Green Synthesis of NiO Nanoflakes Using Bitter Gourd Peel, and Their Electrochemical Urea Sensing Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals Used
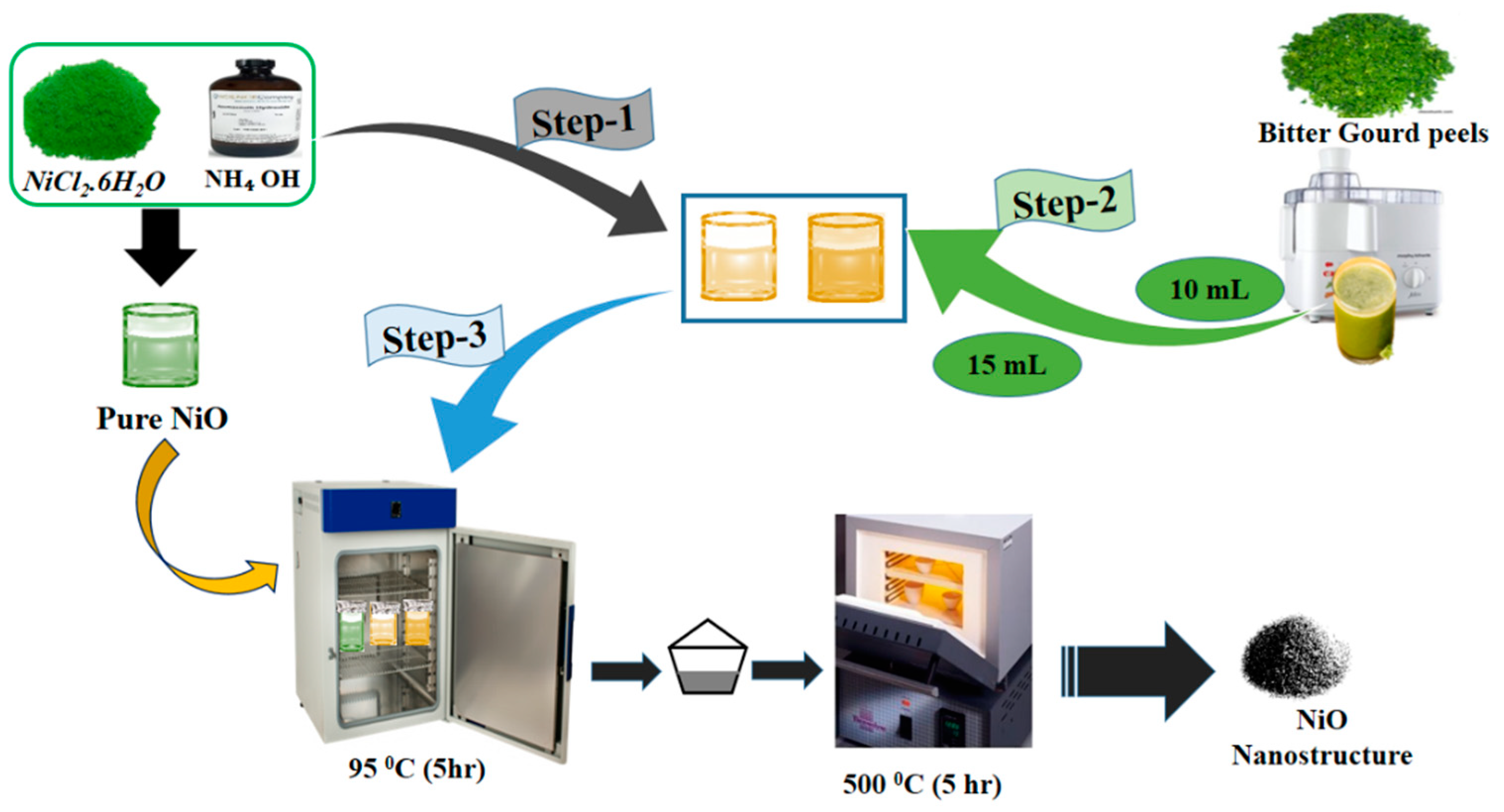
2.2. Phytochemical Synthesis of NiO Nanostructures Using Low-Temperature Aqueous Chemical Growth Method
2.3. Structural Characterization of NiO Nanostructures
2.4. Electrochemical Oxidation of Urea in Alkaline Media Using Modified Glassy Carbon Electrode (GCE)
3. Results and Discussion
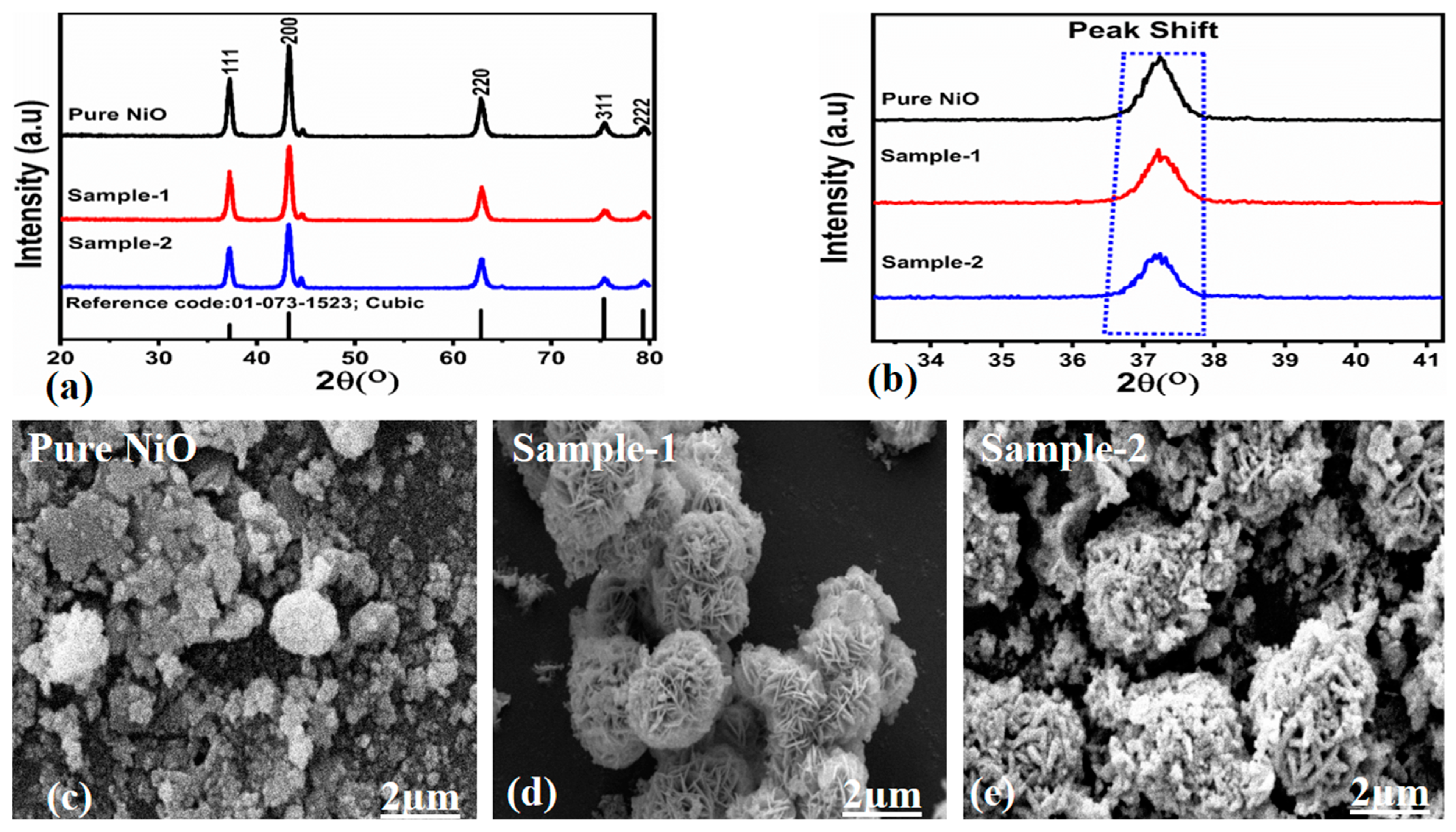
3.1. The Structural Characterization of NiO Nanostructures Prepared with Bitter Gourd Peel Extract
3.2. Non-Enzymatic Urea Sensor Characterization Based on the Bitter Gourd Peel Extract Assisted NiO Nanostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsaee, Z. Synthesis of novel amperometric urea-sensor using hybrid synthesized NiO-NPs/GO modified GCE in aqueous solution of cetrimonium bromide. Ultrason. Sonochemistry 2018, 44, 120–128. [Google Scholar] [CrossRef]
- Li, L.; Long, Y.; Gao, J.M.; Song, K.; Yang, G. Label-free and pHsensitive colourimetric materials for the sensing of urea. Nanoscale 2016, 8, 4458–4462. [Google Scholar] [CrossRef]
- Ahuja, T.; Mir, I.A.; Kumar, D. Potentiometric urea biosensor based on BSA embedded surface modified polypyrrole film. Sens. Actuators B 2008, 134, 140–145. [Google Scholar] [CrossRef]
- Dutta, D.; Chandra, S.; Swain, A.K.; Bahadur, D. SnO2 quantum dots-reduced graphene oxide composite for enzyme-free ultrasensitive electrochemical detection of urea. Anal. Chem. 2014, 86, 5914–5921. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative analysis of urea in human urine and serum by 1H nuclear magnetic resonance. Analyst 2012, 137, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Verma, N.; Garg, A.K.; Redhu, N. Urea biosensors. Sens. Actuators B Chem. 2008, 134, 345–351. [Google Scholar] [CrossRef]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
- Rahmanian, R.; Mozaffari, S.A. Electrochemical fabrication of ZnO-polyvinyl alcohol nanostructured hybrid film for application to urea biosensor. Sens. Actuators B Chem. 2015, 207, 772–781. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Srivastava, S.; Narayanan, T.N.; Mahlotra, B.D.; Vajtai, R.; Ajayan, P.M.; Srivastava, A. Functionalized multilayered graphene platform for urea sensor. ACS Nano 2011, 6, 168–175. [Google Scholar] [CrossRef]
