Analysis of Degradation of Electromigration Reliability of Au-Al and OPM Wire Bonded Contacts at 250 °C Using Resistance Monitoring Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Test Sample and Protocol
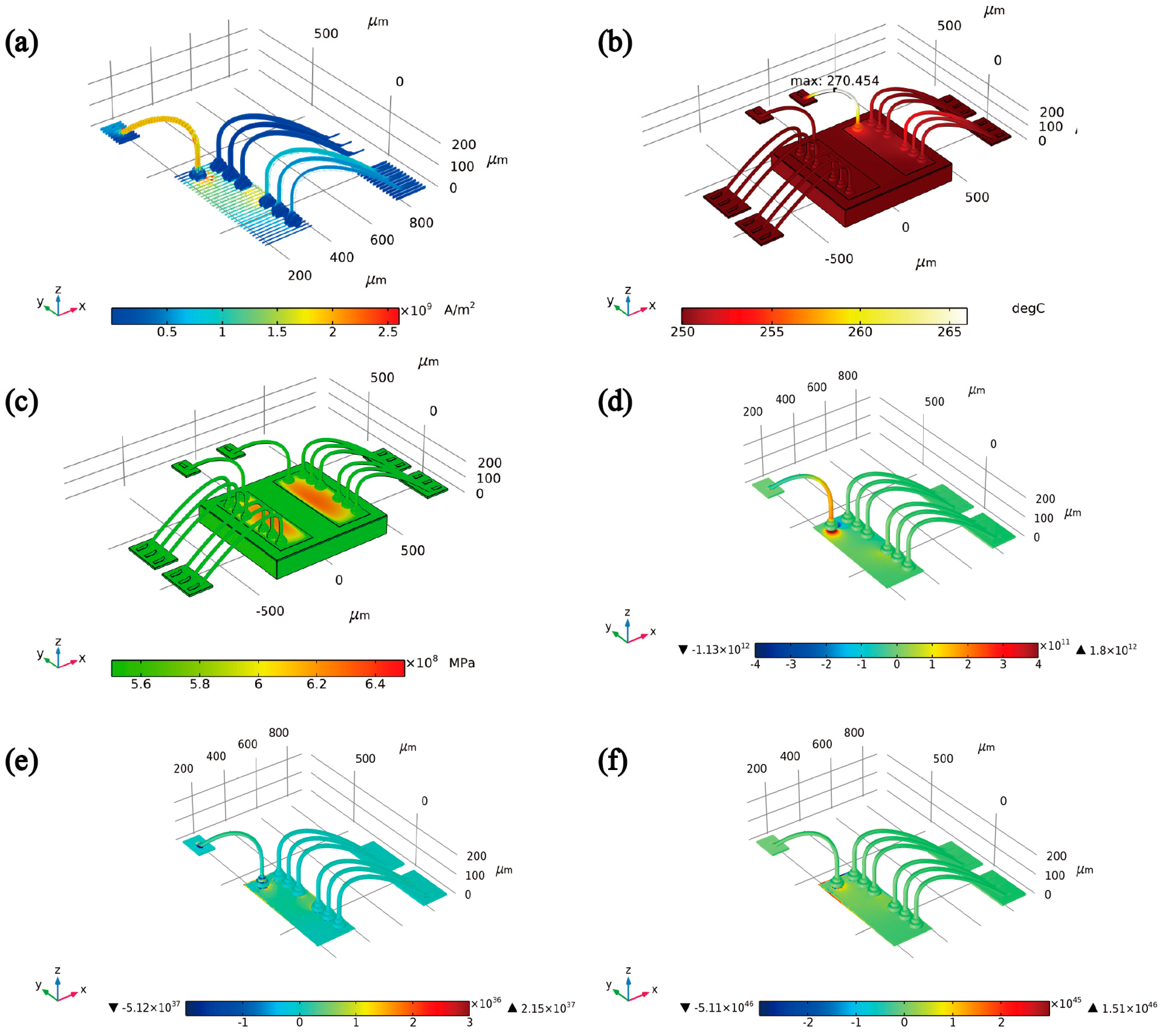
2.2. Principles of Finite Element Simulation
3. Results
3.1. Effect of Current Parameters on the Reliability of Au-Al Bonding
3.2. Electromigration Testing of Au-Al and OPM Structures
3.3. Microscopic Characterization
3.4. Finite Element Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Passagrilli, C.; Gobbato, L.; Tiziani, R. Reliability of Au/Al bonding in plastic packages for high temperature (200 °C) and high current applications. Microelectron. Reliab. 2002, 42, 1523–1528. [Google Scholar] [CrossRef]
- Johal, K.; Lamprecht, S.; Roberts, H. Electroless Nickel/Electroless Palladium/Immersion Gold Plating Process for Gold-and Aluminum-Wire Bonding Designed for High-Temperature Applications; Asia Pacific Press: Canberra, Australia, 2002. [Google Scholar]
- Mustain, H.A.; Brown, W.D.; Lostetter, A.B. Evaluation of gold and aluminum wire bond performance for high temperature (500 °C) silicon carbide (SiC) power modules. In Proceedings of the Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 31 May–3 June 2005. [Google Scholar]
- Li, F. 3-D Stacking of SiC Integrated Circuit Chips with Gold Wire Bonded Interconnects for Long-Duration High-Temperature Applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 1601–1608. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Li, M.; Zhang, H. High Quality Pt-Pt Metal Bonding for High Temperature Packaging. Micromachines 2022, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Cao, J.; Su, C.; Li, C.; Chang, A.; An, B. Research Progress on Bonding Wire for Microelectronic Packaging. Micromachines 2023, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; Acoff, V.L. New mechanisms of void growth in Au-Al wire bonds: Volumetric shrinkage and intermetallic oxidation. Scr. Mater. 2011, 65, 642–645. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z.; Acoff, V.L. Intermetallic phase transformations in AueAl wire bonds. Intermetallics 2011, 19, 1808–1816. [Google Scholar] [CrossRef]
- Zin, E.; Michael, N.; Kang, S.H.; Oh, K.H.; Kim, C.U. Mechanism of Electromigration in Au/Al Wirebond and its Effects. In Proceedings of the 2009 59th Electronic Components and Technology Conference, San Diego, CA, USA, 26–29 May 2009; pp. 943–947. [Google Scholar]
