Sub PPM Detection of NO2 Using Strontium Doped Bismuth Ferrite Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bi1−xSrxFeO3 (x = 0, 0.20) Nanopowder
2.2. Characterization
2.3. Sensor Fabrication
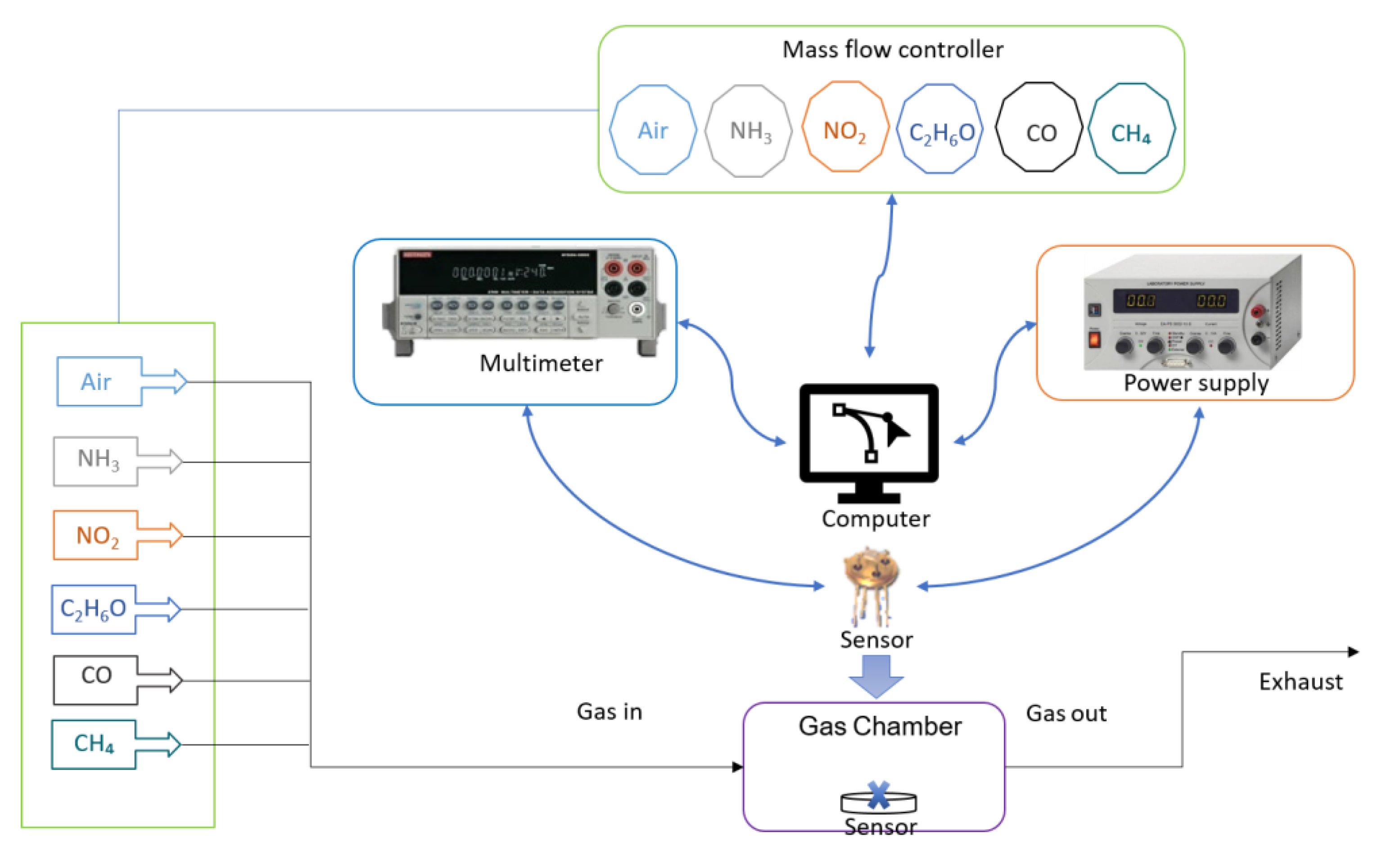
2.4. Gas-Sensing System
3. Results and Discussion
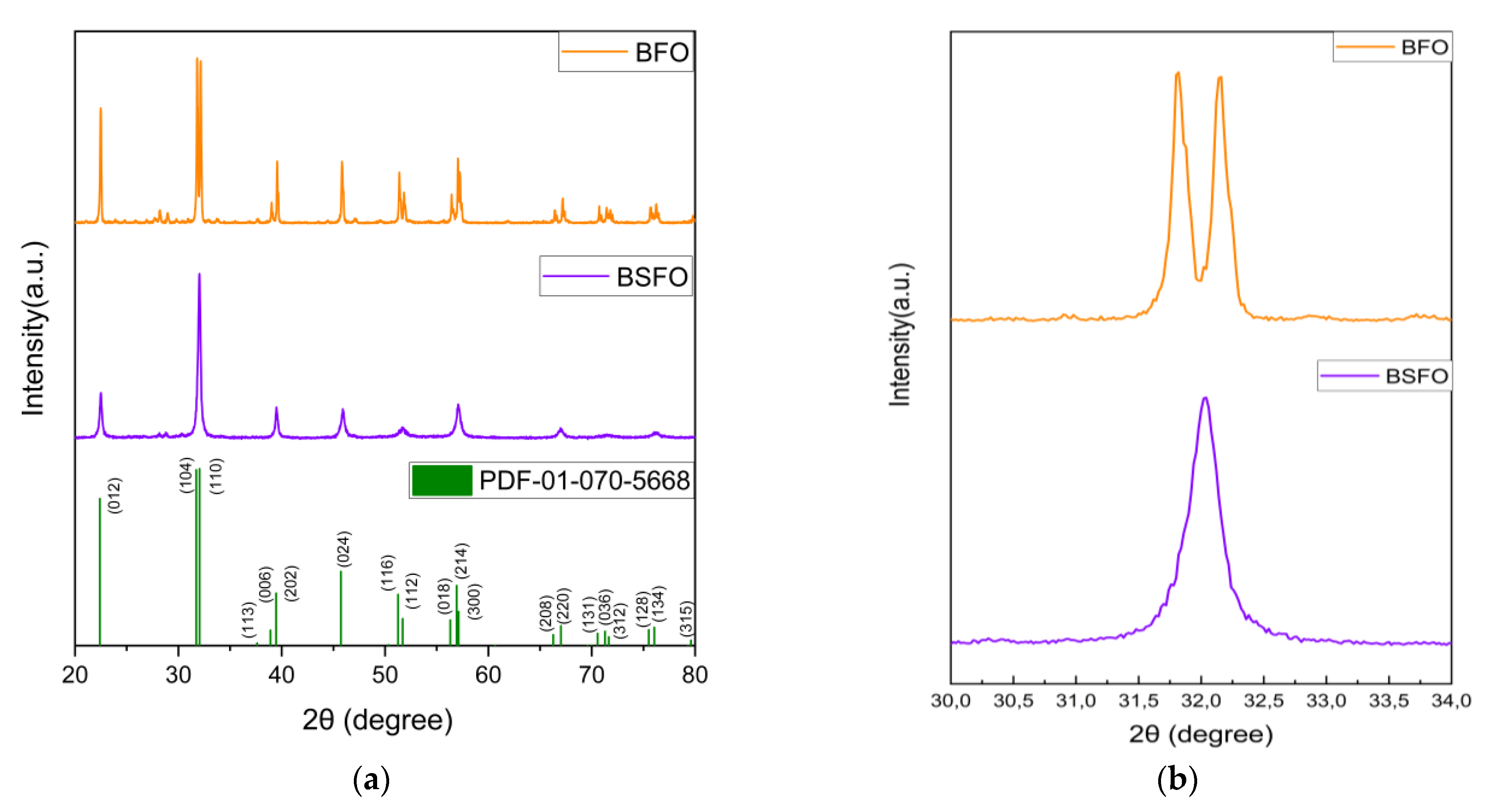
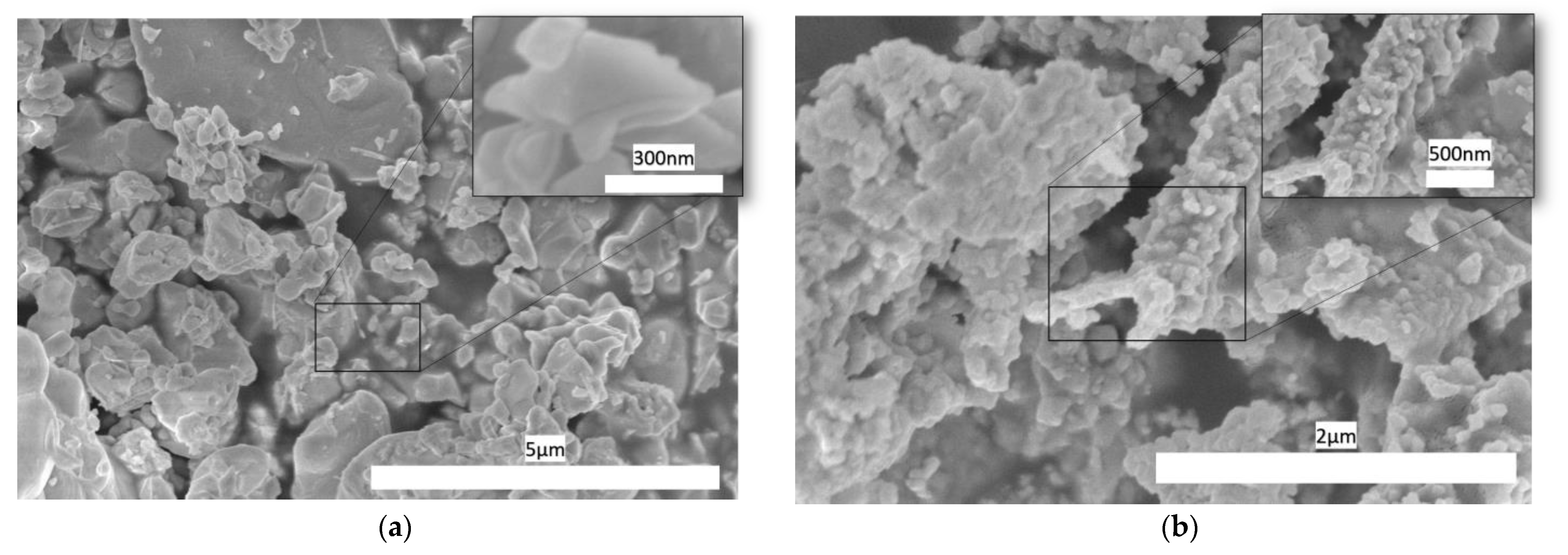
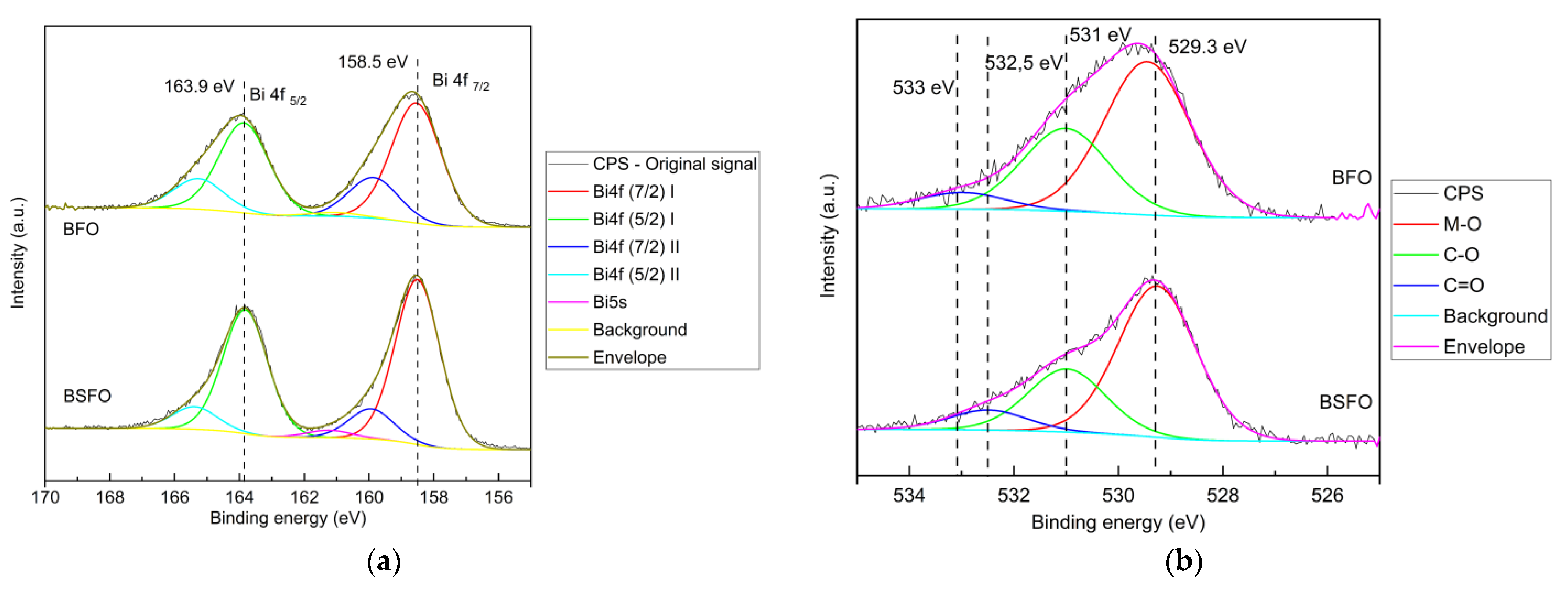
3.1. Physical Properties
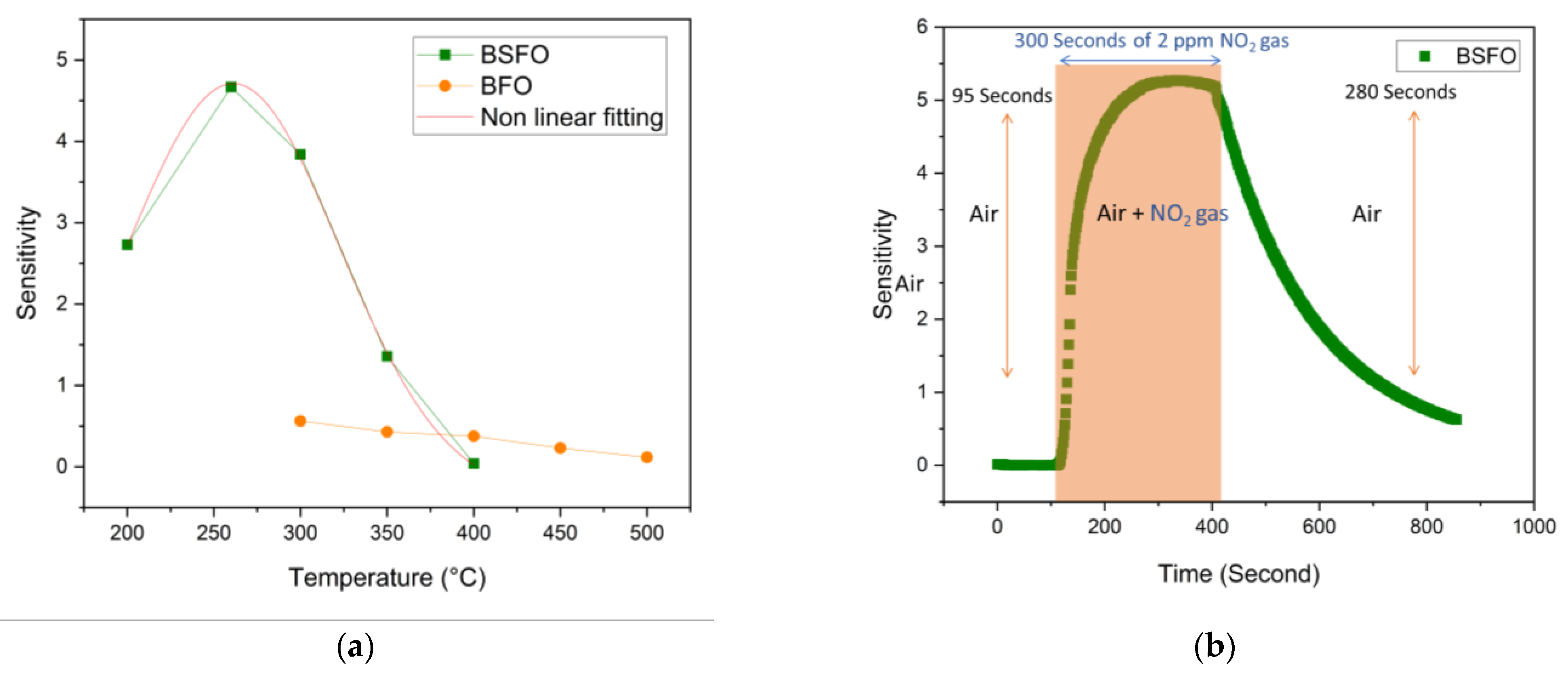
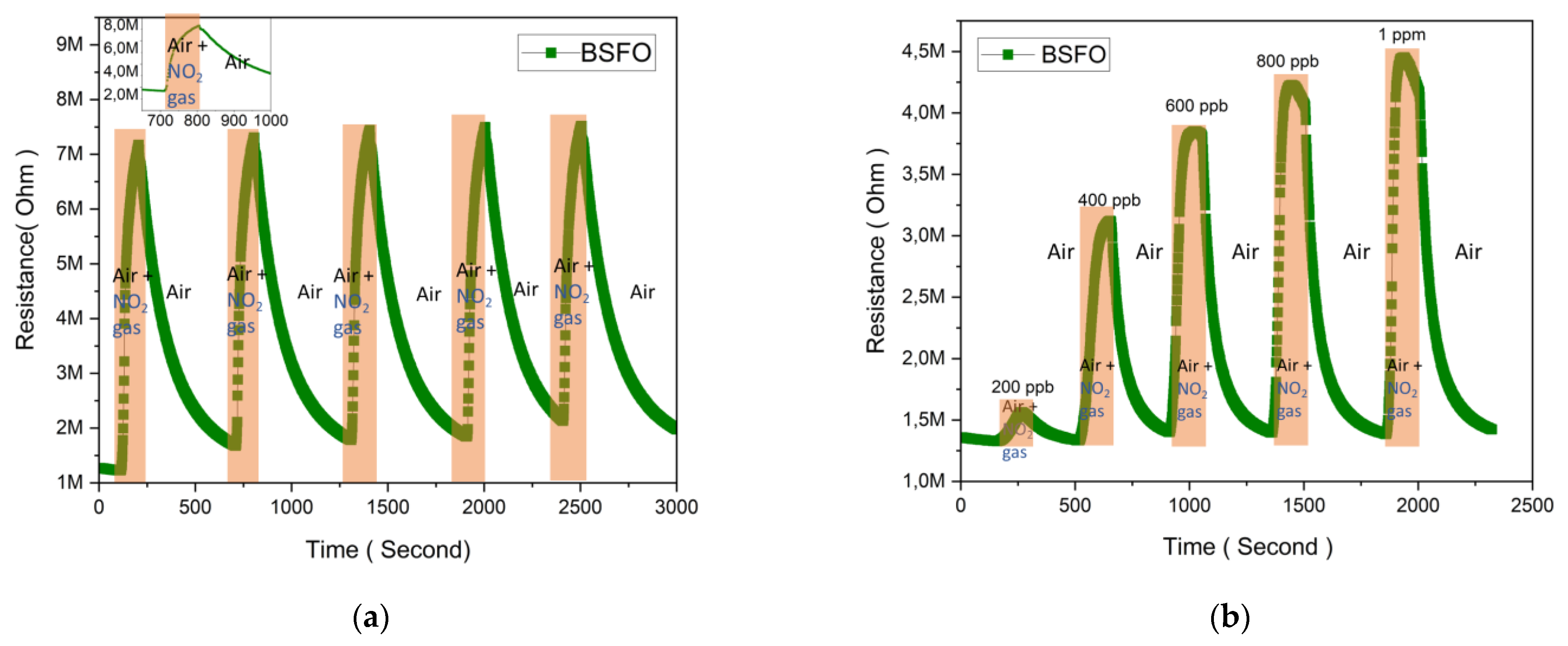
3.2. Gas-Sensing Properties
3.3. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Zhang, Q.; Qin, W.; Pan, R.; Yi, W.; Xu, Z.; Cheng, J.; Su, H. Premature Mortality Attributable to NO2 Exposure in Cities and the Role of Built Environment: A Global Analysis. Sci. Total Environ. 2023, 866, 161395. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor Air Pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Dou, X.; Liao, C.; Wang, H.; Huang, Y.; Tu, Y.; Huang, X.; Peng, Y.; Zhu, B.; Tan, J.; Deng, Z.; et al. Estimates of Daily Ground-Level NO2 Concentrations in China Based on Random Forest Model Integrated K-Means. Adv. Appl. Energy 2021, 2, 100017. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-Based Charge Transfer in Two-Dimensional SnS2 for Selective and Reversible NO2 Gas Sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Oldham, G.; Gupta, A.; Shafiei, M.; Langford, S.J. Enhancement in Room Temperature Ammonia Sensing Properties of Naphthalene Diimides through Core Expansion. J. Mater. Chem. C Mater. 