- Sharma, A.; Rawat, K.; Bohidar, H.B.; Solanki, P.R. Studies on claygelatin nanocomposite as urea sensor. Appl. Clay Sci. 2017, 146, 297–305. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Rafiei, F. Trace level and highly selective determination of urea in various real samples based upon voltammetric analysis of diacetylmonoxime-urea reaction product on the carbon nanotube/carbon paste electrode. Anal. Chim. Acta 2017, 974, 54–62. [Google Scholar] [CrossRef]
- Khan, K.M.; Krishna, H.; Majumder, S.K.; Gupta, P.K. Gupta, Detection of urea adulteration in milk using near-infrared Raman spectroscopy. Food Anal. Methods 2015, 8, 93–102. [Google Scholar] [CrossRef]
- Clark, S.; Francis, P.S.; Conlan, X.A.; Barnett, N.W. Determination of urea using highperformance liquid chromatography with fluorescence detection after automated derivatisation with xanthydrol. J. Chromatogr. A 2007, 1161, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Takenaka, N.; Kitano, M.; Bandow, H.; Maeda, Y.; Hattori, M. Determination of trace amounts of urea by using flow injection with chemiluminescence detection. Analyst 1994, 119, 1829–1833. [Google Scholar] [CrossRef]
- Tyagi, M.; Tomar, M.; Gupta, V. NiO nanoparticle-based urea biosensor. Biosens. Bioelectron. 2013, 41, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Puhazhendi, P.; Kumar, J.; Gowthaman, M.K.; D’Souza, S.F.; Kamini, N.R. Potentiometric biensor for determination of urea in milk using immobilized Arthrobacter creatinolyticus urease. Mater. Sci. Eng. C 2015, 49, 786–792. [Google Scholar] [CrossRef]
- Pan, T.M.; Huang, M.D.; Lin, W.Y.; Wu, M.H. A urea biosensor based on pH-sensitive Sm2TiO5 electrolyte–insulator–semiconductor. Anal. Chim. Acta 2010, 669, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Hahn, Y.B. Highly stable urea sensor based on ZnO nanorods directly grown on Ag/glass electrodes. Sens. Actuators B 2014, 194, 290–295. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Buron, C.C.; Quinart, M.; Vrlinic, T.; Yunus, S.; Glinel, K.; Jonas, A.M.; Lakard, B. Application of original assemblies of polyelectrolytes, urease and electrodeposited polyaniline as sensitive films of potentiometric urea biosensors. Electrochim. Acta 2014, 148, 53–61. [Google Scholar] [CrossRef]