- Hook, M.; Di, X.; Mayer, M. Electromigration testing of wire bondsIn Proceedings of the IEEE Canadian Conference on Electrical & Computer Engineering, Toronto, ON, Canada, 4–7 May 2014.
- Hsu, T.Y.; Chang, J.Y.; Leu, F.J.; Chang, H.M.; Ouyang, F.Y. Ag alloy wire bonding under electromigration test. In Proceedings of the International Conference on Electronics Packaging and iMAPS All Asia Conference, Kyoto, Japan, 14–17 April 2015. [Google Scholar]
- Hang, C.J.; Wang, C.Q.; Mayer, M.; Tian, Y.H.; Zhou, Y.; Wang, H.H. Growth behavior of Cu/Al intermetallic compounds and cracks in copper ball bonds during isothermal aging. Microelectron. Reliab. 2008, 48, 416–424. [Google Scholar] [CrossRef]
- Gam, S.A.; Kim, H.J.; Cho, J.S.; Park, Y.J.; Paik, K.W. Effects of Cu and Pd addition on Au bonding wire/Al pad interfacial reactions and bond reliability. J. Electron. Mater. 2006, 35, 2048–2055. [Google Scholar] [CrossRef]
- Hai, L.; Qi, C.; Zhao, Z.; Qian, W.; Lee, J. Reliability of Au-Ag Alloy Wire Bonding. In Proceedings of the Electronic Components & Technology Conference, Las Vegas, NV, USA, 1–4 June 2010; pp. 234–239. [Google Scholar]
- Ng, B.; Ganesh, V.P.; Lee, C. Impact of Electroless Nickel/Palladium/Immersion Gold Plating on Gold Ball Bond Reliability. In Proceedings of the International Conference on Electronic Materials & Packaging, Hong Kong, China, 11–14 December 2006. [Google Scholar]
- Wu, C.; Wang, J.; Liu, X.; Li, M.; Zhu, Z.; Qi, Y. Au Wire Ball Welding and Its Reliability Test for High-Temperature Environment. Micromachines 2022, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Mavinkurve, A.; Goumans, L.; O’Halloran, G.M.; Rongen, R.T.H.; Farrugia, M.L. Copper wire interconnect reliability evaluation using in-situ High Temperature Storage Life (HTSL) tests. Microelectron. Reliab. 2014, 54, 1661–1665. [Google Scholar] [CrossRef]
- Lin, M.; Basaran, C. Electromigration induced stress analysis using fully coupled mechanical–diffusion equations with nonlinear material properties. Comput. Mater. Sci. 2005, 34, 82–98. [Google Scholar] [CrossRef]
- Basaran, C. Introduction to Unified Mechanics Theory with Applications; Springer: Berlin, Germany, 2021. [Google Scholar]
- Lin, Q.; Zhao, P.-f.; Wu, H.-F.; Chen, S.-w. Interconnect reliability investigation for a high power GaN MMIC PA based on APDL. Int. J. RF Microw. Comput.-Aided Eng. 2022, 32, e23552. [Google Scholar] [CrossRef]
- Dalleau, D.; Weide-Zaage, K.; Danto, Y. Simulation of time depending void formation in copper, aluminum and tungsten plugged via structures. Microelectron. Reliab. 2003, 43, 1821–1826. [Google Scholar] [CrossRef]
