Multi-Channel MEMS-FAIMS Gas Sensor for VOCs Detection
Abstract
:1. Introduction
2. Sensor Principle and Sensor Design
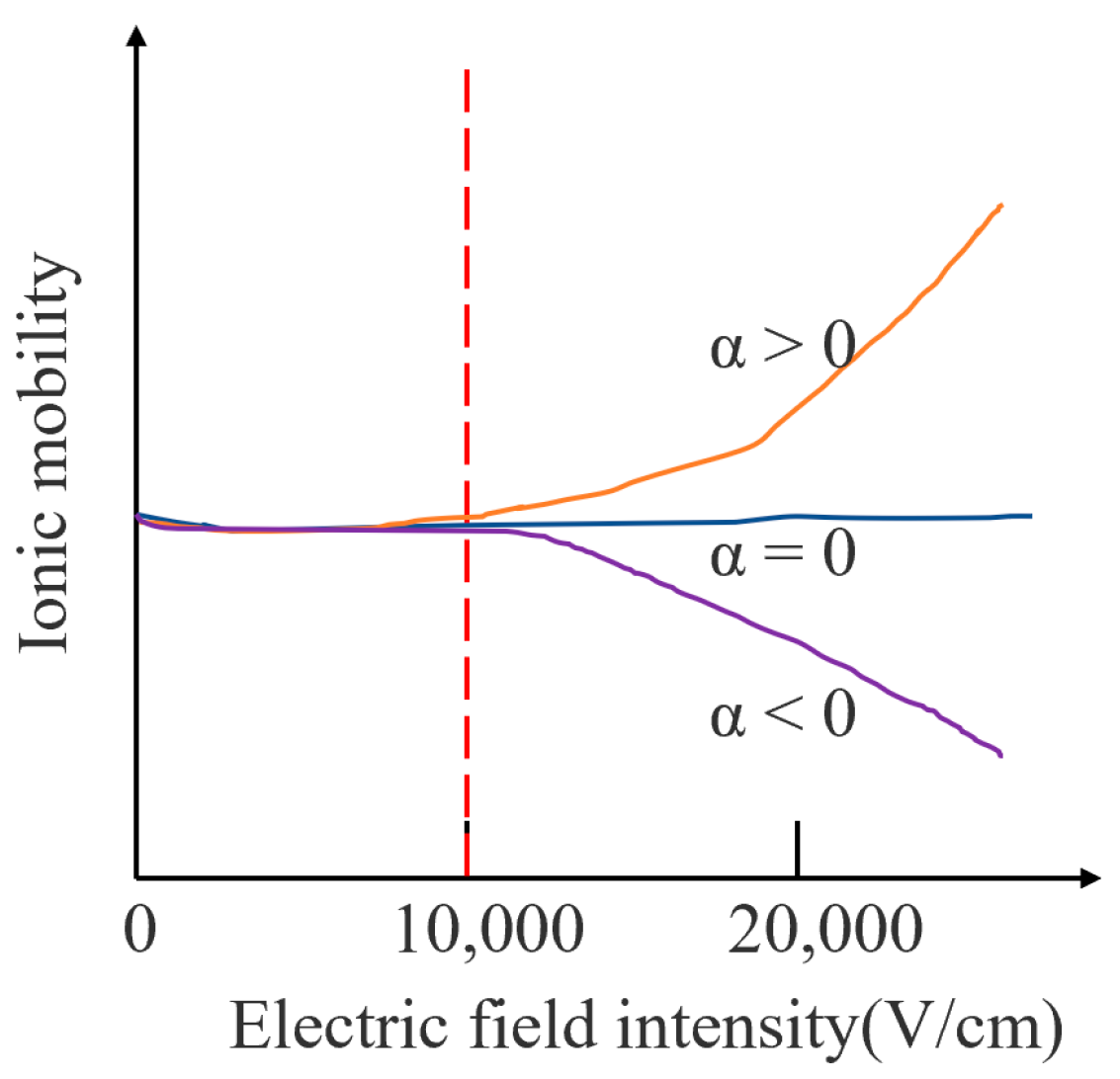
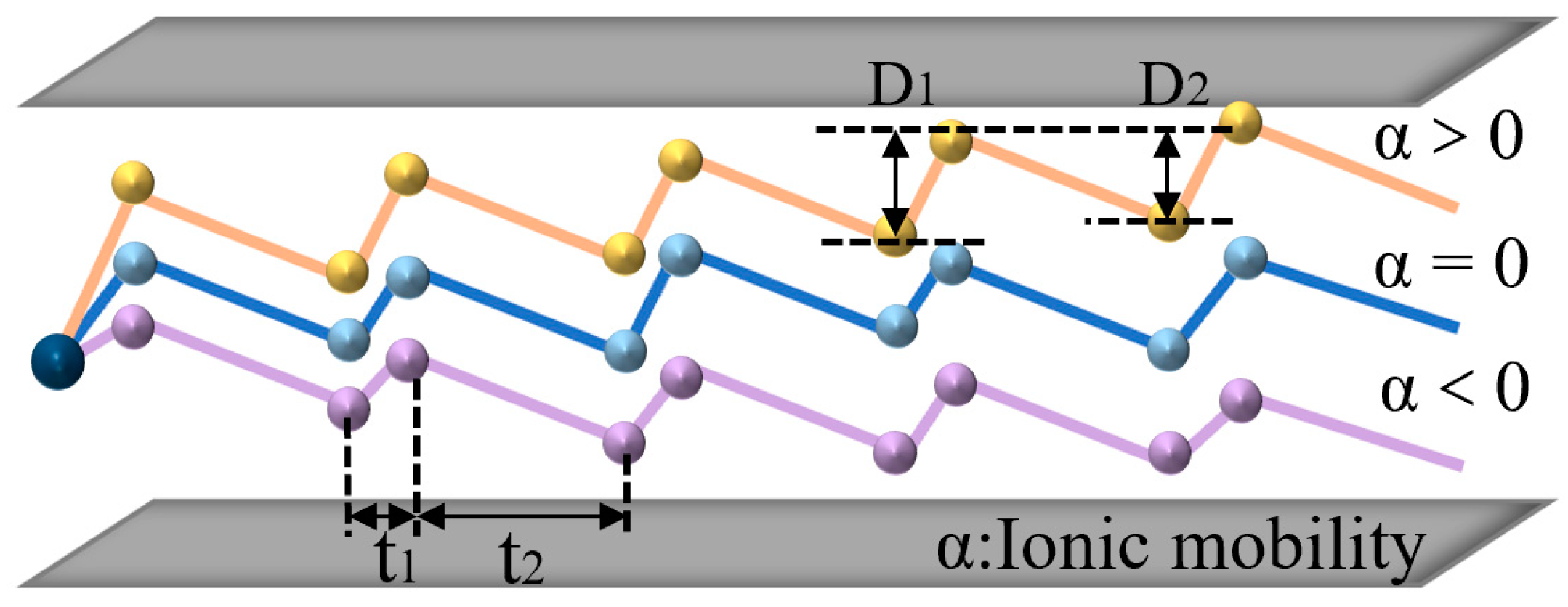
2.1. Effect of Electric Field on Ion Mobility
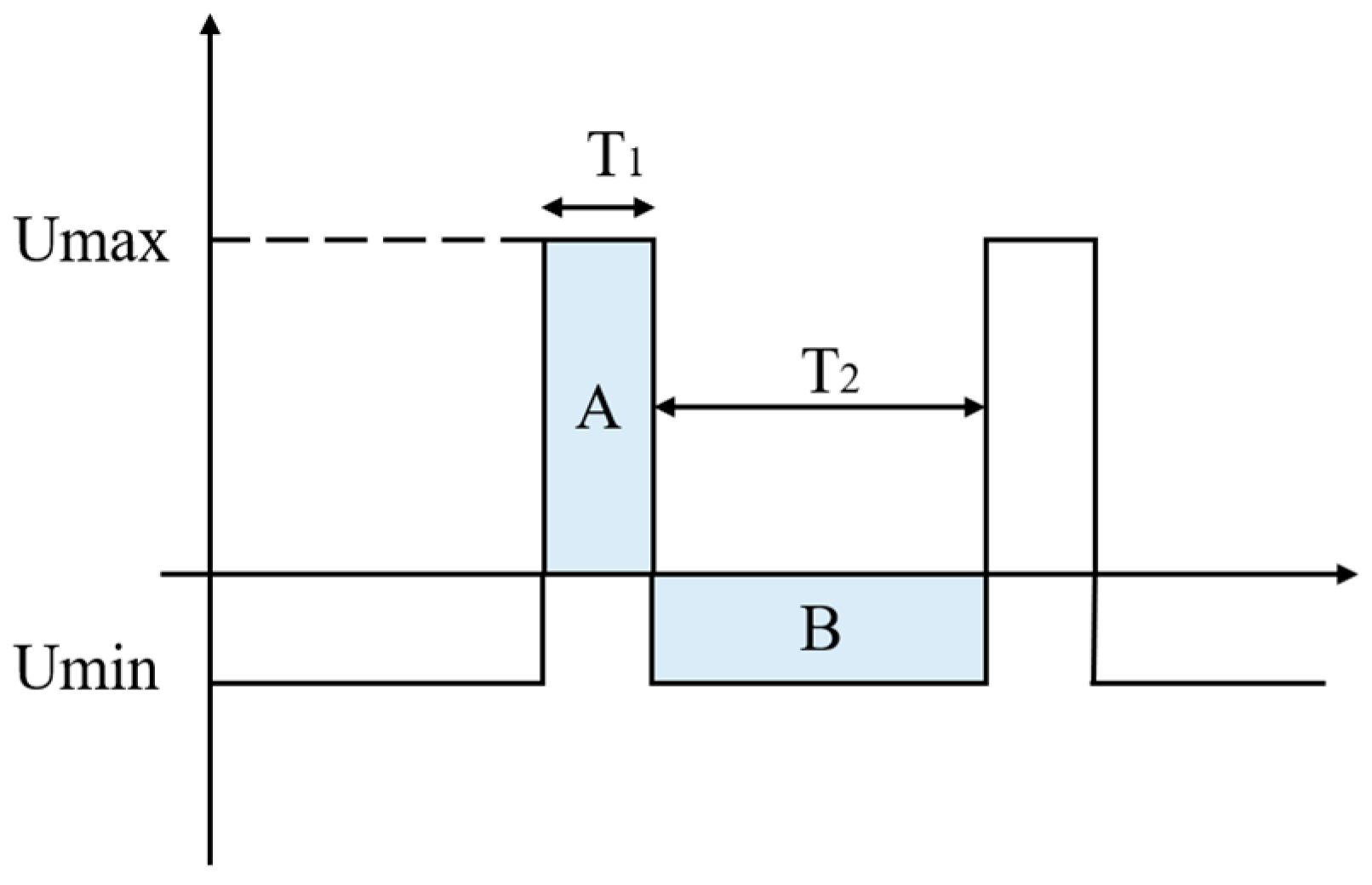
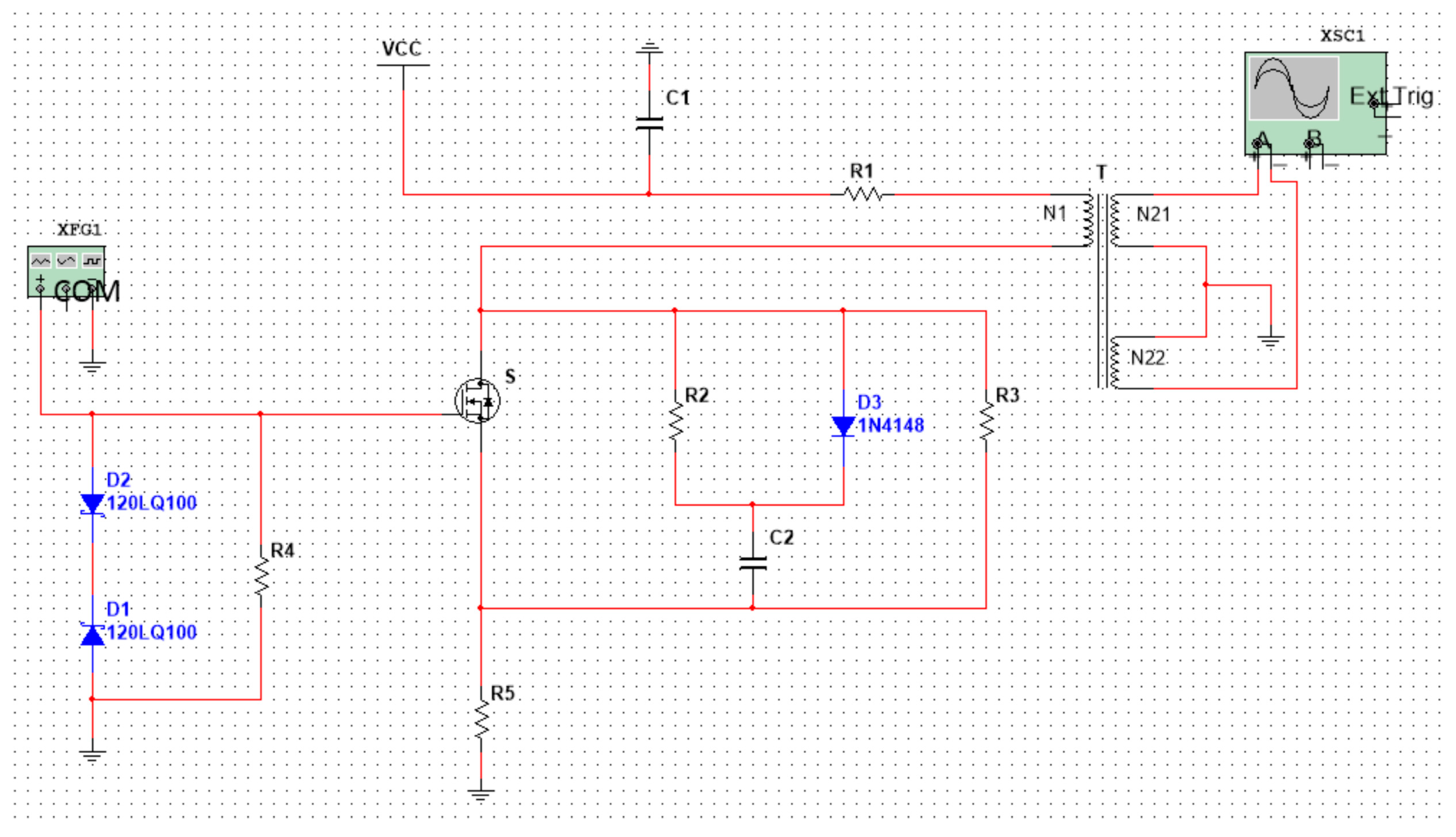
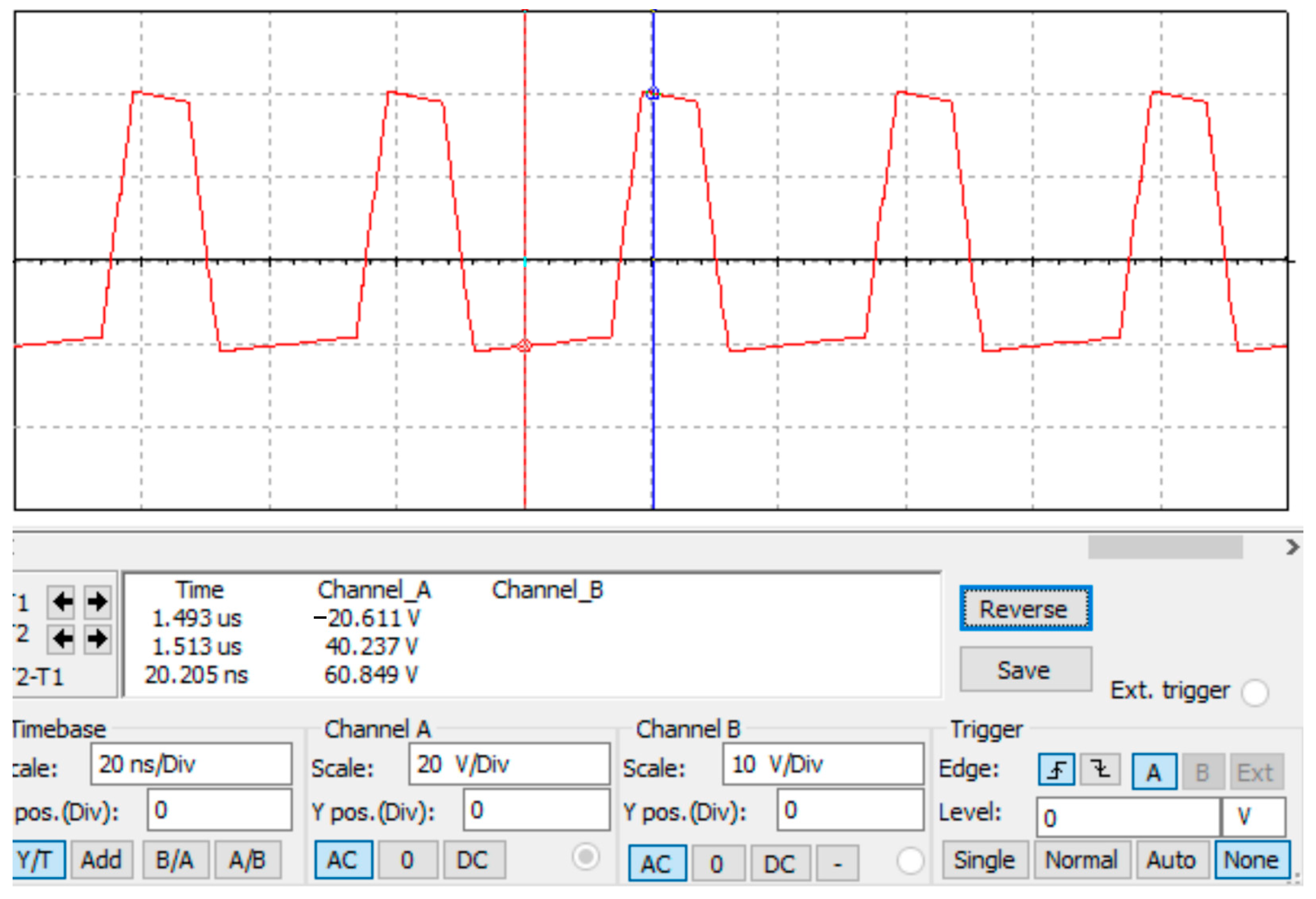
2.2. Circuit Design
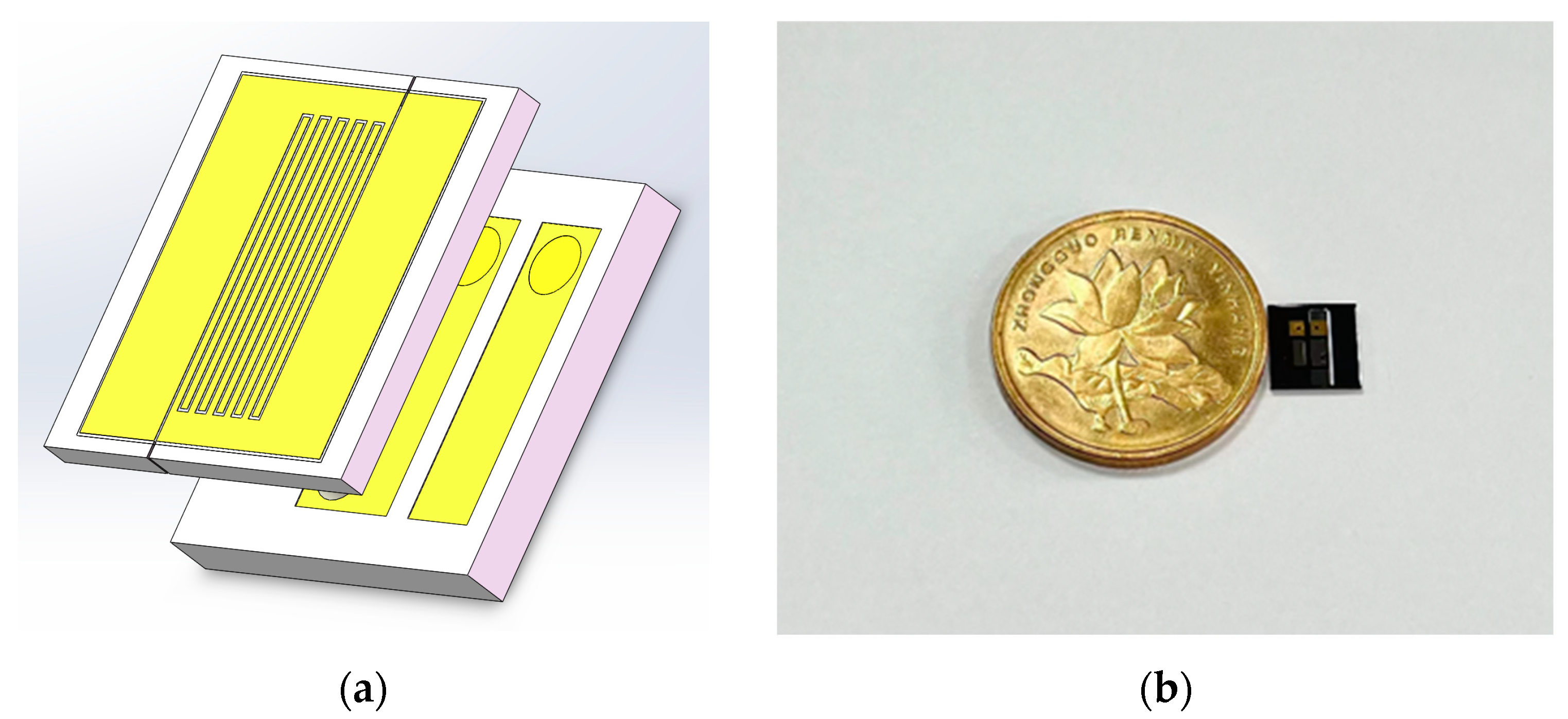
2.3. Manufacturing Steps of the Sensor
3. Results and Discussion
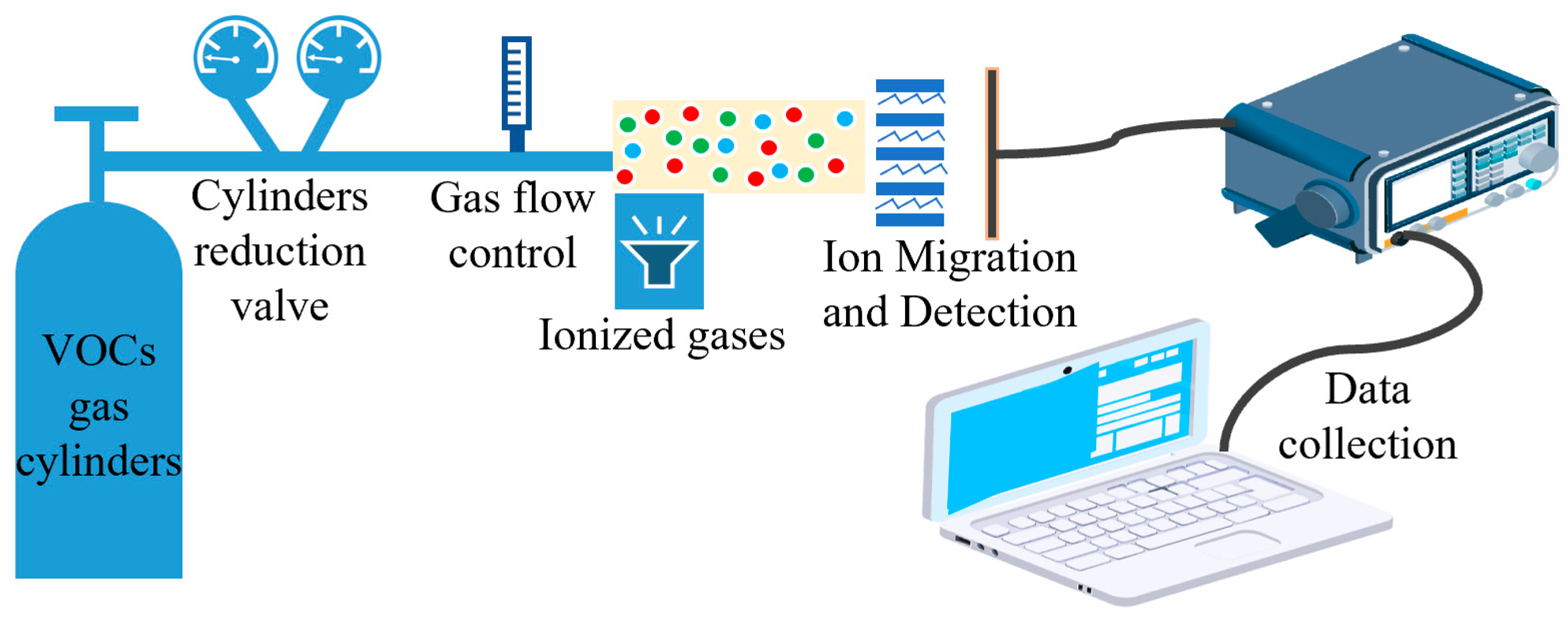
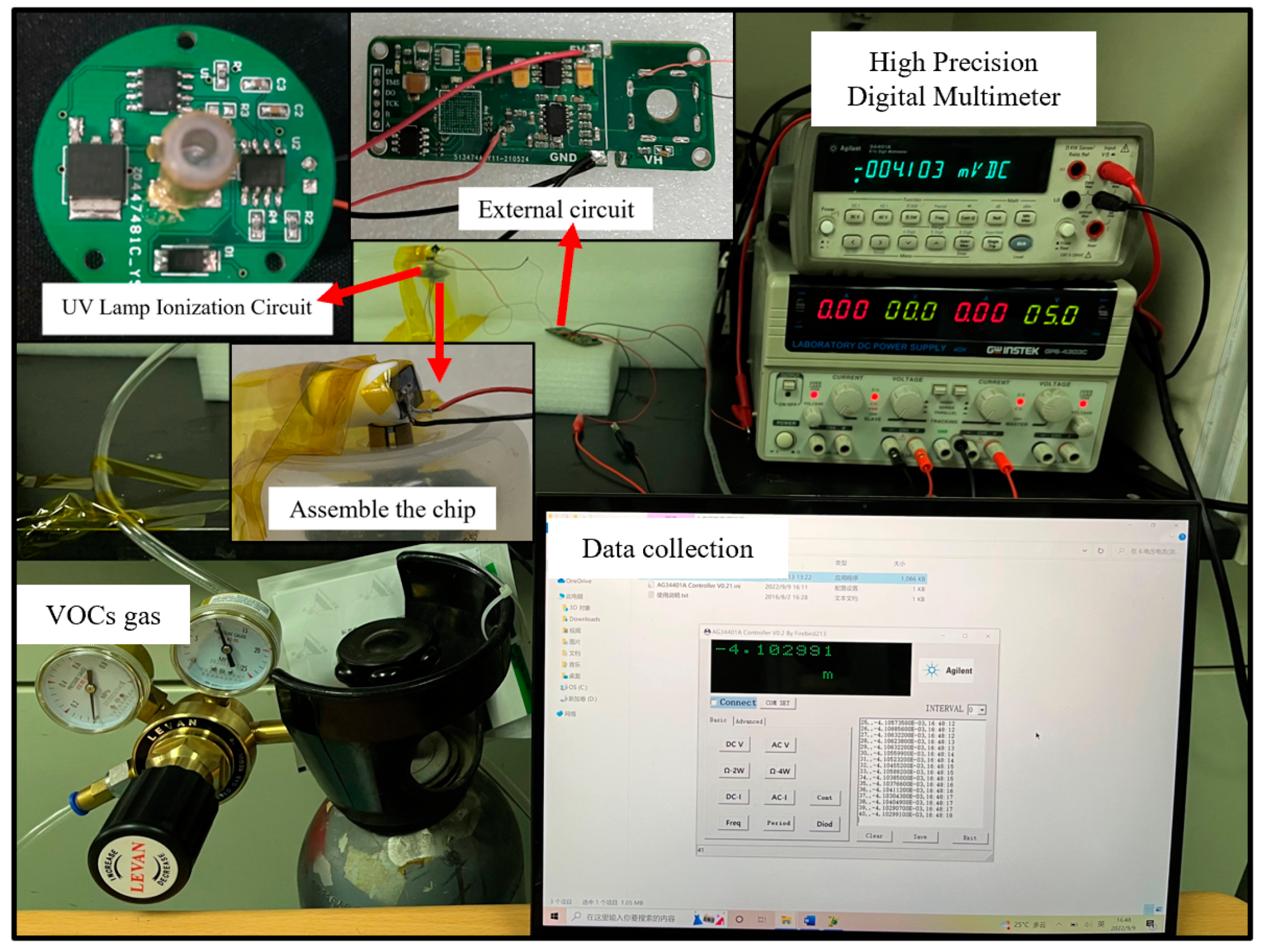
3.1. VOC Test System
3.2. Testing Method
3.2.1. Isobutylene Test
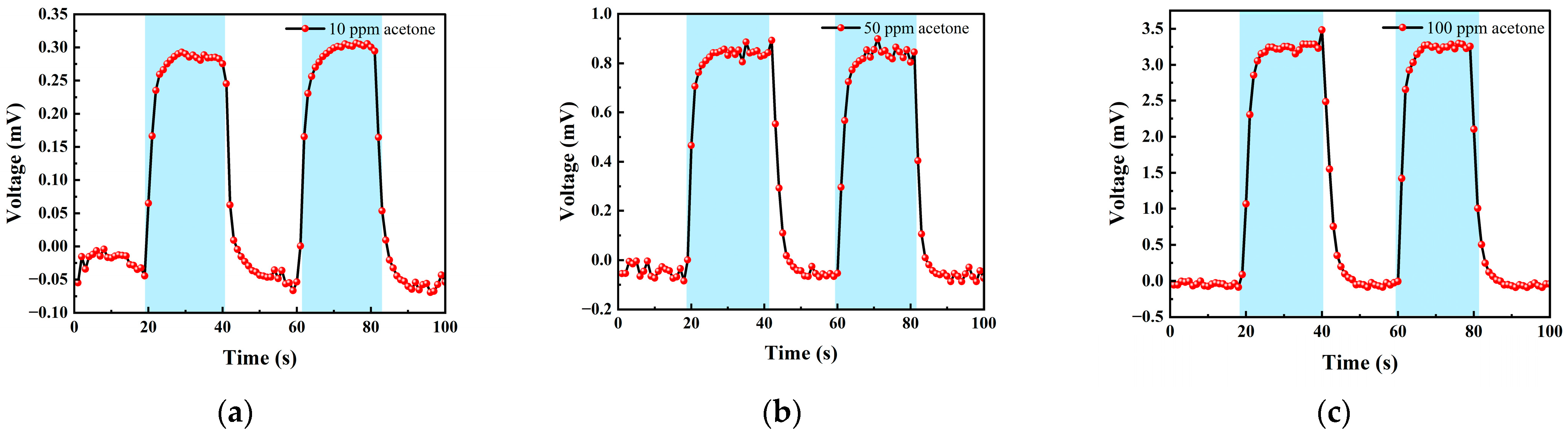
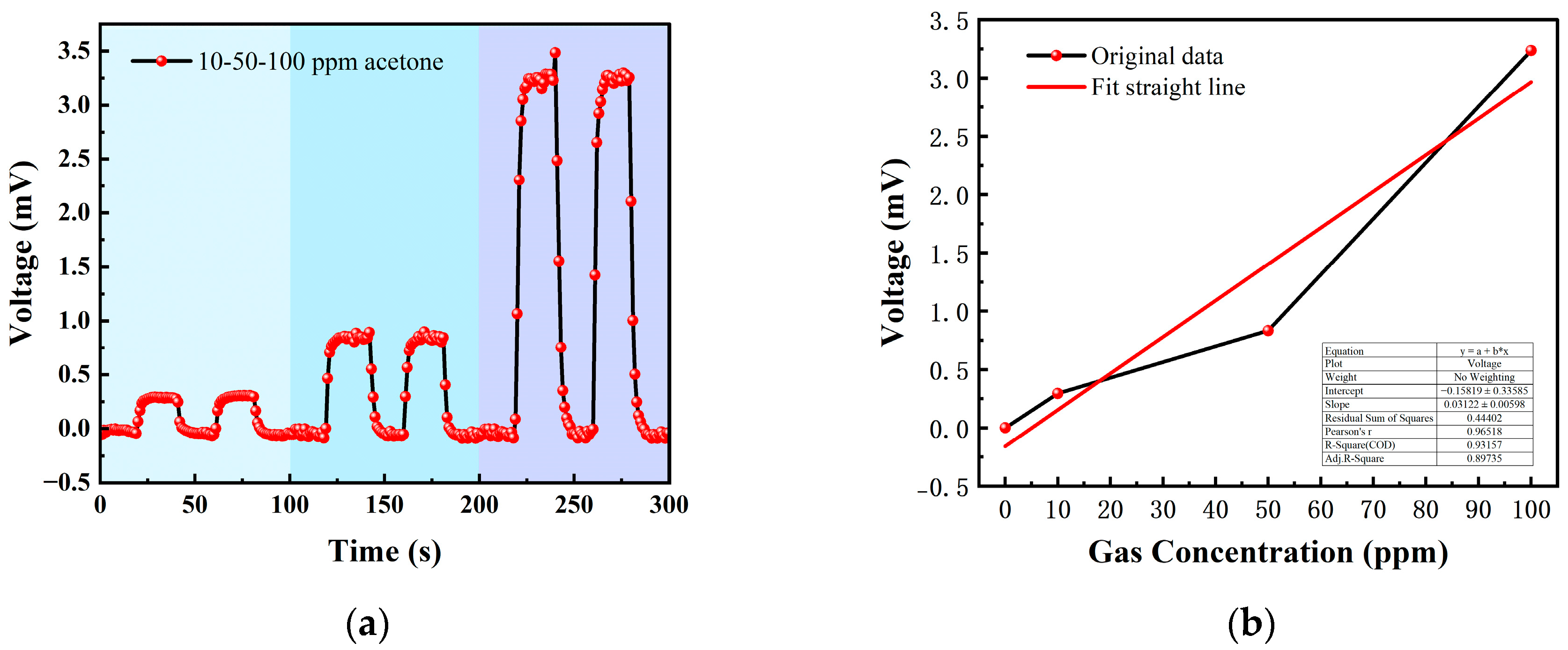
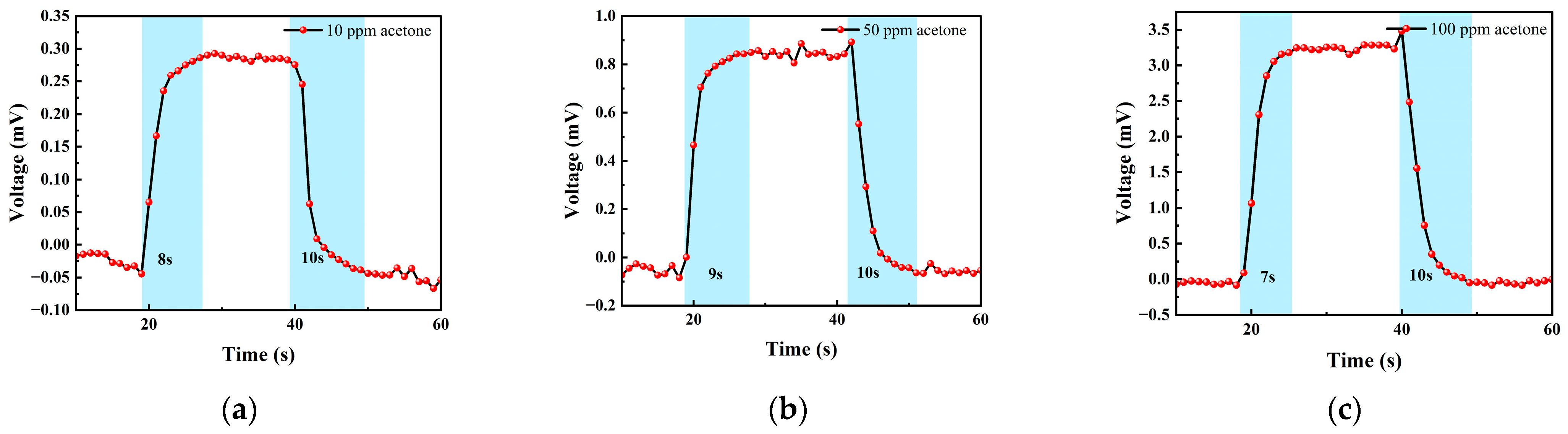
3.2.2. Acetone Test
3.2.3. Ar Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sverre, B.H.; Aileen, Y.; Merethe, L.; Kari, T.; Peter, S.; Mads, M. VOC emission rates in newly built and renovated buildings, and the influence of ventilation—A review and meta-analysis. Int. J. Vent. 2019, 18, 153–166. [Google Scholar]
- Liu, C. Analysis on the treatment of VOC pollution hazards. IOP Conf. Ser. Earth Environ. Sci. 2021, 791, 012201. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.H.; Wang, X.C.; Wang, J.X. Experimental research on alcohols, aldehydes, aromatic hydrocarbons and olefins emissions from alcohols fuelled vehicles. Huanjing Kexue 2013, 34, 2539–2545. [Google Scholar]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, A.; Pokorski, M.; Sartucci, F.; Domenici, L.; Giulio, C.D. Volatile organic compounds (VOCs) fingerprint of Alzheimer’s disease. Respir. Physiol. Neurobiol. 2015, 209, 81–84. [Google Scholar] [CrossRef]
- Tan, D.S. Prospect of VOCs-GC/MS Navigation Monitoring Technology Applied to Environmental Monitoring. Energy Conserv. Environ. Prot. 2019, 302, 98–99. [Google Scholar]
- Laurent, S.; Michel, G.; Gertjan, K.; Stefan, P.; Tilman, S. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar]
- Xu, Y.; Lou, C.; Zheng, L.; Zheng, W.; Liu, X.; Kumar, M.; Zhang, J. Highly sensitive and selective detection of acetone based on platinum sensitized porous tungsten oxide nanospheres. Sens. Actuators B 2020, 307, 127616. [Google Scholar] [CrossRef]
- Ding, Q.; Jianjun, L.; Zou, Z.; Sun, K.; Wang, Y.; He, D. Fluoride-assisted highly-active tungsten oxide with modulating exposed facets and defect sites for efficient ppb-level acetone detection. Appl. Surf. Sci. 2022, 584, 152554. [Google Scholar] [CrossRef]