- Arain, M.; Nafady, A.; Ibupoto, Z.H.; Sherazi, S.T.H.; Shaikh, T.; Khan, H.; Alsalme, A.; Niaz, A.; Willander, M. Simpler and highly sensitive enzyme-free sensing of urea via NiO nanostructures modified electrode. RSC Adv. 2016, 6, 39001–39006. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.S.; Das, G.; Yoon, H.H. Nickel/cobalt oxide-decorated 3D graphene nanocomposite electrode for enhanced electrochemical detection of urea. Biosens. Bioelectron. 2016, 77, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Komori, K.; Badhulika, S. Graphene–Polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, H.; Li, G.; Wu, Z. Nickel-cobalt bimetallic anode catalysts for direct urea fuel cell. Sci. Rep. 2014, 4, 5863. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Cao, D.; Du, M.; Ye, K.; Wang, G.; Zhang, W.; Gao, Y.; Cheng, K. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel-cobalt anode. J. Power Sources 2016, 307, 697–704. [Google Scholar] [CrossRef]
- Guo, F.; Cheng, K.; Ye, K.; Wang, G.; Cao, D. Preparation of nickel-cobalt nanowire arrays anode electro-catalyst and its application in direct urea/hydrogen peroxide fuel cell. Electrochim. Acta 2016, 199, 290–296. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, H.; Zhao, L.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Facile preparation of three-dimensional Ni(OH)2/Ni foam anode with low cost and its application in a direct urea fuel cell. New J. Chem. 2016, 40, 8673–8680. [Google Scholar] [CrossRef]
- Wang, L.; Du, T.; Cheng, J.; Xie, X.; Yang, B.; Li, M. Enhanced activity of urea electro oxidation on nickel catalysts supported on tungsten carbides/carbon nanotubes. J. Power Sources 2015, 280, 550–554. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Yoon, H.H. Nickel oxide-deposited cellulose/CNT composite electrode for non-enzymatic urea detection. Sens. Actuators B 2016, 236, 304–310. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: Direct hydrogen production from urine. Chem. Commun. 2009, 32, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef] [Green Version]
- López-Naranjo, E.; Hernández-Rosales, I.; Bueno-Durán, A.; Martínez-Aguilar, M.; González-Ortiz, L.; Pérez-Fonseca, A.; Robledo-Ortiz, J.; Sánchez-Peña, M.; Manzano-Ramírez, A. Biosynthesis of silver nanoparticles using a natural extract obtained from an agroindustrial residue of the tequila industry. Mater. Lett. 2018, 213, 278–281. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.G.N.; Delerue-Matos, C.; De Oliveira, R.D.C.M.; et al. In situ synthesis of silver nanoparticles in a hydrogel of carboxymethyl cellulose with phthalated-cashew gum as a promising antibacterial and healing agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef] [Green Version]
