Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants
Abstract
1. Introduction
2. Materials and Methods
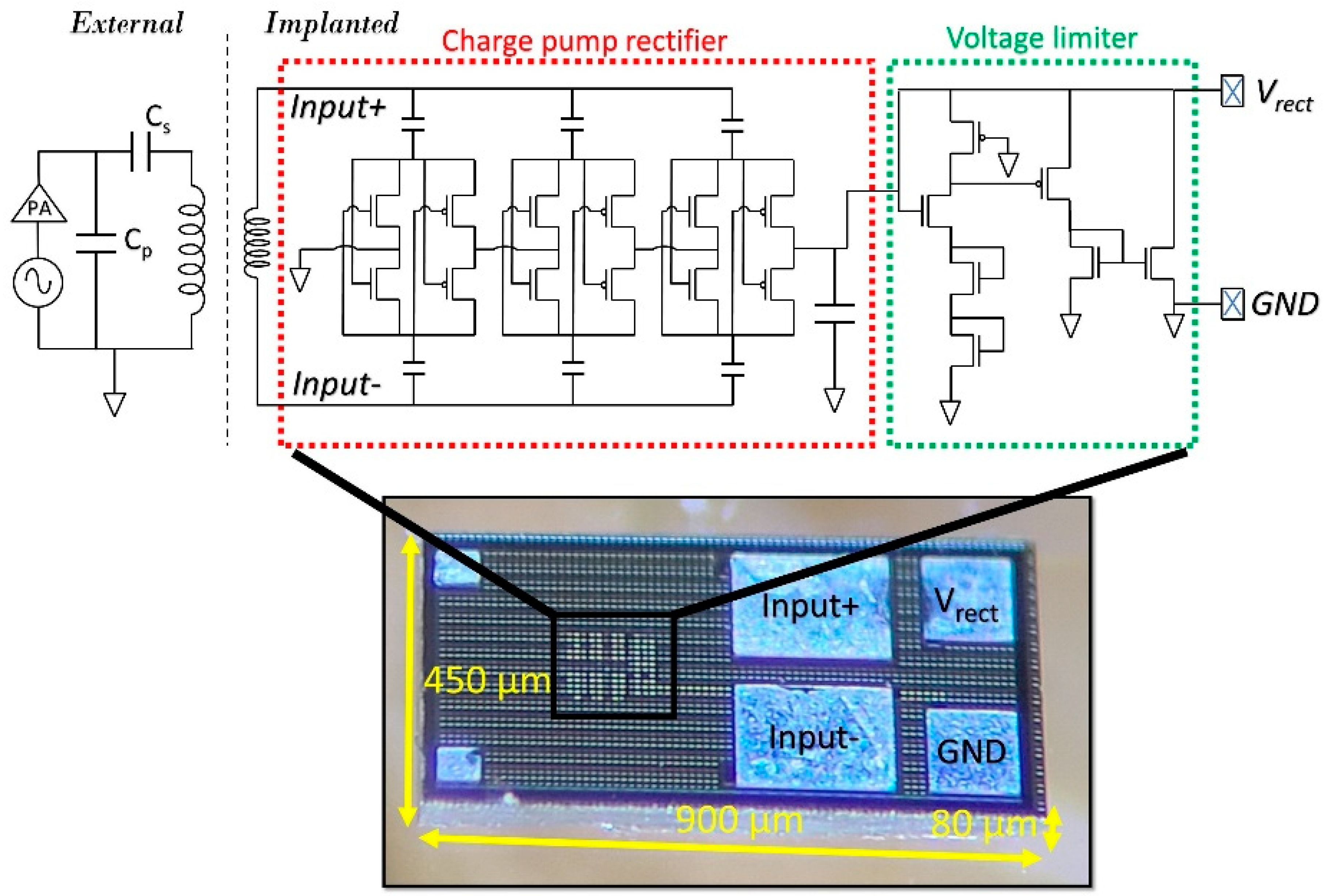
2.1. ASIC
2.2. Wireless Link
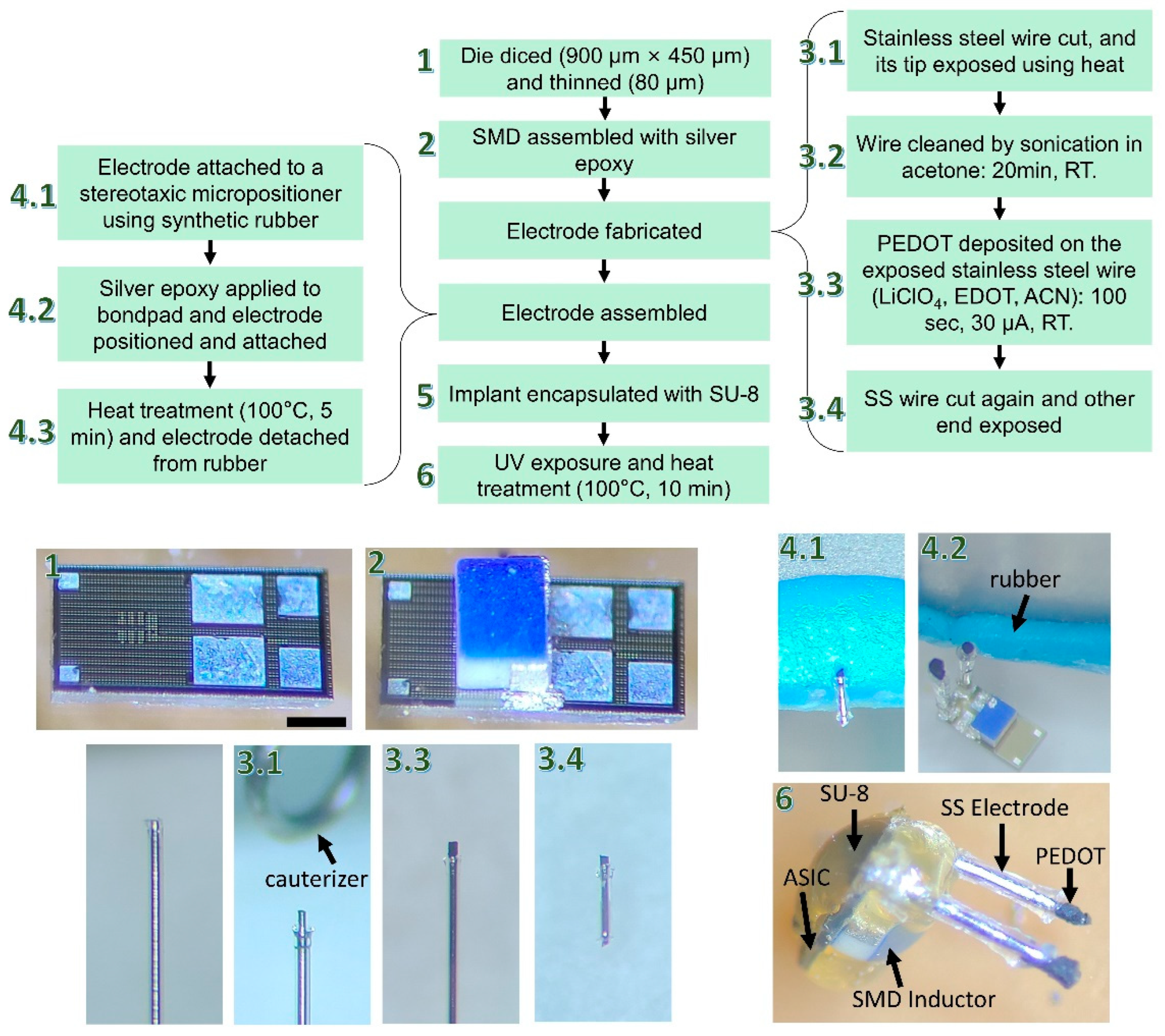
2.3. Fabrication and Assembly Process Flow
2.4. Electrode Impedance Characterization
2.5. In Vivo Experimental Design
3. Results
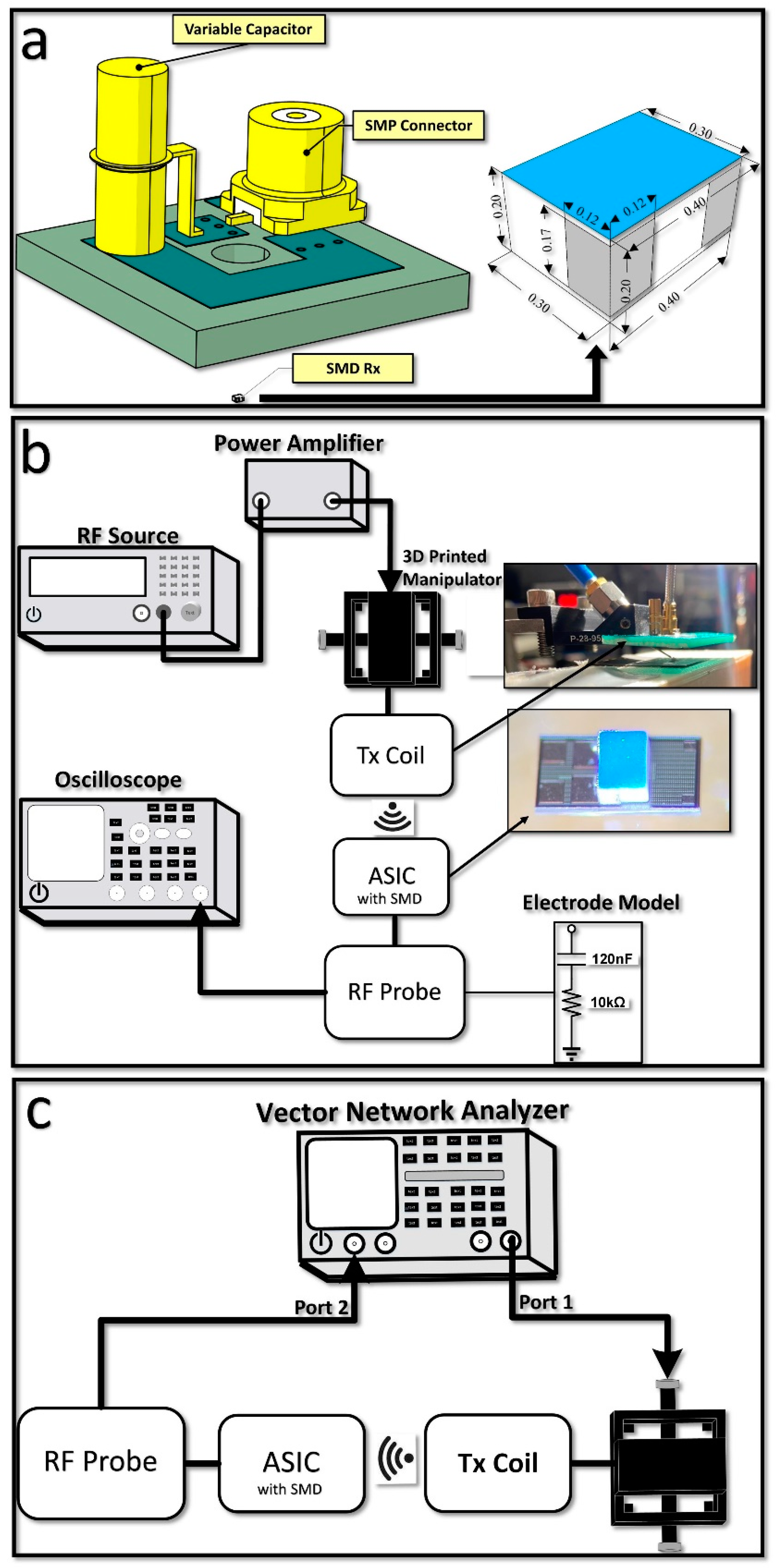
3.1. Benchtop Measurement Results
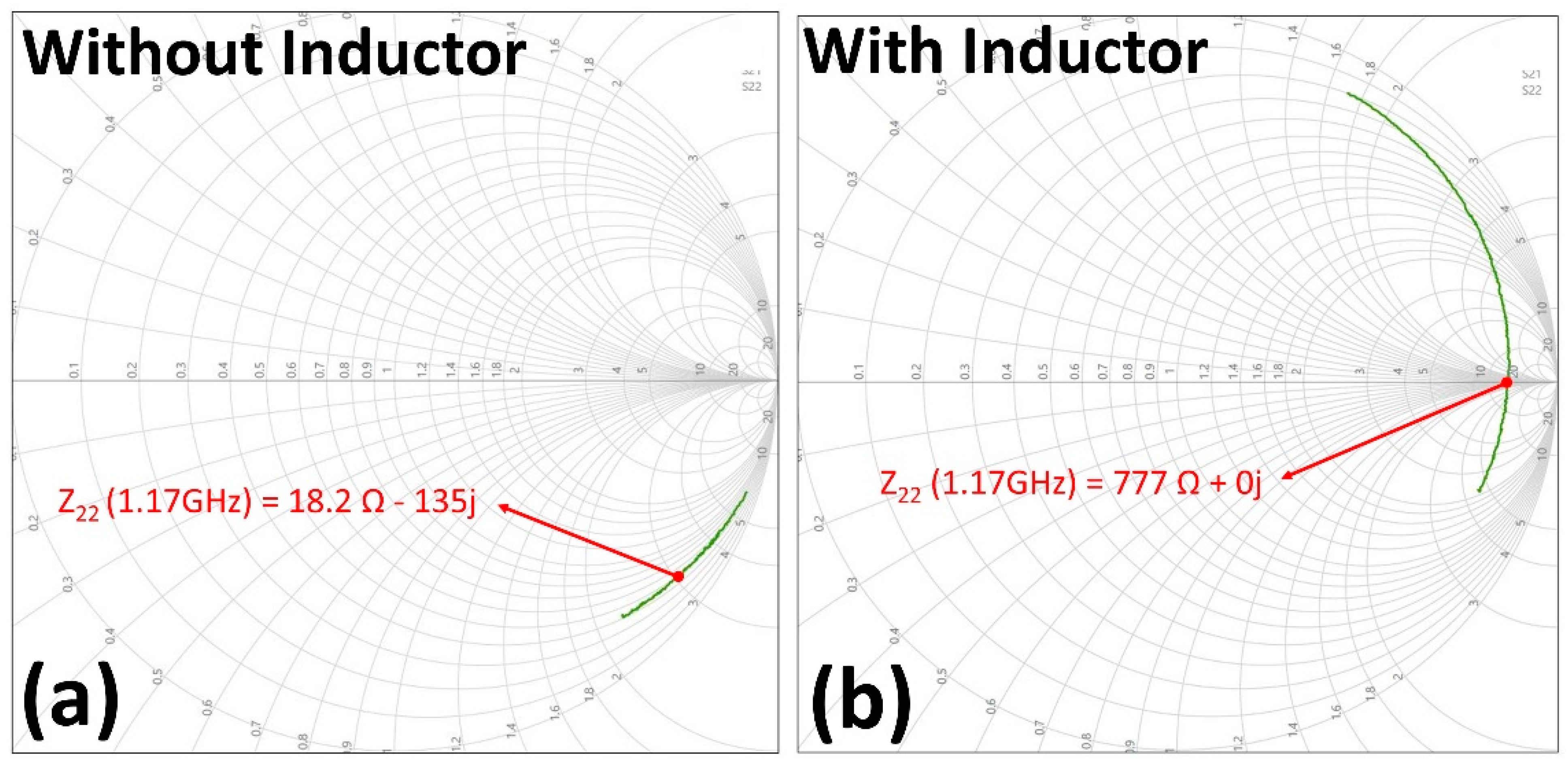
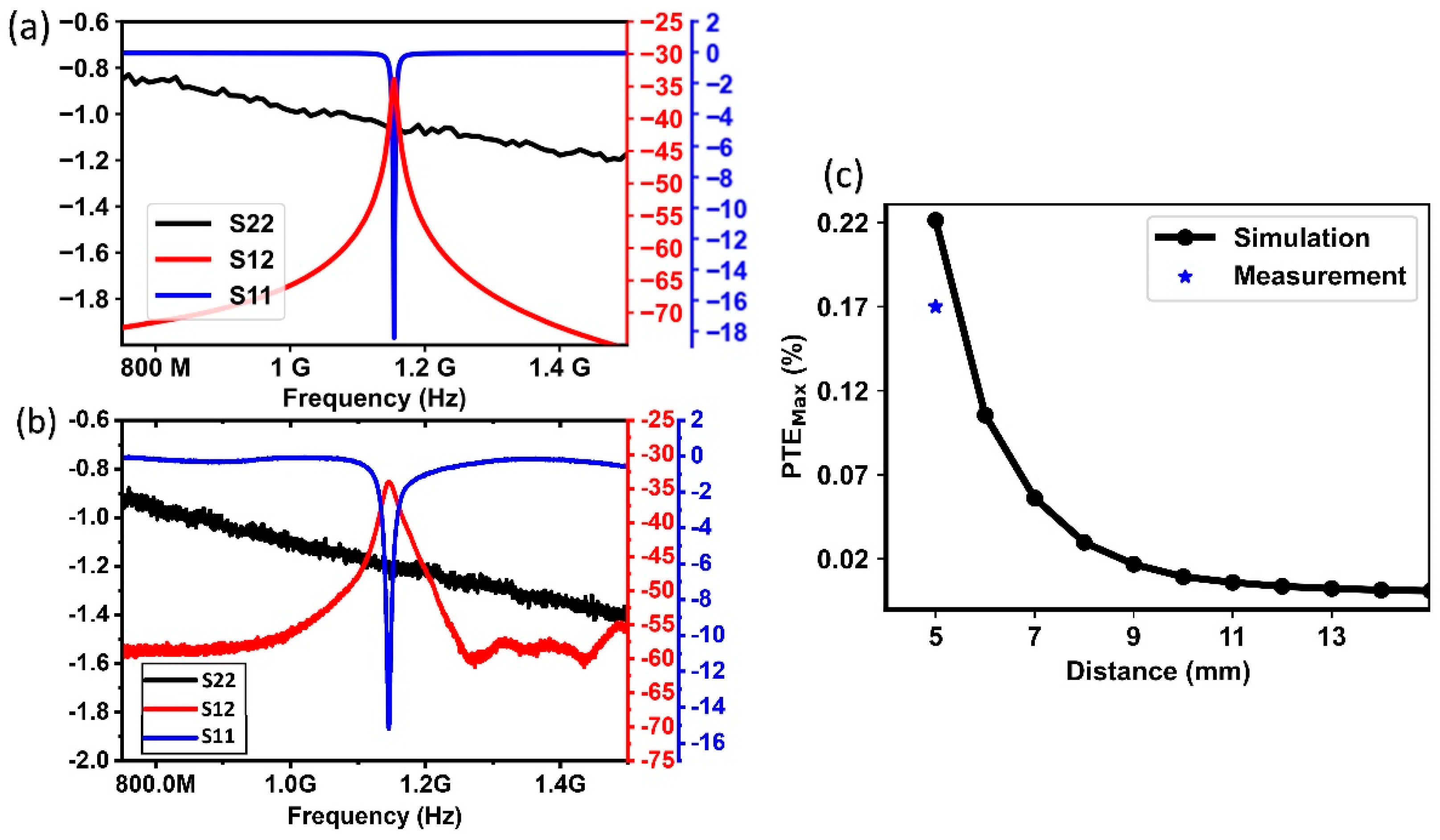
3.1.1. ASIC and Wireless Link Characterization
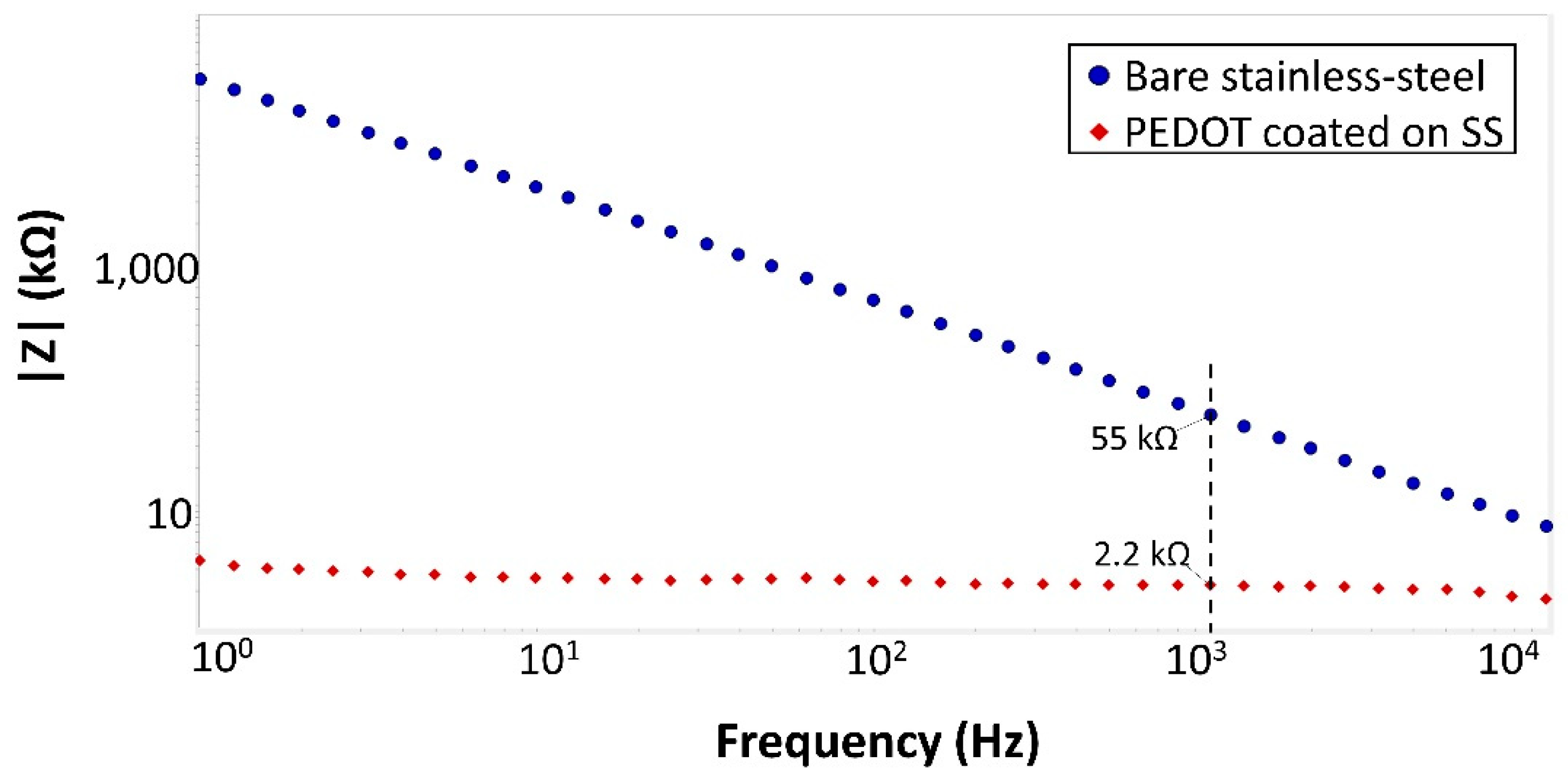
3.1.2. Electrode Characterization
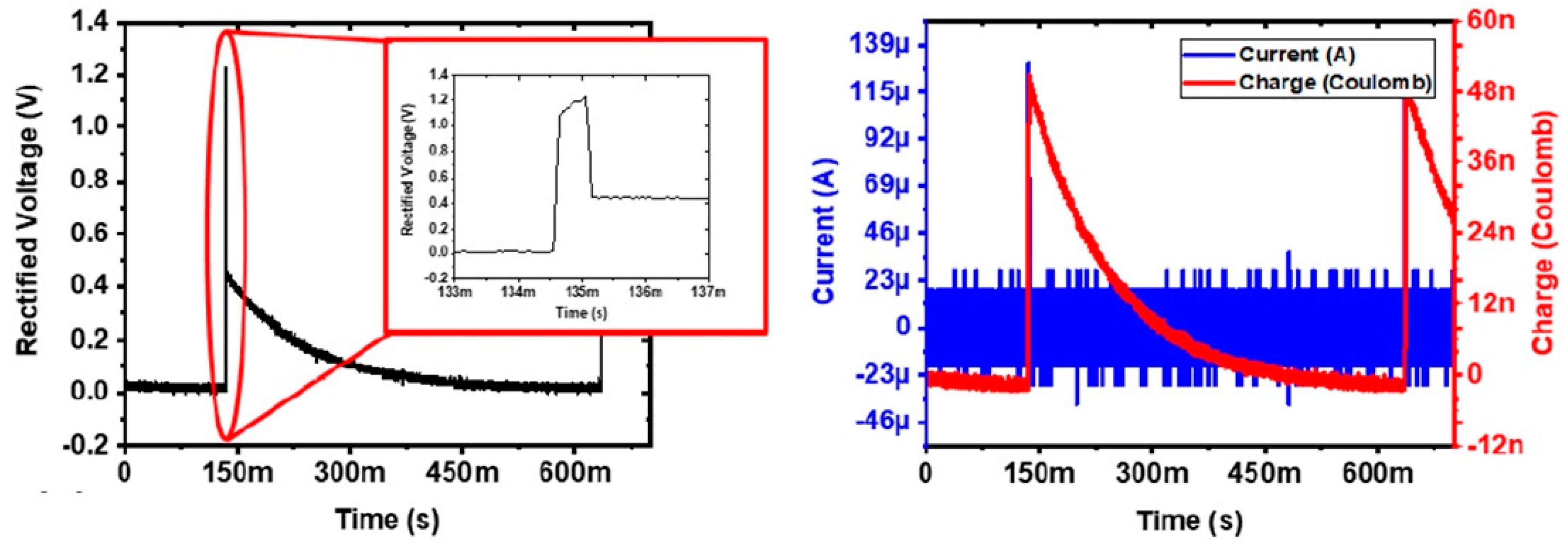
3.2. In Vivo Experimental Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Leung, V.; Lee, A.H.; Huang, J.; Asbeck, P.; Mercier, P.P.; Shellhammer, S.; Larson, L.; Laiwalla, F.; Nurmikko, A. Neural recording and stimulation using wireless networks of microimplants. Nat. Electron. 