- Lekshmi, M.S.; Arun, K.; Suja, K.J. A microcontroller-based signal conditioning circuitry for acetone concentration detection using a metal oxide-based gas sensor. J. Comput. Electron. 2022, 21, 1017–1025. [Google Scholar] [CrossRef]
- Gardner, J.W.; Wei, G.; Vincent, T.; Volans, K.; Tremlett, P.; Wotherspoon, T.; Dyer, D.C. A Gas Sensor System for Harsh Environment Applications. Procedia Eng. 2015, 120, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, M.; Lehmann, M. (Eds.) Solid State Gas Sensors–Industrial Application; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–12. [Google Scholar]
- Peng, F.M.; Xie, P.H.; Shi, Y.G.; Wang, J.D.; Liu, W.Q.; Li, H.Y. Photoionization Detector for Portable Rapid GC. Chromatographia 2007, 65, 331–336. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, H.; Zhang, Y.J.; Bi, F.; Meng, L.S.; Zhang, X.M.; Mao, J.Y.; Cheng, N.L.; Fang, B.; Yang, Y.; et al. Fast Determination of Monocyclic Aromatic Hydrocarbons in Ambient Air Using a Portable Gas Chromatography–Photoionization Detector. Chromatographia 2017, 80, 1233–1247. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, P.; Harrington, P.B. Comparison of differential mobility spectrometry and mass spectrometry for gas chromatographic detection of ignitable liquids from fire debris using projected difference resolution. Anal. Bioanal. Chem. 2009, 394, 2061–2067. [Google Scholar] [CrossRef]