- Govindaraju, K.; Basha, S.K.; Kumar, V.G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Scarano, G.; Morelli, E. Properties of phytochelatin-coated CdS nanocrystallites formed in a marine phytoplanktonic alga (Phaeodactylum tricornutum, Bohlin) in response to Cd. Plant Sci. 2003, 165, 803–810. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C.; Jayakumar, S.J. Synthesis of CeO2-NPs by chemical and biological methods and their photocatalytic, antibacterial and in vitro antioxidant activity. Res. Chem. Intermed. 2020, 46, 2705–2729. [Google Scholar] [CrossRef]
- Mathew, S.; Mathew, B. Biomass Derived Carbon Dot as Nanoswitch, Logic Gate Operation, and Electrochemical Sensor for Flavonoids. New J. Chem. 2023, 47, 2383–2395. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Palanikumar, L.; Ramasamy, S.N.; Balachandran, C. Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnology 2014, 8, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Raina, K.; Kumar, D.; Agarwal, R. October. Promise of bitter melon (Momordica charantia) bioactives in cancer prevention and therapy. Semin. Cancer Biol. 2016, 40, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Steele, R.; Aurora, R.; Varvares, M.; Schwetye, K.E.; Ray, R.B. Bitter Melon Prevents the Development of 4-NQO–Induced Oral Squamous Cell Carcinoma in an Immunocompetent Mouse Model by Modulating Immune SignalingBME Prevents Oral Cancer. Cancer Prev. Res. 2018, 11, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Madhura, T.R.; Kumar, G.G.; Ramaraj, R. Reduced graphene oxide supported 2D-NiO nanosheets modified electrode for urea detection. J. Solid State Electrochem. 2020, 24, 3073–3081. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Al-Omair, M.A.; Dao, V.D.; Mohamed, I.M. Chemical synthesis of NiO nanostructure by surfactant-assisted sol–gel methodology for urea electrocatalytic oxidation. Mater. Lett. 2020, 276, 128192. [Google Scholar] [CrossRef]
- Tyagi, M.; Tomar, M.; Gupta, V. Enhanced electron transfer properties of NiO thin film for the efficient detection of urea. Mater. Sci. Eng. B 2019, 240, 147–155. [Google Scholar] [CrossRef]
