Analysis of Homogeneous/Heterogeneous Reactions in an Electrohydrodynamic Environment Utilizing the Second Law
Abstract
1. Introduction
2. Mathematical Formulation
2.1. Geometric Model
2.2. Eyring–Powell Fluid Model
2.3. Electrical Potential Distribution
2.4. Flow Analysis
3. Entropy Generation
4. Solution of the Problem
5. Computational Results and Discussion
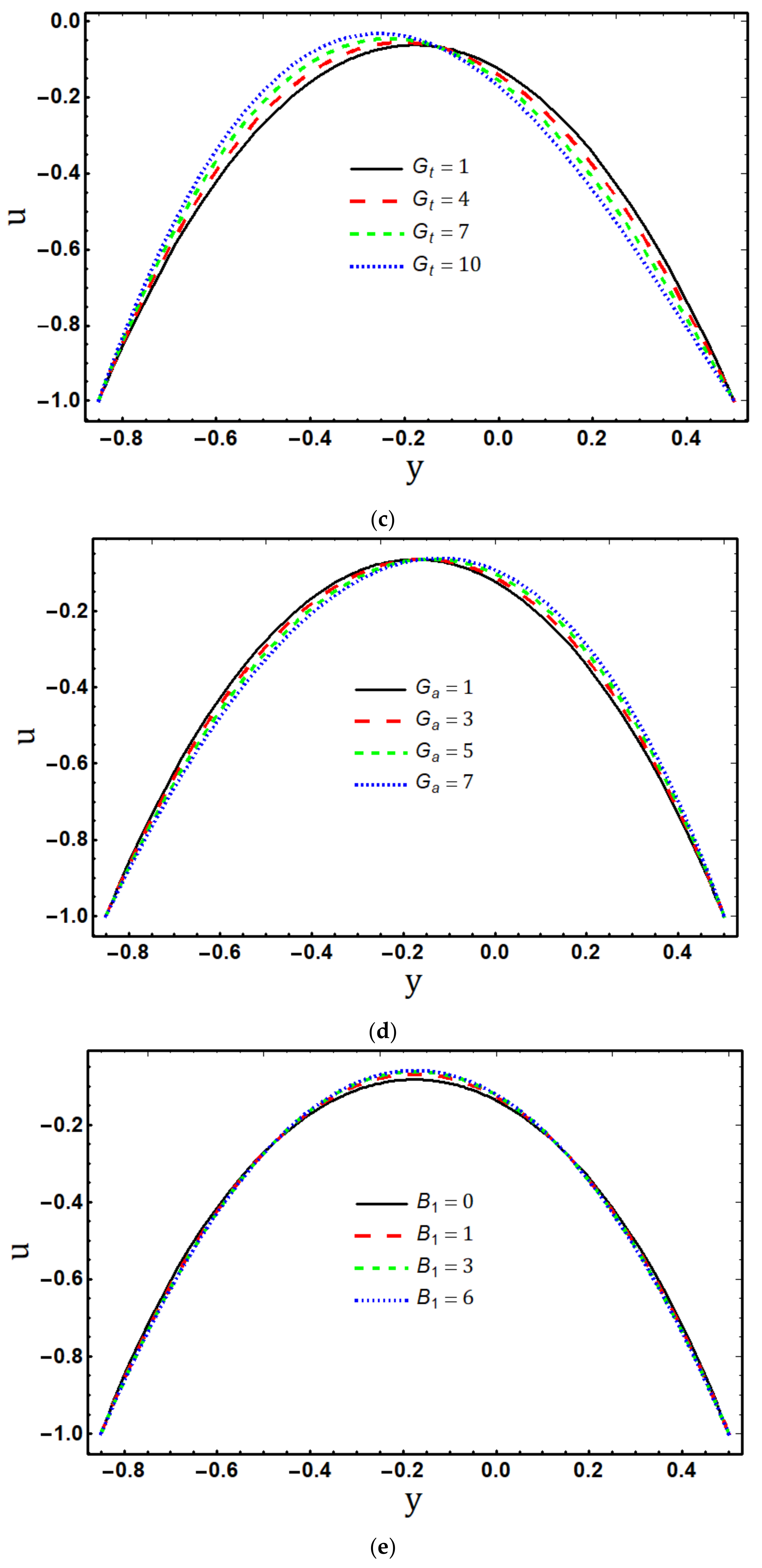
