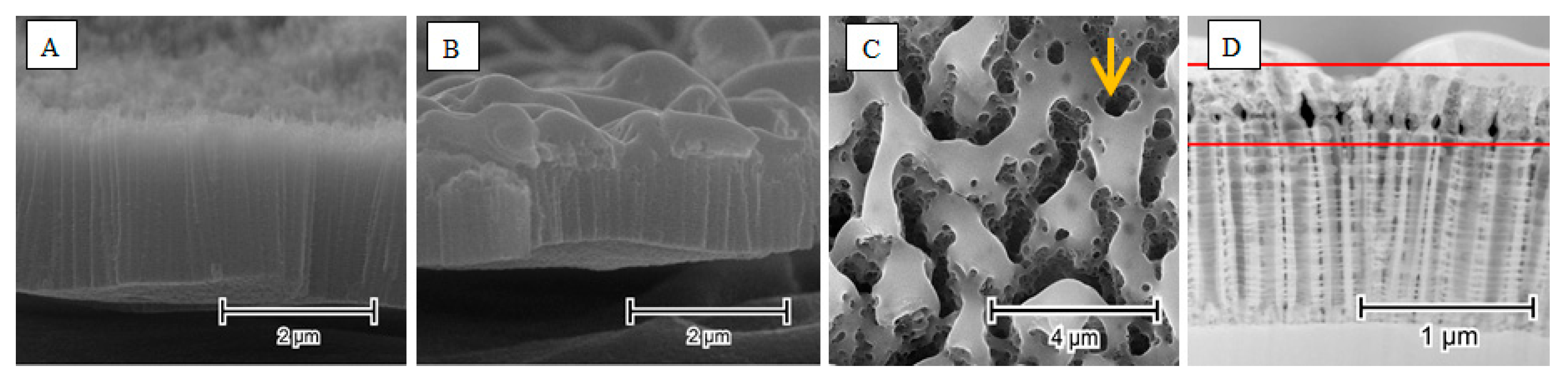
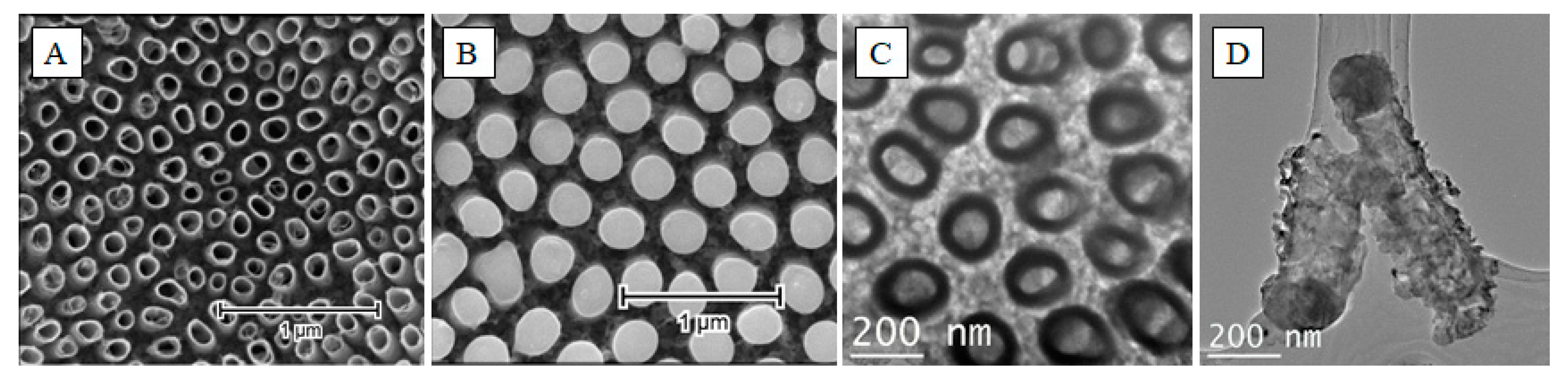
| Used Laser | Wavelength [nm] | Achievement | Drawback | Reference |
|---|---|---|---|---|
| Ti-sapphire femtosecond laser | 800 | 15-fold photocurrent enhancement and double-MB photodegradation in visible light | n.d. | [41] |
| KrF pulsed excimer laser | 248 | 1.5-fold photocurrent improvement under AM1.5G solar irradiation | Decrease of photoinduced charge carriers when energy fluence was >0.3 J/cm2 | [35] |
| KrF pulsed excimer laser | 248 | Enhanced catalytic activity | Decrease of crystallinity | [40] |
| Picosecond diode pumped thin-disk laser | 257.5 | Amorphous to anatase phase transition | More structural defects, lower photocurrent densities, lower flat band potential | [39] |
| Nd:YAG laser | 266 and 355 | Multicyclic stability and time-effective phase transition | n.d. | [42] |
| Nd:YAG laser | 266, 355 and 532 | Creation of spaced, hollow nanopillars with photonic properties | Increase of oxygen vacancies for 4ω harmonics samples | [43] |
| DPSS laser | 532 | Amorphous-to-anatase phase transition | Possibility of rutile formation | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siuzdak, K.; Wawrzyniak, J.; Haryński, Ł.; Bielan, Z.; Grochowska, K. The Impact of Side-Selective Laser Tailoring of Titania Nanotubes on Changes in Photoelectrocatalytic Activity. Micromachines 2023, 14, 274. https://doi.org/10.3390/mi14020274
Siuzdak K, Wawrzyniak J, Haryński Ł, Bielan Z, Grochowska K. The Impact of Side-Selective Laser Tailoring of Titania Nanotubes on Changes in Photoelectrocatalytic Activity. Micromachines. 2023; 14(2):274. https://doi.org/10.3390/mi14020274
Chicago/Turabian StyleSiuzdak, Katarzyna, Jakub Wawrzyniak, Łukasz Haryński, Zuzanna Bielan, and Katarzyna Grochowska. 2023. "The Impact of Side-Selective Laser Tailoring of Titania Nanotubes on Changes in Photoelectrocatalytic Activity" Micromachines 14, no. 2: 274. https://doi.org/10.3390/mi14020274
APA StyleSiuzdak, K., Wawrzyniak, J., Haryński, Ł., Bielan, Z., & Grochowska, K. (2023). The Impact of Side-Selective Laser Tailoring of Titania Nanotubes on Changes in Photoelectrocatalytic Activity. Micromachines, 14(2), 274. https://doi.org/10.3390/mi14020274