- Narwade, S.S.; Mali, S.M.; Digraskar, R.V.; Sapner, V.S.; Sathe, B.R. Ni/NiO@ rGO as an efficient bifunctional electrocatalyst for enhanced overall water splitting reactions. Int. J. Hydrog. Energy 2019, 44, 27001–27009. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim. Acta. 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Du, M.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim. Acta. 2016, 210, 474–482. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta. 2012, 8, 292–300. [Google Scholar] [CrossRef]
- Daramola, D.A.; Singh, D.; Botte, G.G. Dissociation rates of urea in the presence of niooh catalyst: A DFT analysis. J. Phys. Chem. 2010, 114, 11513–11521. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C. Single-enzyme nanoparticles based urea biosensor. Sens. Actuators B 2013, 188, 313–317. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, T.; Niu, Y.; Zhang, Y.; Zhang, C.; Li, P.; Zhu, M.; Jia, Y.; Li, Q. Flexible visible-light-driven photoelectrochemical biosensor based on molecularly imprinted nanoparticle intercalation-modulated graphene fiber for ultrasensitive urea detection. Carbon 2020, 157, 457–465. [Google Scholar] [CrossRef]
- Yang, Y.; Yoon, S.G.; Shin, C.; Jin, H.; Lee, W.H.; Park, J.; Kim, Y.S. Ionovoltaic urea sensor. Nano Energy 2019, 57, 195–201. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, M.; Niu, Y.; Kozliak, E.; Yao, B.; Zhang, Y.; Zhang, C.; Qin, T.; Jia, Y.; Li, Q. A graphene-based coaxial fibrous photo fuel cell powered by mine gas. Adv. Funct. Mater. 2019, 29, 1906813. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.; Beni, V.; Morante, J.R.; Liu, X.; Falhman, M.; Mazzaro, R.; Ibupoto, Z.H.; Vomiero, A. A practical non-enzymatic urea sensor based on NiCo2O4 nanoneedles. RSC Adv. 2019, 9, 14443–14451. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.; Tahira, A.; Solangi, A.R.; Mazzaro, R.; Ibupoto, Z.H.; Fatima, A.; Vomiero, A. Functional Nickel Oxide Nanostructures for Ethanol Oxidation in Alkaline Media. Electroanalysis 2020, 32, 1052–1059. [Google Scholar] [CrossRef]
- Naik, T.S.K.; Mwaurah, M.M.; Swamy, B.K. Fabrication of poly (Sudan III) modified carbon paste electrode sensor for dopamine: A voltammetric study. J. Electroanal. Chem. 2019, 834, 71–78. [Google Scholar] [CrossRef]