2021, 4, 604–614. [Google Scholar] [CrossRef]
- Yeon, P.; Mirbozorgi, S.A.; Ash, B.; Eckhardt, H.; Ghovanloo, M. Fabrication and microassembly of a mm-sized floating probe for a distributedwireless neural interface. Micromachines 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cortese, A.J.; Mok, A.; Wu, C.; Wang, T.; Park, J.U.; Smart, C.; Ghajari, S.; Khilwani, D.; Sadeghi, S.; et al. Fabrication of Injectable Micro-Scale Opto-Electronically Transduced Electrodes (MOTEs) for Physiological Monitoring. J. Microelectromechanical Syst. 2020, 29, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.C.; Shen, K.; Piech, D.; Ghanbari, M.M.; Li, K.Y.; Neely, R.; Carmena, J.M.; Maharbiz, M.M.; Muller, R. timDust: A 6.5mm3, wireless ultrasonic peripheral nerve stimulator with 82% peak chip efficiency. In Proceedings of the 2018 IEEE Custom Integrated Circuits Conference (CICC), San Diego, CA, USA, 8–11 April 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Khalifa, A.; Liu, Y.; Karimi, Y.; Wang, Q.; Eisape, A.; Stanaćević, M.; Thakor, N.; Bao, Z.; Etienne-Cummings, R. The Microbead: A 0.009 mm3 Implantable Wireless Neural Stimulator. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Lee, S.; Molnar, A.C.; Cash, S. Injectable wireless microdevices: Challenges and opportunities. Bioelectron. Med. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Lee, J.; Laiwalla, F.; Leung, V.; Huang, J.; Nurmikko, A.; Song, Y.K. A scalable and low stress post-cmos processing technique for implantable microsensors. Micromachines 