2022, 10, 1326–1333. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kim, I.; Hong, J.; Kumar, A.; Song, S.-J. Transition Metal Oxide (Ni, Co, Fe)-Tin Oxide Nanocomposite Sensing Electrodes for a Mixed-Potential Based NO2 Sensor. Sens. Actuators B Chem. 2019, 284, 534–544. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Joshi, M.; Myung, J.H. Sputtered SnO2/ZnO Heterostructures for Improved NO2 Gas Sensing Properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, J.W.; Hong, Y.J.; Kang, Y.C.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.H. Highly Sensitive and Selective Detection of Ppb-Level NO2 Using Multi-Shelled WO3 Yolk-Shell Spheres. Sens. Actuators B Chem. 2016, 229, 561–569. [Google Scholar] [CrossRef]
- Addabbo, T.; Bruzzi, M.; Fort, A.; Mugnaini, M.; Vignoli, V. Gas Sensing Properties of In2O3 Nano-Films Obtained by Low Temperature Pulsed Electron Deposition Technique on Alumina Substrates. Sensors 2018, 18, 4410. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined Chemoresistive and in Situ FTIR Spectroscopy Study of Nanoporous NiO Films for Light-Activated Nitrogen Dioxide and Acetone Gas Sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal Oxide Gas Sensors for Detecting NO2 in Industrial Exhaust Gas: Recent Developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In Situ Synthesis of Lead-Free Halide Perovskite–COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Bulemo, P.M.; Kim, I.D. Recent Advances in ABO3 Perovskites: Their Gas-Sensing Performance as Resistive-Type Gas Sensors. J. Korean Ceram. Soc. 2020, 57, 24–39. [Google Scholar] [CrossRef]