- Kannan, P.K.; Rout, C.S. High performance non-enzymatic glucose sensor based on one-step electrodeposited nickel sulfide. Chem. Eur. J. 2015, 21, 9355–9359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.V.; Sundramoorthy, A.K. Non-enzymatic electrochemical detection of urea on silver nanoparticles anchored nitrogen-doped single-walled carbon nanotube modified electrode. J. Electrochem. Soc. 2018, 165, B3006. [Google Scholar] [CrossRef] [Green Version]
- Salarizadeh, N.; Habibi-Rezaei, M.; Zargar, S.J. NiO–MoO3 nanocomposite: A sensitive non-enzymatic sensor for glucose and urea monitoring. Mater. Chem. Phys. 2022, 281, 125870. [Google Scholar] [CrossRef]
- Naik, T.S.K.; Saravanan, S.; Saravana, K.S.; Pratiush, U.; Ramamurthy, P.C. A non-enzymatic urea sensor based on the nickel sulfide/graphene oxide modified glassy carbon electrode. Mater. Chem. Phys. 2020, 245, 122798. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Ahmad, R.; Umar, A.; Al-Assiri, M.S.; Al-Salami, A.E.; Kumar, R.; Ansari, S.G.; Baskoutas, S. Two-dimensional ytterbium oxide nanodisks based biosensor for selective detection of urea. Biosens. Bioelectron. 2017, 98, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Dervisevic, E.; Şenel, M. Design of amperometric urea biosensor based on self-assembled monolayer of cystamine/PAMAM-grafted MWCNT/Urease. Sens. Actuators B Chem. 2018, 254, 93–101. [Google Scholar] [CrossRef]
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-nanoflowers decorating a plastic electrode for the non-enzymatic amperometric detection of H2O2 in milk: Old issue, new challenge. Food Control. 2022, 132, 108549. [Google Scholar] [CrossRef]
- Tyagi, M.; Tomar, M.; Gupta, V. Glad assisted synthesis of NiO nanorods for realization of enzymatic reagentless urea biosensor. Biosens. Bioelectron. 2014, 52, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Yoon, Y.S.; Kim, D.J. Silver-nanoparticle-decorated NiOOH nanorods for electrocatalytic urea sensing. ACS Appl. Nano Mater. 2020, 3, 7651–7658. [Google Scholar] [CrossRef]
- Farithkhan, A.; John, S.A. Three-Dimensional Coral-Like NiFe-Layered Double Hydroxides on Biomass-Derived Nitrogen-Doped Carbonized Wood as a Sensitive Probe for Nonenzymatic Urea Determination. ACS Sustain. Chem. Eng. 2022, 10, 6952–6962. [Google Scholar] [CrossRef]
- Zhybak, M.T.; Fayura, L.Y.; Boretsky, Y.R.; Gonchar, M.V.; Sibirny, A.A.; Dempsey, E.; Turner, A.P.; Korpan, Y.I. Amperometric L-arginine biosensor based on a novel recombinant arginine deiminase. Microchim. Acta 2017, 184, 2679–2686. [Google Scholar] [CrossRef] [Green Version]