2020, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Zhang, J.; Leistner, M.; Etienne-Cummings, R. A compact, low-power, fully analog implantable microstimulator. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Montreal, QC, Canada, 22–25 May 2016; pp. 2435–2438. [Google Scholar]
- Feng, P.; Yeon, P.; Cheng, Y.; Ghovanloo, M.; Constandinou, T.G. Chip-Scale Coils for Millimeter-Sized Bio-Implants. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Nasrollahpour, M.; Sun, N.; Zaeimbashi, M.; Chen, H.; Liang, X.; Alemohammad, M.; Etienne-Cummings, R.; Sun, N.X.; Cash, S. Magnetoelectric versus inductive power delivery for sub-mm receivers. In Proceedings of the IEEE Wireless Power Transfer Conference (WPTC), San Diego, CA, USA, 2–4 June 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Khalifa, A.; Karimi, Y.; Huang, Y.; Stanacevic, M.; Etienne-Cummings, R. The Challenges of Designing an Inductively Coupled Power Link for μm-sized On-Chip Coils. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Zargham, M.; Gulak, P.G. Maximum achievable efficiency in near-field coupled power-transfer systems. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, N.; Luthra, P.; Hagler, J.E.; Jae Lee, A.H.; Bodart, C.; Li, X.; Ducharme, G.; Soavi, F.; Amilhon, B.; Cicoira, F. Poly(3,4-ethylenedioxythiophene) (PEDOT) Coatings for High-Quality Electromyography Recording. ACS Appl. Bio Mater. 2019, 2, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Xue, N.; Cauller, L.; Rosellini, W.; Lee, J.-B. A SU-8-based fully integrated biocompatible inductively powered wireless neurostimulator. J. Microelectromechanical Syst. 2013, 22, 170–176. [Google Scholar] [CrossRef]