- Basaran, C.; Lin, M.; Hua, Y. A thermodynamic model for electrical current induced damage. Int. J. Solids Struct. 2003, 40, 7315–7327. [Google Scholar] [CrossRef]
- Ng, B.T.; Ganesh, V.P.; Lee, C. Optimization of gold wire bonding on electroless nickel immersion gold for high temperature applications. In Proceedings of the Electronics Packaging Technology Conference, Singapore, 6–8 December 2006. [Google Scholar]
- Chen, H.; Chen, C.; Zhou, Y.; Yang, C.; Song, G.; Hou, F.; Jiao, B.; Liu, R. Evaluation and Optimization of a Cross-Rib Micro-Channel Heat Sink. Micromachines 2022, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tan, C.M. Circuit level interconnect reliability study using 3D circuit model. Microelectron. Reliab. 2010, 50, 376–390. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Y.; Rioux, M.; Zhang, Y.; Liang, L. Electromigration prediction and test for 0.18μm power technology in wafer level reliability. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar]
- Yong, L.; Liang, L.; Irving, S.; Luk, T. 3D Modeling of electromigration combined with thermal–mechanical effect for IC device and package. Microelectron. Reliab. 2008, 48, 811–824. [Google Scholar]
- Chan, Y.C.; Yang, D. Failure mechanisms of solder interconnects under current stressing in advanced electronic packages. Prog. Mater. Sci. 2010, 55, 428–475. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Liang, L.; Yong, L. Investigation for electromigration-induced hillock in a wafer level interconnect device. In Proceedings of the Electronic Components & Technology Conference, Las Vegas, NV, USA, 1–4 June 2010; pp. 617–624. [Google Scholar]
- Neumann, G.; Tuijn, C. Self-diffusion and Impurity Diffusion in Pure Metals-Handbook of Experimental Data; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Li, W.; Kou, H.; Zhang, X.; Ma, J.; Fang, D. Temperature-dependent elastic modulus model for metallic bulk materials. Mech. Mater. 2019, 139, 103194. [Google Scholar] [CrossRef]
- Belko, V.; Glivenko, D.; Emelyanov, O.; Ivanov, I.; Plotnikov, A. Current Pulse Polarity Effect on Metallized Film Capacitors Failure. IEEE Trans. Plasma Sci. 2017, 45, 1020–1025. [Google Scholar] [CrossRef]
- Son, K.; Ryu, H.; Kim, G.; Lee, J.; Park, Y.B. Electromigration polarity effect of Cu/Ni/Sn-Ag microbumps for three-dimensional integrated circuits. In Proceedings of the 2017 IEEE 19th Electronics Packaging Technology Conference (EPTC), Singapore, 6–9 December 2017. [Google Scholar]