- Soni, A.; Surana, R.K.; Jha, S.K. Smartphone based optical biosensor for the detection of urea in saliva. Sens. Actuators B Chem. 2018, 269, 346–353. [Google Scholar] [CrossRef]

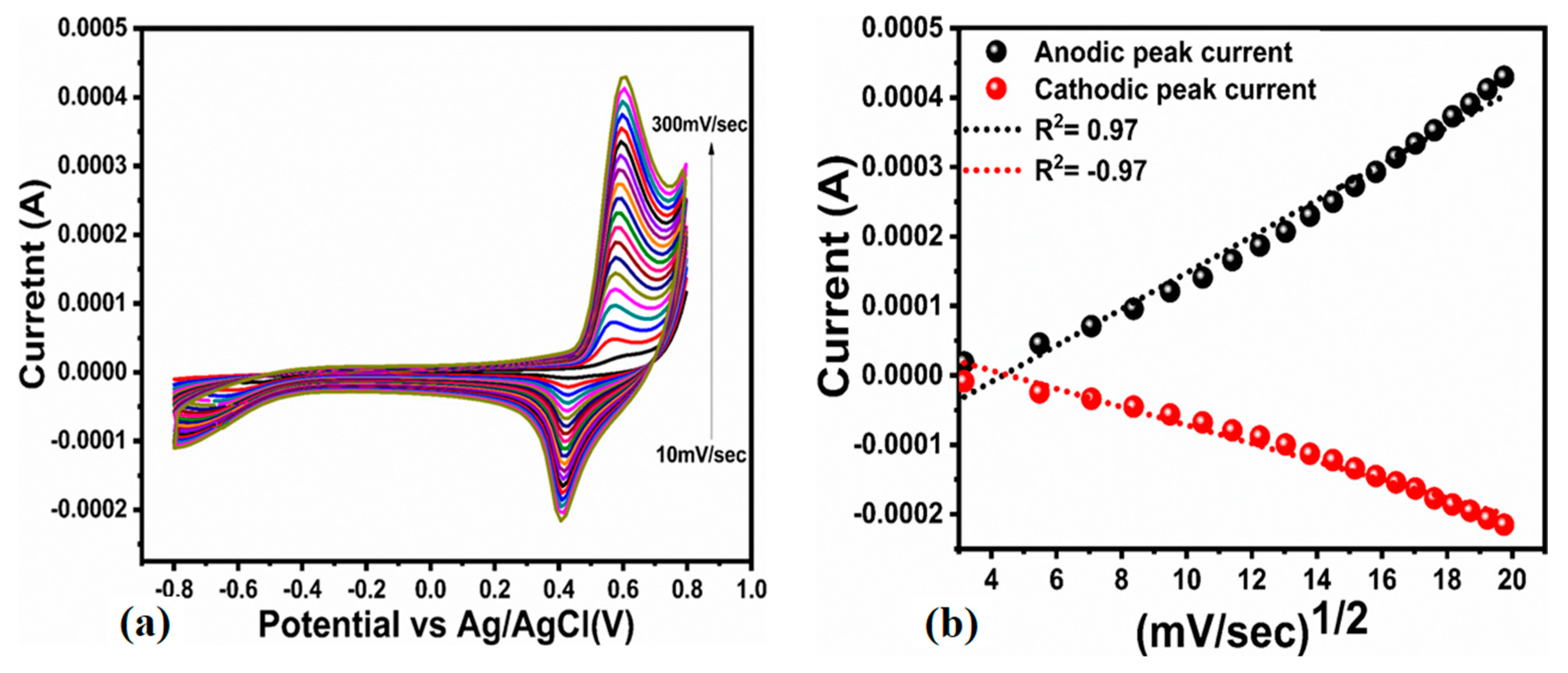
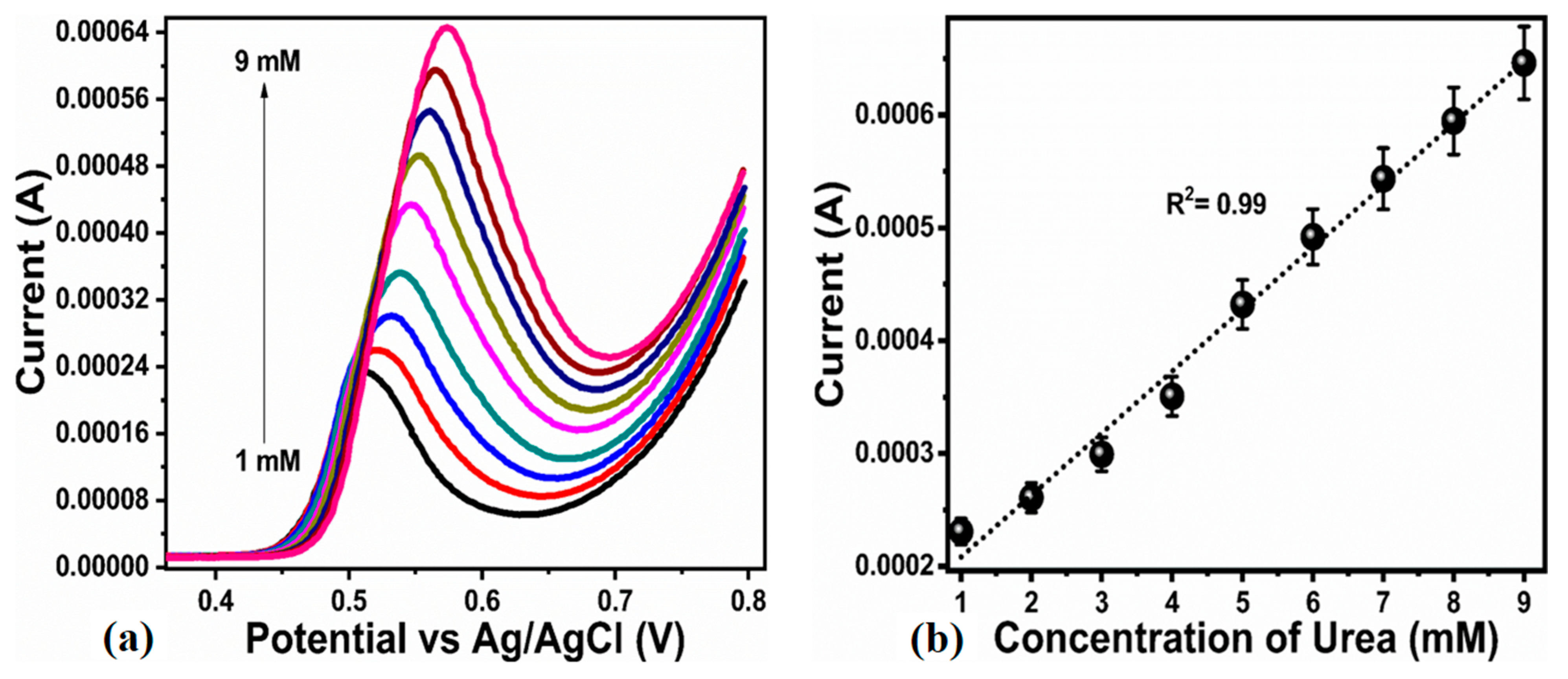
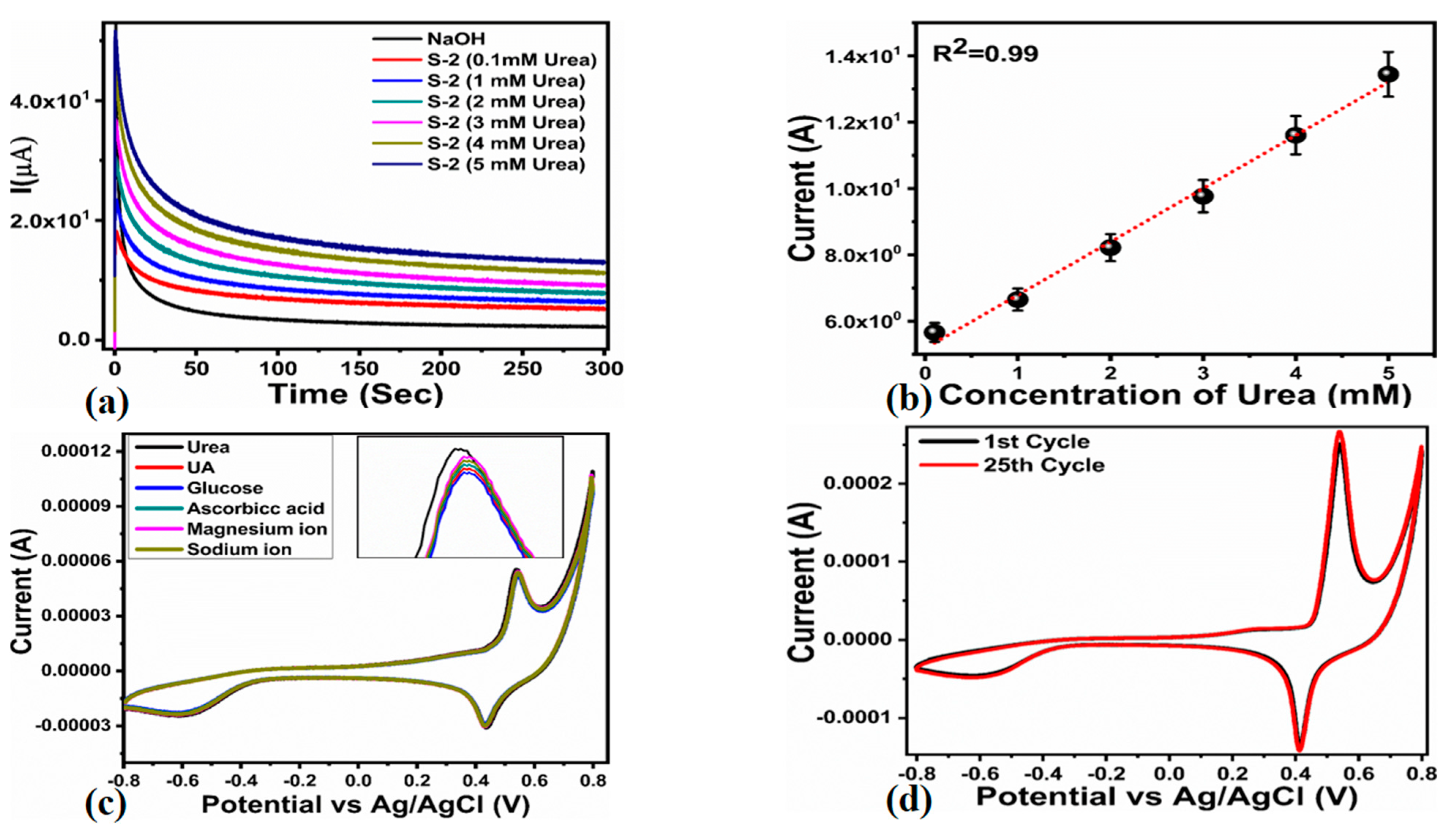
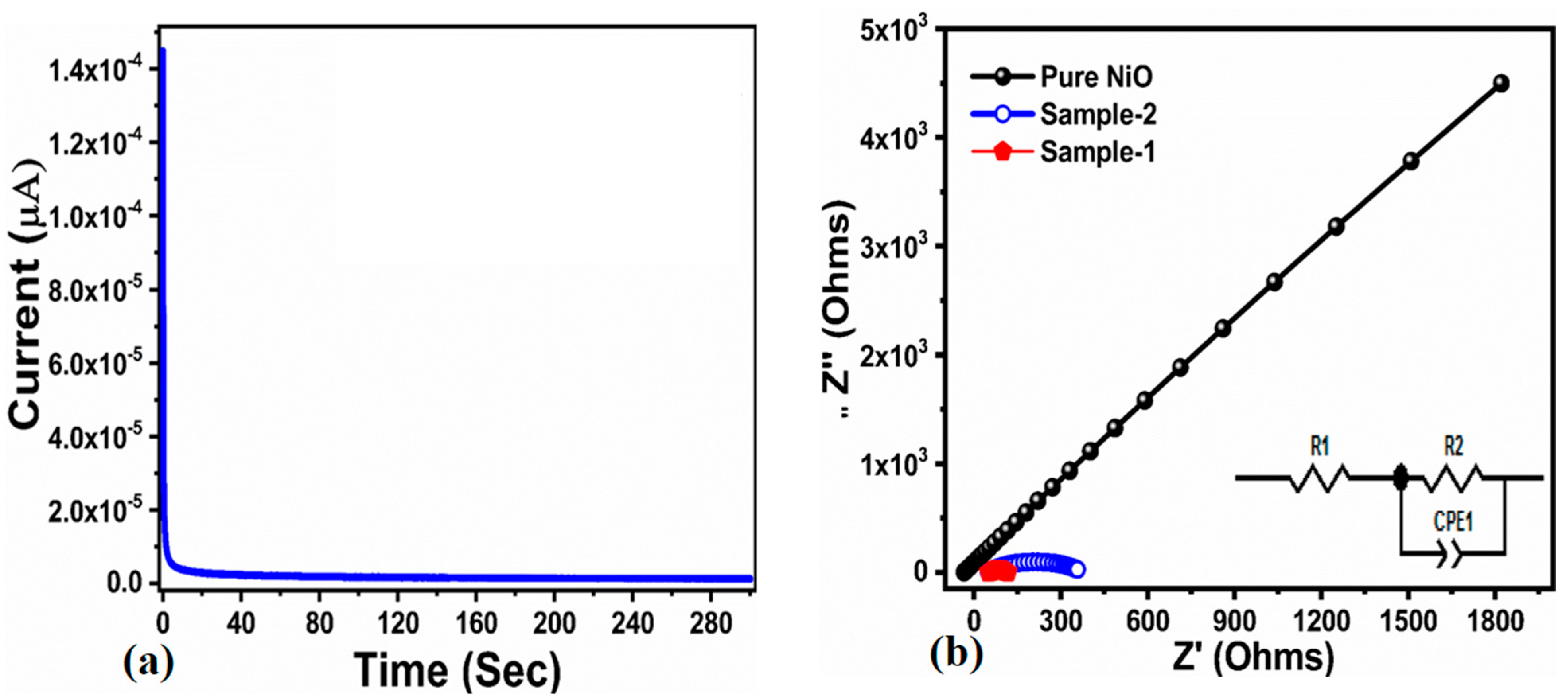
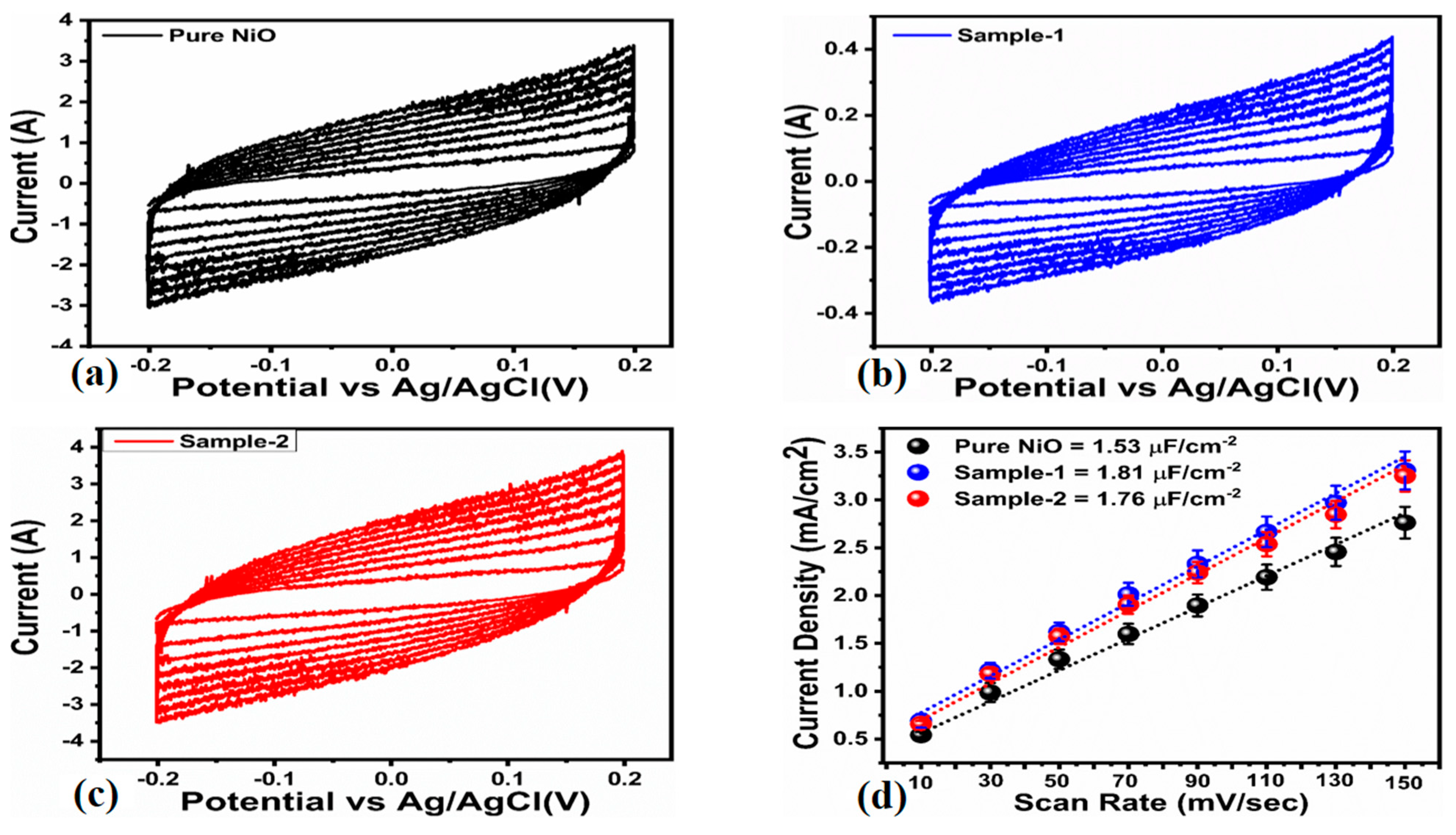
| Scan Rate (mV/S) | Peak Separation Potential (mV) | Peak Current Ratio |
|---|---|---|
| 10 | 143 | 2.5210631 |
| 30 | 136 | 2.1818611 |
| 50 | 140 | 2.1720295 |
| 70 | 142 | 2.1576472 |
| 90 | 149 | 2.1611629 |
| 110 | 153 | 2.1153078 |
| 130 | 157 | 2.1042438 |
| 150 | 159 | 2.1252675 |
| 170 | 165 | 2.1040786 |
| 190 | 168 | 2.0843702 |
| 210 | 170 | 2.0709691 |
| 230 | 168 | 2.0379324 |
| 250 | 170 | 2.0389048 |
| 270 | 179 | 2.0315545 |
| 290 | 182 | 2.0172478 |
| 310 | 186 | 2.0193316 |
| 330 | 188 | 2.0142131 |
| 350 | 192 | 2.0170487 |
| 370 | 190 | 2.0202359 |
| 390 | 198 | 1.984715 |
| Sensing Material | Linear Range (mM) | Limit of Detection | Method of Detection | Reference |
|---|---|---|---|---|
| NiO–MoO3 | 0.2–1 | 0.86–0.85 μM | Non-enzymatic | [64] |
| NiCo2O4 | 0.01–5 | 1.0 μM | Non-enzymatic | [65] |
| NiS/GO/MGCE | 0.1–1.0 | 3.79–12.6 μM | Non-enzymatic sensor | [66] |
| NF/Ag-N-SWCNTs/GCE | 66 nM–20.6 mM | 4.7 nM | Non-enzymatic | [67] |
| NF/urease/Yb2O3/ GCE | 0.05–19 mM | 2 μM | Enzymatic | [68] |
| Au/MWCNT-PAMAM (G5)/Urease | 1–20 mM | 0.4 mM | Enzymatic | [69] |
| NiO-PE(A4) | Up to 4 mM | 5 μM | Non-enzymatic | [70] |
| Ur/Nr- NiO/ITO/glass | 0.83–16.65 mM | 0.47 mM | Enzymatic | [71] |
| Ag/NiOOH/C nanorod electrode | 0.2 to 26.0 mM | 5.0 μM | Non-enzymatic | [72] |
| NF-LDH | 0.5 to 8 mM | 0.114 mM | Non-enzymatic | [73] |
| ZnO NRs | 0.001–24.0 mM | 10 μM | Enzymatic | [19] |
| Nafion®(urease)/ PANI-Nafion® | 3–30 mM | 1 μM | Enzymatic | [74] |
| nano-PANI:PSS | 0.2–0.9 mM | 10.4 mgdL−1 | Enzymatic | [75] |
| NiO nanoflakes | 1–9 mM | 0.02 mM | Non-enzymatic | This work |
| Experiment | Linear Range (mM) | Limit of Detection (mM) | Limit of Quantification (mM) |
|---|---|---|---|
| 1 | 0.98–9.02 ± 0.001 | 0.021 ± 0.005 | 0.088 ± 0.006 |
| 2 | 1–9.01 ± 0.003 | 0.009 ± 0.003 | 0.086 ± 0.008 |
| 3 | 0.99–9.03 ± 0.004 | 0.022 ± 0.002 | 0.091 ± 0.005 |
| Sample | Added (mM) | Found (mM) | (%) Recovery |