- Wu, K.Y. Principle and Classification of Gas Chromatography Mass Spectrometer. Sci. Technol. Vis. 2014, 26, 270. [Google Scholar]
- Saifur, R.; Abdullah, S.A.; Muhammed, I.; Rabia, N.; Mohsin, R.; Ehtasham, J.; Muhammad, A. Wireless E-Nose Sensors to Detect Volatile Organic Gases through Multivariate Analysis. Micromachines 2020, 11, 597. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.C.; Chou, T.I.; Tang, K.T. A Gas Mixture Prediction Model Based on the Dynamic Response of a Metal-Oxide Sensor. Micromachines 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timothy, S.B.W.; Roger, N. Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Network. Sensors 2020, 20, 2851. [Google Scholar]
- Ihokure, K.; Watson, J. The Stannic Oxide Gas Sensor Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Mishra, R.K.; Choi, G.; Choi, H.; Gwag, J. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, L.; Yang, C.; Liu, X.; Zhang, J. Highly sensitive and selective electronic sensor based on Co catalyzed SnO2 nanospheres for acetone detection. Sens. Actuators B 2020, 304, 127237. [Google Scholar] [CrossRef]
- Wang, J.P.; Peng, B.Y.; Wu, W.; Gong, Y.D. Synthesis of W-doped Cr2O3 thin films and their application in isobutylene sensing. Acta Mater. Compos. Sin. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhao, C.H.; Lan, W.Z.; Gong, H.M.; Bai, J.L.; Ramachandran, R.; Liu, S.; Wang, F. Highly sensitive acetone-sensing properties of Pt-decorated CuFe2O4 nanotubes prepared by electrospinning. Ceram. Int. 2018, 44, 2856–2863. [Google Scholar] [CrossRef]
- Gong, H.M.; Zhao, C.H.; Niu, G.Q.; Zhang, W.; Wang, F. Construction of 1D/2D α -Fe2O3/SnO2 Hybrid Nanoarrays for Sub-ppm Acetone Detection. Research 2020, 2020, 2196063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, E.S.; Raanan, A.M. Application of mobility theory to the interpretation of data generated by linear and RF excited ion mobility spectrometers. Int. J. Mass Spectrom. 2002, 214, 95–104. [Google Scholar]
- Carr, K.K.M. Detection of Contaminants Using a MEMS FAIMS Sensor. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005. [Google Scholar]
- Miller, R.A.; Nazarov, E.G.; Eiceman, G.A.; King, A.T. A MEMS radio-frequency ion mobility spectrometer for chemical vapor detection. Sens. Actuators A. Phys. 2001, 91, 301–312. [Google Scholar] [CrossRef]