- Li, J.; Zhao, Z.; Lee, J. Wire bonding performance and solder joint reliability investigation on ENEPIG finish substrate. In Proceedings of the 2010 11th International Conference on Electronic Packaging Technology & High Density Packaging, Xi’an, China, 16–19 August 2010. [Google Scholar]
- Qu, S.; Pham, K.; Nguyen, L.; Prabhu, A.; Poddar, A.; Athavale, S.; Xu, A. Electroless Over Pad Metallization for High Temperature Interconnections. In Proceedings of the 2010 IEMT Conference, Melaka, Malaysia, 30 November–2 December 2010. [Google Scholar]
- Shi, L.; Qian, W.; Yu, C.; Lin, T.; Yang, H. Performance and reliability study of Pd-coated copper wire bonding on ENEPIG substrate. In Proceedings of the 2013 14th International Conference on Electronic Packaging Technology, Dalian, China, 11–14 August 2013. [Google Scholar]












| Materials | Sample | Thickness | Temperature | Current | Time |
|---|---|---|---|---|---|
| Al | S1 | 250 °C | 0.6 A | 165 h | |
| NiPdAu | S2 | 200/100/50 nm | 250 °C | 0.6 A | 165 h |
| CrAu | S3 | 5/50 nm | 250 °C | 0.6 A | 165 h |
| Parameter | Unit | Value | |
|---|---|---|---|
| Au | Al [29] | ||
| 0.785 | 0.87 | ||
| 3.7 | −4 | ||
| [30] | |||
| 176.9 [30] | −0.0867 | ||
| Material | Elastic Modulus (GPa) [31] | Poisson’s Ration | Thermal Conductivity (W·m−1·K−1) | Resistivity (10−8·Ω·m) | Thermal Expansion (K−1) [29] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 K | 300 K | 400 K | 500 K | 600 K | 700 K | |||||
| Al | 0.35 | 240.0 | 20.3 | 23.23 | 25.20 | 26.4 | 28.4 | 30.90 | ||
| Au | 0.44 | 317 | 2.2 | |||||||
| Si | 71 | 0.16 | 1.75 | 4.4 | 2.24 | 2.64 | 3.20 | 3.50 | 3.70 | 3.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gao, L.; Ni, T.; Zhou, J.; Li, X.; Li, Y.; Xu, L.; Wang, R.; Zeng, C.; Li, B.; et al. Analysis of Degradation of Electromigration Reliability of Au-Al and OPM Wire Bonded Contacts at 250 °C Using Resistance Monitoring Method. Micromachines 2023, 14, 640. https://doi.org/10.3390/mi14030640
Li X, Gao L, Ni T, Zhou J, Li X, Li Y, Xu L, Wang R, Zeng C, Li B, et al. Analysis of Degradation of Electromigration Reliability of Au-Al and OPM Wire Bonded Contacts at 250 °C Using Resistance Monitoring Method. Micromachines. 2023; 14(3):640. https://doi.org/10.3390/mi14030640
Chicago/Turabian StyleLi, Xueqin, Linchun Gao, Tao Ni, Jingnan Zhou, Xiaojing Li, Yifan Li, Lida Xu, Runjian Wang, Chuanbin Zeng, Bo Li, and et al. 2023. "Analysis of Degradation of Electromigration Reliability of Au-Al and OPM Wire Bonded Contacts at 250 °C Using Resistance Monitoring Method" Micromachines 14, no. 3: 640. https://doi.org/10.3390/mi14030640