|---|---|---|---|
| 1 | - | 3 ± 0.001 | - |
| - | 0.5 | 3.48 ± 0.003 | 99% |
| - | 1 | 4.01 ± 0.001 | 100% |
| 2 | - | 3.5 ± 0.001 | - |
| - | 0.5 | 4.02 ± 0.001 | 100% |
| - | 1 | 4.49 ± 0.001 | 0.99% |
| Sample | Added(mM) | Found (mM) | (%) Recovery |
|---|---|---|---|
| 1 | - | 1 ± 0.002 | - |
| - | 0.3 | 1.31 ± 0.003 | 100 |
| - | 0.6 | 1.88 ± 0.003 | 98 |
| 2 | - | 3 ± 0.003 | - |
| - | 1 | 4.05 ± 0.001 | 101.25 |
| - | 1.5 | 4.52 ± 0.002 | 100.44 |
| Sample | Added (mM) | Found (mM) | (%) Recovery |
|---|---|---|---|
| 1 | - | 3.5 ± 0.002 | - |
| - | 0.5 | 4.06 ± 0.003 | 101.50 |
| - | 1 | 4.53 ± 0.001 | 100.60 |
| 2 | - | 1.5 ± 0.001 | - |
| - | 1 | 2.51 ± 0.003 | 100.40 |
| - | 2 | 3.53 ± 0.002 | 100.85 |
| Sample | Added (mM) | Found (mM) | % Recovery |
|---|---|---|---|
| 1 | - | 2 ± 0.004 | - |
| - | 0.5 | 2.49 ± 0.003 | 99 |
| - | 1.5 | 3.54 ± 0.002 | 101.11 |
| 2 | - | 2.4 ± 0.001 | - |
| - | 1 | 3.43 ± 0.002 | 100.8 |
| - | 1.5 | 3.9 ± 0.004 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naz, I.; Tahira, A.; Shah, A.A.; Bhatti, M.A.; Mahar, I.A.; Markhand, M.P.; Mastoi, G.M.; Nafady, A.; Medany, S.S.; Dawi, E.A.; et al. Green Synthesis of NiO Nanoflakes Using Bitter Gourd Peel, and Their Electrochemical Urea Sensing Application. Micromachines 2023, 14, 677. https://doi.org/10.3390/mi14030677
Naz I, Tahira A, Shah AA, Bhatti MA, Mahar IA, Markhand MP, Mastoi GM, Nafady A, Medany SS, Dawi EA, et al. Green Synthesis of NiO Nanoflakes Using Bitter Gourd Peel, and Their Electrochemical Urea Sensing Application. Micromachines. 2023; 14(3):677. https://doi.org/10.3390/mi14030677
Chicago/Turabian StyleNaz, Irum, Aneela Tahira, Aqeel Ahmed Shah, Muhammad Ali Bhatti, Ihsan Ali Mahar, Mehnaz Parveen Markhand, Ghulam Murtaza Mastoi, Ayman Nafady, Shymaa S. Medany, Elmuez A. Dawi, and et al. 2023. "Green Synthesis of NiO Nanoflakes Using Bitter Gourd Peel, and Their Electrochemical Urea Sensing Application" Micromachines 14, no. 3: 677. https://doi.org/10.3390/mi14030677
APA StyleNaz, I., Tahira, A., Shah, A. A., Bhatti, M. A., Mahar, I. A., Markhand, M. P., Mastoi, G. M., Nafady, A., Medany, S. S., Dawi, E. A., Saleem, L. M., Vigolo, B., & Ibupoto, Z. H. (2023). Green Synthesis of NiO Nanoflakes Using Bitter Gourd Peel, and Their Electrochemical Urea Sensing Application. Micromachines, 14(3), 677. https://doi.org/10.3390/mi14030677