- Hua, L.; Hongmei, Y.; Xiaoxia, D.; Chaoqun, G.; Ruosheng, Z.; Yongrong, J.; Zhencheng, C. Design, Fabrication and Mass-spectrometric Studies of a Micro Ion Source for High-Field Asymmetric Waveform Ion Mobility Spectrometry. Micromachines 2019, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Kao, K.W.; Hsu, M.C.; Chang, Y.H.; Gwo, S.; Yeh, J.A. A Sub-ppm Acetone Gas Sensor for Diabetes Detection Using 10 nm Thick Ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroy, J.G.; Gonzalez-Jimenez, J. Gas classification in motion: An experimental analysis. Sens. Actuators B Chem. 2017, 240, 1205–1215. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Li, K.; Chen, C. Rapid Identification and On-Site Detection of Different Labeled Gasoline by the Integrated MEMS-FAIMS. Micronanoelectron. Technol. 2019, 56, 745–753. [Google Scholar]
- Yokoshiki, Y.; Nakamoto, T. Ternary Gas Mixture Quantification Using Field Asymmetric Ion Mobility Spectrometry (FAIMS). Sensors 2019, 19, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.N.; Lei, C.; Liang, T.; Liu, R.F.; Zhao, Z.J.; Qi, L.; Ghaffar, A.; Xiong, J.J. Acetone Sensor Based on FAIMS-MEMS. Micromachines 2021, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Ortiz, M.; Sankaran, S. Development of a semi-automated volatile organic compounds (VOCs) sampling system for field asymmetric ion mobility spectrometry (FAIMS) analysis. HardwareX 2022, 12, e00344. [Google Scholar] [CrossRef]