- Fergus, J.W. Perovskite Oxides for Semiconductor-Based Gas Sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Itagaki, Y.; Mori, M.; Hosoya, Y.; Aono, H.; Sadaoka, Y. O3 and NO2 Sensing Properties of SmFe1-XCoxO3 Perovskite Oxides. Sens. Actuators B Chem. 2007, 122, 315–320. [Google Scholar] [CrossRef]
- Vijjapu, M.T.; Surya, S.G.; He, J.H.; Salama, K.N. Highly Selective Self-Powered Organic-Inorganic Hybrid Heterojunction of a Halide Perovskite and InGaZnO NO2 Sensor. ACS Appl. Mater. Interfaces 2021, 13, 40460–40470. [Google Scholar] [CrossRef]
- Casanova-Chafer, J.; Garcia-Aboal, R.; Atienzar, P.; Llobet, E. Unraveling the Gas-Sensing Mechanisms of Lead-Free Perovskites Supported on Graphene. ACS Sens. 2022, 7, 3753–3763. [Google Scholar] [CrossRef]
- Ghuge, R.S.; Shinde, M.D.; Rane, S.B. Bismuth-Based Gas Sensors: A Comprehensive Review. J. Electron. Mater. 2021, 50, 6060–6072. [Google Scholar] [CrossRef]
- Yu, X.L.; Wang, Y.; Hu, Y.M.; Cao, C.B.; Chan, H.L.W. Gas-Sensing Properties of Perovskite BiFeO3 Nanoparticles. J. Am. Ceram. Soc. 2009, 92, 3105–3107. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Li, H.; Zhang, W.; Zhang, Y.; Zhai, Z. Highly Sensitive Ethanol and Acetone Gas Sensors with Reduced Working Temperature Based on Sr-Doped BiFeO3 Nanomaterial. J. Mater. Res. Technol. 2022, 17, 1955–1963. [Google Scholar] [CrossRef]