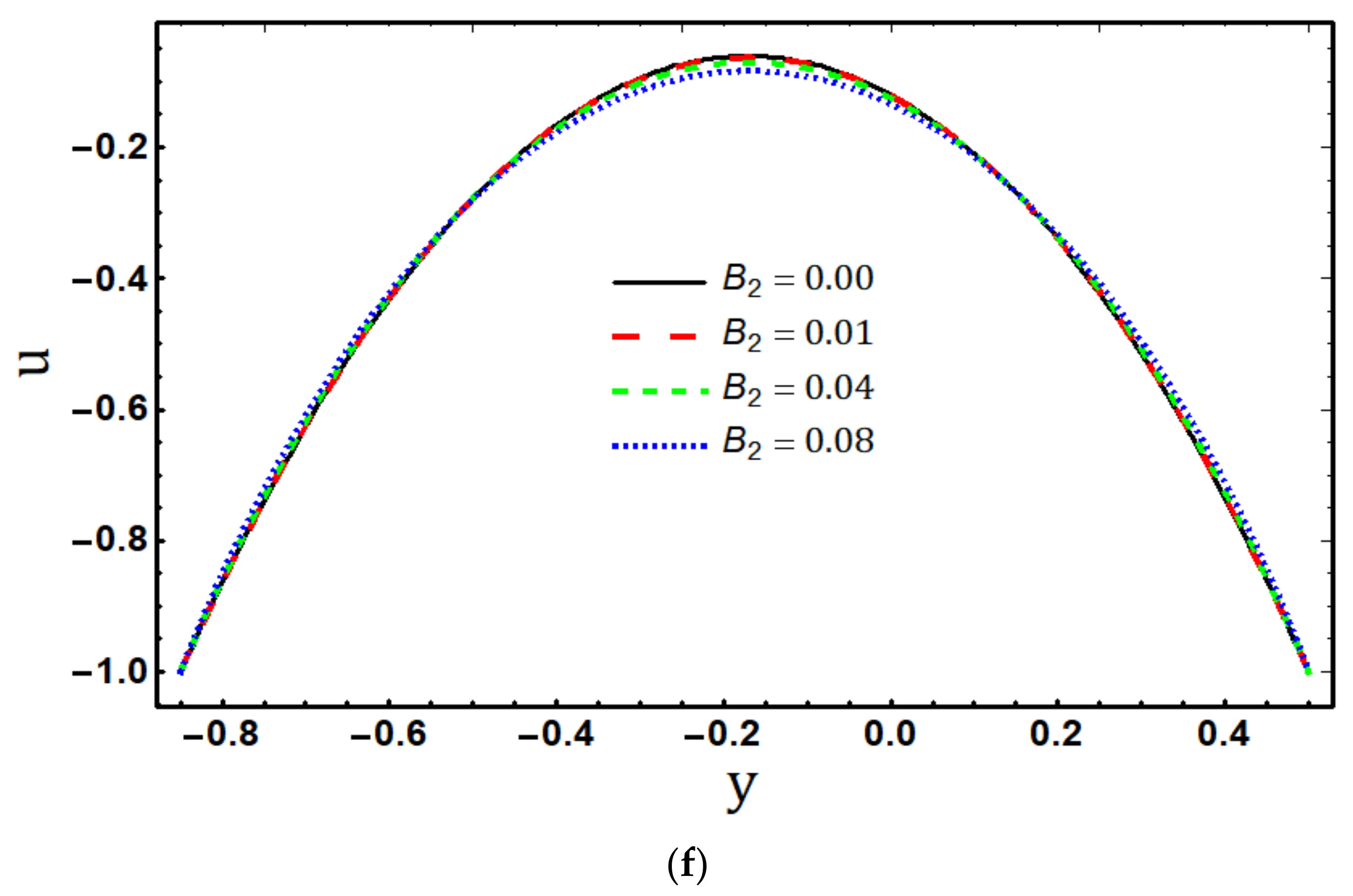
5.1. Velocity Profile
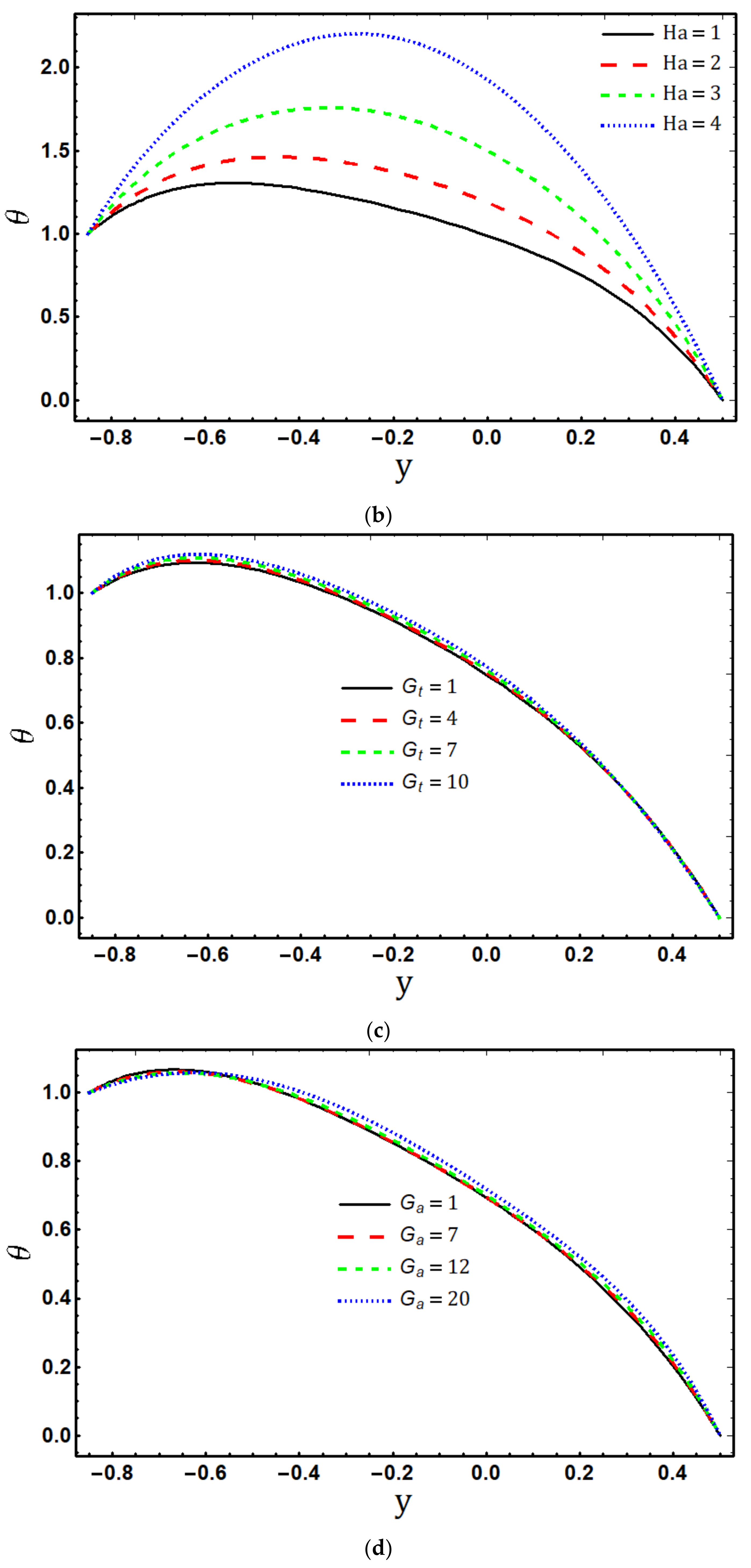