- Zheng, P.C.; Xu, G.J.; He, Y.T.; Lu, J.S.; Wang, J.M. Design of portable photoionization VOCs gas detector. Instrum. Tech. Sens. 2021, 467, 45–50. [Google Scholar]
- Zhang, C.Y.; Lei, C.; Liang, T.; Liu, R.F.; Feng, W.; Shan, C.L.; Xue, S.F. Preparation and testing of a miniaturized FAIMS gas sensor. Micronanoelectron. Technol. 2021, 58, 219–224. [Google Scholar] [CrossRef]
- Kakati, N.; Jee, S.H.; Kim, S.H.; Oh, J.Y.; Yoon, Y.S. Thickness dependency of sol-gel derived ZnO thin films on gas sensing behaviors. Thin Solid Film. 2010, 519, 494–498. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, K.H.; Chang, Y.M.; Yeh, J.A. Epitaxy of m-plane GaN on nanoscale patterned c-plane sapphire substrates. Surf. Sci. 2012, 606, L1–L4. [Google Scholar] [CrossRef]


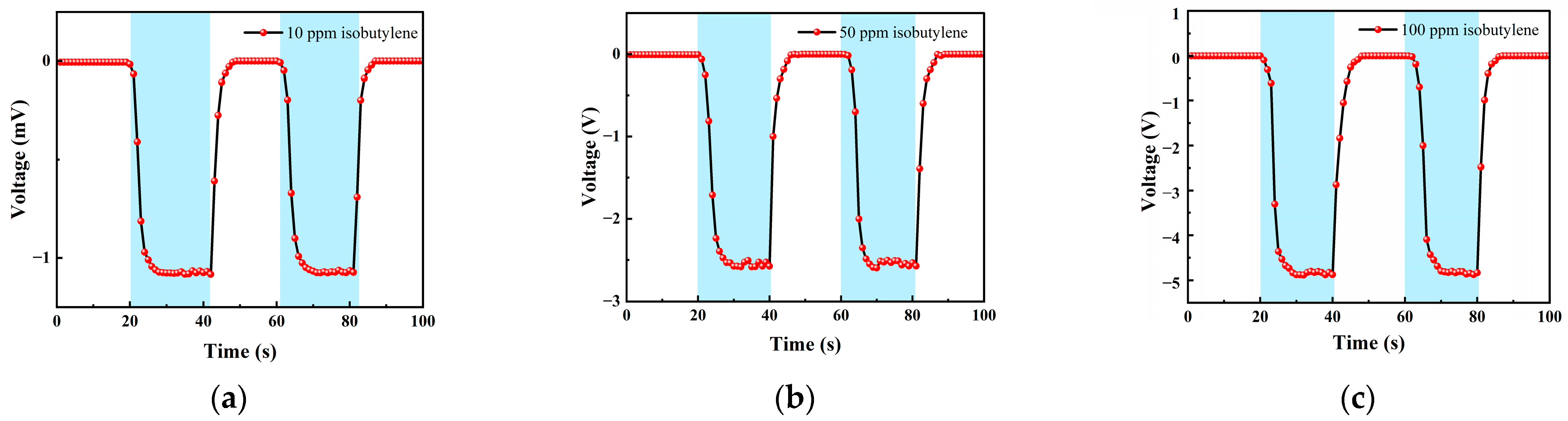

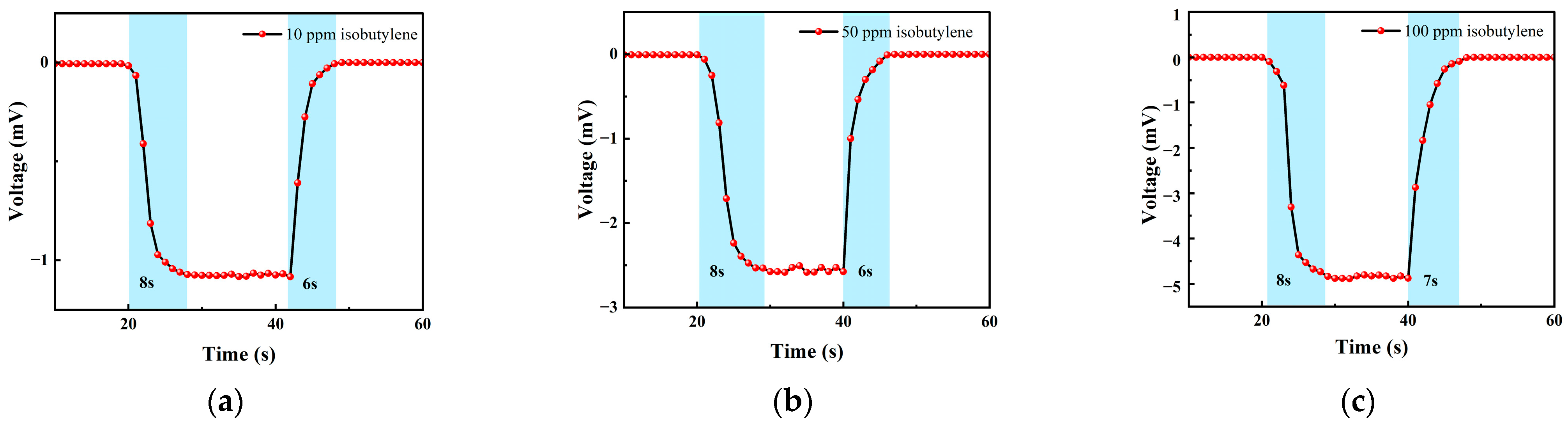

| 0–20 s | 21–40 s | 41–60 s | 61–80 s | 80–100 s |
|---|---|---|---|---|
| Ar | 10 ppm isobutylene | Ar | 10 ppm isobutylene | Ar |
| Ar | 50 ppm isobutylene | Ar | 50 ppm isobutylene | Ar |
| Ar | 100 ppm isobutylene | Ar | 100 ppm isobutylene | Ar |
| 0–20 s | 21–40 s | 41–60 s | 61–80 s | 80–100 s |
|---|---|---|---|---|
| Ar | 10 ppm acetone | Ar | 10 ppm acetone | Ar |