- Hussain, T.; Siddiqi, S.A.; Atiq, S.; Awan, M.S. Induced Modifications in the Properties of Sr Doped BiFeO3 Multiferroics. Prog. Nat. Sci. Mater. Int. 2013, 23, 487–492. [Google Scholar] [CrossRef]
- Hussain, S.; Hasanain, S.K.; Hassnain Jaffari, G.; Ali, N.Z.; Siddique, M.; Ismat Shah, S. Correlation between Structure, Oxygen Content and the Multiferroic Properties of Sr Doped BiFeO3. J. Alloys Compd. 2015, 622, 8–16. [Google Scholar] [CrossRef]
- Waghmare, S.D.; Raut, S.D.; Ghule, B.G.; Jadhav, V.V.; Shaikh, S.F.; Al-Enizi, A.M.; Ubaidullah, M.; Nafady, A.; Thamer, B.M.; Mane, R.S. Pristine and Palladium-Doped Perovskite Bismuth Ferrites and Their Nitrogen Dioxide Gas Sensor Studies. J. King Saud Univ. Sci. 2020, 32, 3125–3130. [Google Scholar] [CrossRef]
- Patil, R.P.; Gaikwad, S.S.; Karanjekar, A.N.; Khanna, P.K.; Jain, G.H.; Gaikwad, V.B.; More, P.V.; Bisht, N. Optimization of Strontium- Doping Concentration in BaTiO3 Nanostructures for Room Temperature NH3 and NO2 Gas Sensing. Mater. Today Chem. 2020, 16, 100240. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Wei, J.; Zhang, Y. Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals. Materials 2020, 13, 3829. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, R. Behavioral Remodeling of Perovskite BiFeO3 by Ag-Doping Strategies for Enhanced and Ppb Level Acetone Sensing Performance: An Advanced Enlightenment for Selectivity and Sensing Mechanism. Appl. Mater. Today 2022, 29, 101611. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, A.; Sim, U.; Im, H.N.; Song, S.J. Synergistic Enhancement in the Sensing Performance of a Mixed-Potential NH3 Sensor Using SnO2@CuFe2O4 Sensing Electrode. Sens. Actuators B Chem. 2020, 308, 127748. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at Fractional Atmosphere Pressures with Iron and Iron Oxide Surfaces: One Possible Mechanism for Surface Contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Queraltó, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 Nanofibers for High Detection of Sulfur-Containing Gases. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Raauf, A.; Graf, D.; Gönüllü, Y.; Sekhar, P.K.; Frank, M.; Mathur, S. Synergistic Acceptor-Donor Interplay of Nd2Sn2O7 Pyrochlore Based Sensor in Selective Detection of Hydrogen. J. Electrochem. Soc. 2021, 168, 047501. [Google Scholar] [CrossRef]
- Ghadage, P.; Kodam, P.; Nadargi, D.; Patil, S.; Tamboli, M.; Bhandari, N.; Mulla, I.; Park, C.; Suryavanshi, S. Pd Loaded Bismuth Ferrite: A Versatile Perovskite for Dual Applications as Acetone Gas Sensor and Photocatalytic Dye Degradation of Malachite Green. Ceram. Int. 2022, 49, 5738–5747. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, R. Ion-Dipole Interaction for Selective Detection of Acetone by Perovskite BiFeO3 Chemi-Resistive Sensor. Anal. Chim. Acta 2022, 1206, 339788. [Google Scholar] [CrossRef]
- Varshney, D.; Kumar, A. Structural, Raman and Dielectric Behavior in Bi1-XSr XFeO3 Multiferroic. J. Mol. Struct. 2013, 1038, 242–249. [Google Scholar] [CrossRef]