- Márton, G.; Tóth, E.Z.; Wittner, L.; Fiáth, R.; Pinke, D.; Orbán, G.; Meszéna, D.; Pál, I.; Győri, E.L.; Bereczki, Z.; et al. The neural tissue around SU-8 implants: A quantitative in vivo biocompatibility study. Mater. Sci. Eng. C 2020, 112, 110870. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Martin, D.C. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens. Actuators B Chem. 2003, 89, 92–102. [Google Scholar] [CrossRef]
- Charthad, J.; Chang, T.C.; Liu, Z.; Sawaby, A.; Weber, M.J.; Baker, S.; Gore, F.; Felt, S.A.; Arbabian, A. A mm-Sized wireless implantable device for electrical stimulation of peripheral nerves. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Piech, D.K.; Johnson, B.C.; Shen, K.; Ghanbari, M.M.; Li, K.Y.; Neely, R.M.; Kay, J.E.; Carmena, J.M.; Maharbiz, M.M.; Muller, R. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 2020, 4, 207–222. [Google Scholar] [CrossRef] [PubMed]


| References | [17] | [18] | [14] | This Work |
|---|---|---|---|---|
| CMOS process | 180 nm HV | 65 nm | N/A | 180 nm SOI |
| Wireless link | Ultrasound | Ultrasound | 2-coil inductive | 2-coil inductive |
| Electrode material | Pt | PEDOT | PtIr | PEDOT |
| Surface area (mm2) | 3.3 | 0.3 | 0.79 | 0.05 |
| Impedance (kΩ) | <1 | 4 | - | 2.2 |
| Encapsulation material | PDMS | Parylene | SU-8 | SU-8 |
| Animal model | Frog sciatic | Rat sciatic | Rat peroneal | Rat brain |
| Reliance on microfabrication techniques | None | Light | Heavy | None |
| Volume (mm3) | 39 | 1.7 | 1.39 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.; Nasrollahpour, M.; Nezaratizadeh, A.; Sha, X.; Stanaćević, M.; Sun, N.X.; Cash, S.S. Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants. Micromachines 2023, 14, 476. https://doi.org/10.3390/mi14020476
Khalifa A, Nasrollahpour M, Nezaratizadeh A, Sha X, Stanaćević M, Sun NX, Cash SS. Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants. Micromachines. 2023; 14(2):476. https://doi.org/10.3390/mi14020476
Chicago/Turabian StyleKhalifa, Adam, Mehdi Nasrollahpour, Ali Nezaratizadeh, Xiao Sha, Milutin Stanaćević, Nian X. Sun, and Sydney S. Cash. 2023. "Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants" Micromachines 14, no. 2: 476. https://doi.org/10.3390/mi14020476
APA StyleKhalifa, A., Nasrollahpour, M., Nezaratizadeh, A., Sha, X., Stanaćević, M., Sun, N. X., & Cash, S. S. (2023). Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants. Micromachines, 14(2), 476. https://doi.org/10.3390/mi14020476