| Ar | 50 ppm acetone | Ar | 50 ppm acetone | Ar |
| Ar | 100 ppm acetone | Ar | 100 ppm acetone | Ar |
| Material | Type | Sensitivity | Detection of Gas Types | Detection Limit (ppm) | Operating Temperature (°C) | References |
|---|---|---|---|---|---|---|
| UV lamp | PID | / | isobutylene | 2.2 | / | [38] |
| Si: BF33 | FAIMS | 1 × 10−7/mV | isobutylene | 10 | RT | [39] |
| ZnO | Metal oxide | 5.71% | acetone | 100 | 200 | [40] |
| GaN | Metal nitride | 23% | acetone | 500 | 350 | [41] |
| Si: BF33 | FAIMS | 4.876 mV/ppm | isobutylene | 10 | RT | This work |
| Si: BF33 | FAIMS | 3.324 mV/ppm | acetone | 10 | RT | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Lei, C.; Liang, T.; Zhang, J.; Liu, Y.; Ghaffar, A.; Xiong, J. Multi-Channel MEMS-FAIMS Gas Sensor for VOCs Detection. Micromachines 2023, 14, 608. https://doi.org/10.3390/mi14030608
Zhao Z, Lei C, Liang T, Zhang J, Liu Y, Ghaffar A, Xiong J. Multi-Channel MEMS-FAIMS Gas Sensor for VOCs Detection. Micromachines. 2023; 14(3):608. https://doi.org/10.3390/mi14030608
Chicago/Turabian StyleZhao, Zhujie, Cheng Lei, Ting Liang, Junna Zhang, Yuqiao Liu, Abdul Ghaffar, and Jijun Xiong. 2023. "Multi-Channel MEMS-FAIMS Gas Sensor for VOCs Detection" Micromachines 14, no. 3: 608. https://doi.org/10.3390/mi14030608