- Li, P.; Chen, Q.; Lin, Y.; Chang, G.; He, Y. Effects of Crystallite Structure and Interface Band Alignment on the Photocatalytic Property of Bismuth Ferrite/ (N-Doped) Graphene Composites. J. Alloys Compd. 2016, 672, 497–504. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Wang, S.; Zhang, N.; Qin, L.; Huang, Y. Facile Synthesis of Sm-Doped BiFeO3 Nanoparticles for Enhanced Visible Light Photocatalytic Performance. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Aslam, M.; Soomro, M.T.; Ismail, I.M.I.; Qari, H.A.; Gondal, M.A.; Hameed, A. The Facile Synthesis, Characterization and Evaluation of Photocatalytic Activity of Bimetallic FeBiO3 in Natural Sunlight Exposure. RSC Adv. 2015, 5, 102663–102673. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surface and Interface Analysis 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Chowdhuri, A.; Singh, S.K.; Sreenivas, K.; Gupta, V. Contribution of Adsorbed Oxygen and Interfacial Space Charge for Enhanced Response of SnO2 Sensors Having CuO Catalyst for H2S Gas. Sens. Actuators B Chem. 2010, 145, 155–166. [Google Scholar] [CrossRef]
- Salaya-Gerónimo, E.; García-Zaleta, D.S.; Jácome-Acatitla, G.; Huerta-García, E.; López-González, R.; Reyes-Montero, A.; Abdel-Mageed, A.M. Structural, Optical and Photocatalytic Properties of Sr-Doped and Ca-Doped BiFeO3 Compounds Prepared by Pechini Method. J. Chem. Technol. Biotechnol. 2022, 97, 2970–2983. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Guo, L.; Wang, G. Highly Sensitive Acetone Gas Sensors Based on Erbium-Doped Bismuth Ferrite Nanoparticles. Nanomaterials 2022, 12, 3679. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, M.; Kumar, R.; Singh, R.; Prasad, B.; Kumar, D. Numerical modelling of chemisorption of oxygen gas molecules on the surface of semiconductor for gas sensors applications. Mater. Today Proc. 2019, 18, 1272–1279. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 143–167. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Sun, F.; Li, T.; Zhang, T.; Qin, S. Humidity-Insensitive NO2 Sensors Based on SnO2/RGO Composites. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Reghu, A.; Legore, L.J.; Vetelino, J.F.; Lad, R.J.; Frederick, B.G. Distinguishing Bulk Conduction from Band Bending Transduction Mechanisms in Chemiresistive Metal Oxide Gas Sensors. J. Phys. Chem. C 2018, 122, 10607–10620. [Google Scholar] [CrossRef]
- Remya, K.P.; Prabhu, D.; Joseyphus, R.J.; Bose, A.C.; Viswanathan, C.; Ponpandian, N. Tailoring the Morphology and Size of Perovskite BiFeO3 Nanostructures for Enhanced Magnetic and Electrical Properties. Mater. Des. 2020, 192, 108694. [Google Scholar] [CrossRef]
- Song, L.; Dou, K.; Wang, R.; Leng, P.; Luo, L.; Xi, Y.; Kaun, C.C.; Han, N.; Wang, F.; Chen, Y. Sr-Doped Cubic In2O3/Rhombohedral In2O3 Homojunction Nanowires for Highly Sensitive and Selective Breath Ethanol Sensing: Experiment and DFT Simulation Studies. ACS Appl. Mater. Interfaces 2020, 12, 1270–1279. [Google Scholar] [CrossRef]
- Zeb, S.; Cui, Y.; Zhao, H.; Sui, Y.; Yang, Z.; Khan, Z.U.; Ahmad, S.M.; Ikram, M.; Gao, Y.; Jiang, X. Synergistic Effect of Au-PdO Modified Cu-Doped K2W4O13 Nanowires for Dual Selectivity High Performance Gas Sensing. ACS Appl. Mater. Interfaces 2022, 14, 13836–13847. [Google Scholar] [CrossRef]



| BFO | BSFO | ||||
|---|---|---|---|---|---|
| Element | Weight % | Atomic % | Element | Weight % | Atomic % |
| O | 12.93 | 55.15 | O | 12.59 | 47.13 |
| Bi | 69.71 | 22.43 | Bi | 49.59 | 14.21 |
| Fe | 18.36 | 22.42 | Fe | 32.92 | 35.3 |
| - | - | - | Sr | 4.9 | 3.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmonte, D.J.; Bhardwaj, A.; Wilhelm, M.; Fischer, T.; Kuřitka, I.; Mathur, S. Sub PPM Detection of NO2 Using Strontium Doped Bismuth Ferrite Nanostructures. Micromachines 2023, 14, 644. https://doi.org/10.3390/mi14030644
Dmonte DJ, Bhardwaj A, Wilhelm M, Fischer T, Kuřitka I, Mathur S. Sub PPM Detection of NO2 Using Strontium Doped Bismuth Ferrite Nanostructures. Micromachines. 2023; 14(3):644. https://doi.org/10.3390/mi14030644
Chicago/Turabian StyleDmonte, David John, Aman Bhardwaj, Michael Wilhelm, Thomas Fischer, Ivo Kuřitka, and Sanjay Mathur. 2023. "Sub PPM Detection of NO2 Using Strontium Doped Bismuth Ferrite Nanostructures" Micromachines 14, no. 3: 644. https://doi.org/10.3390/mi14030644
APA StyleDmonte, D. J., Bhardwaj, A., Wilhelm, M., Fischer, T., Kuřitka, I., & Mathur, S. (2023). Sub PPM Detection of NO2 Using Strontium Doped Bismuth Ferrite Nanostructures. Micromachines, 14(3), 644. https://doi.org/10.3390/mi14030644