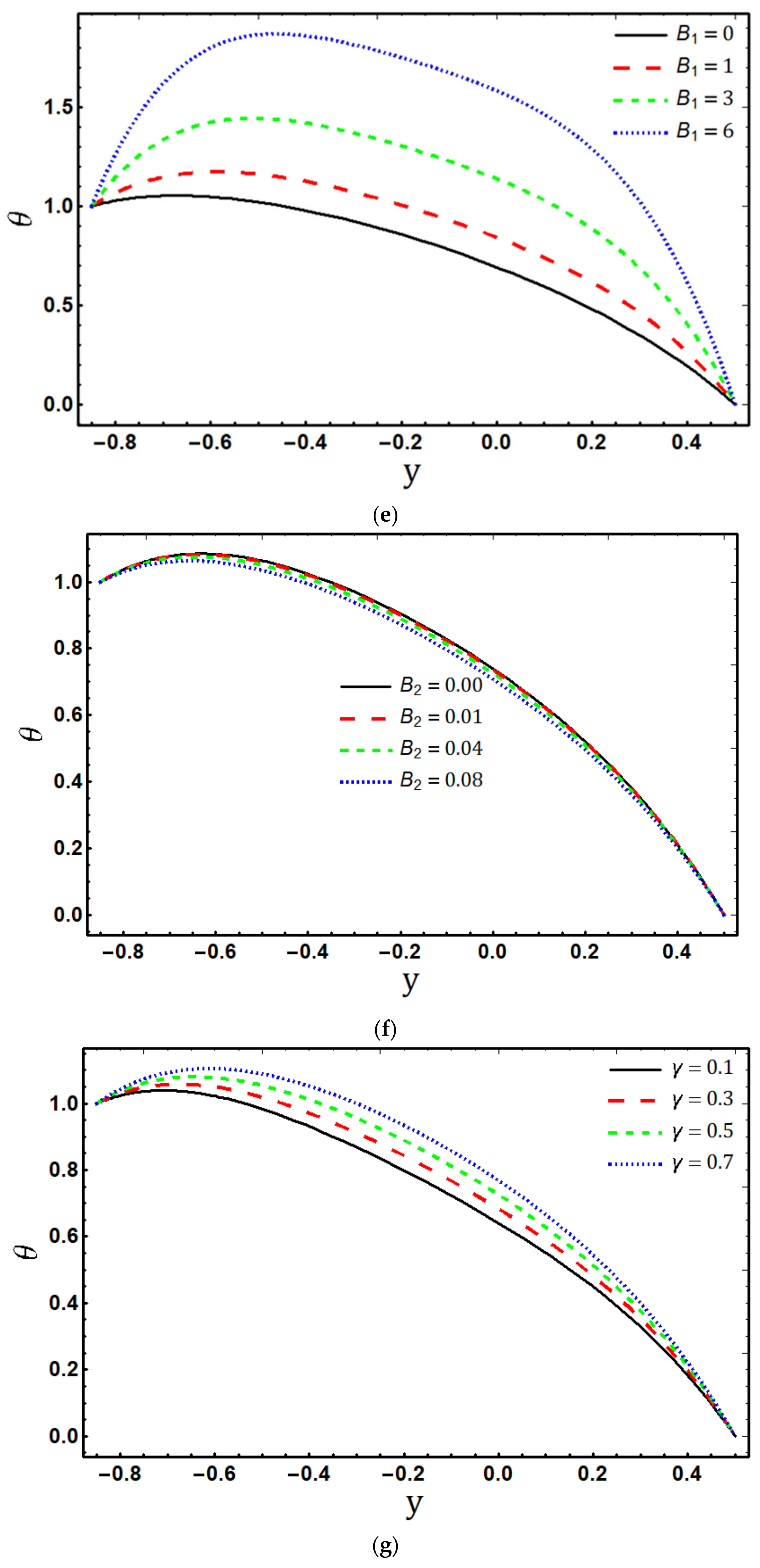
5.2. Temperature Profile
5.3. Concentration Profile
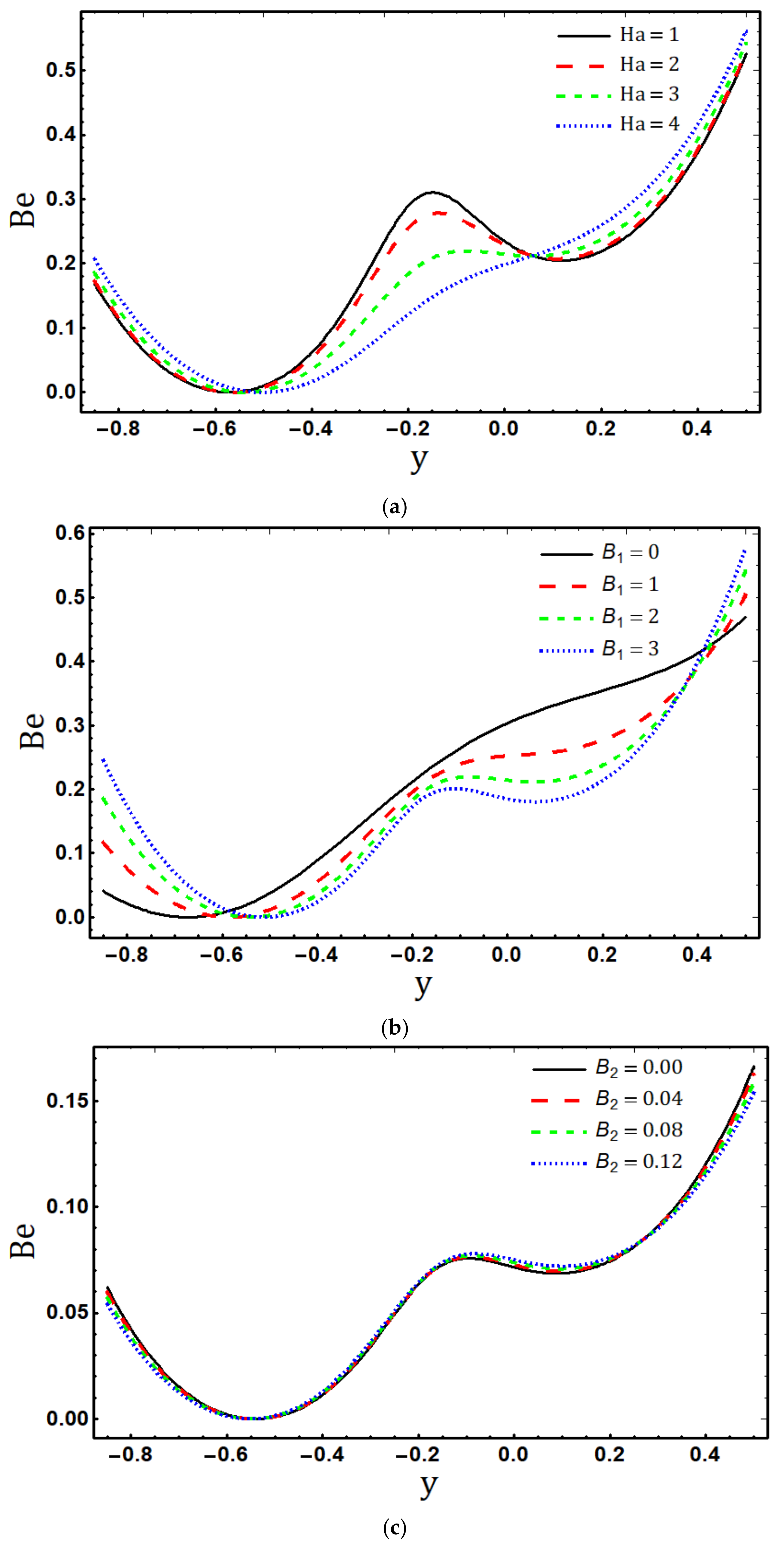
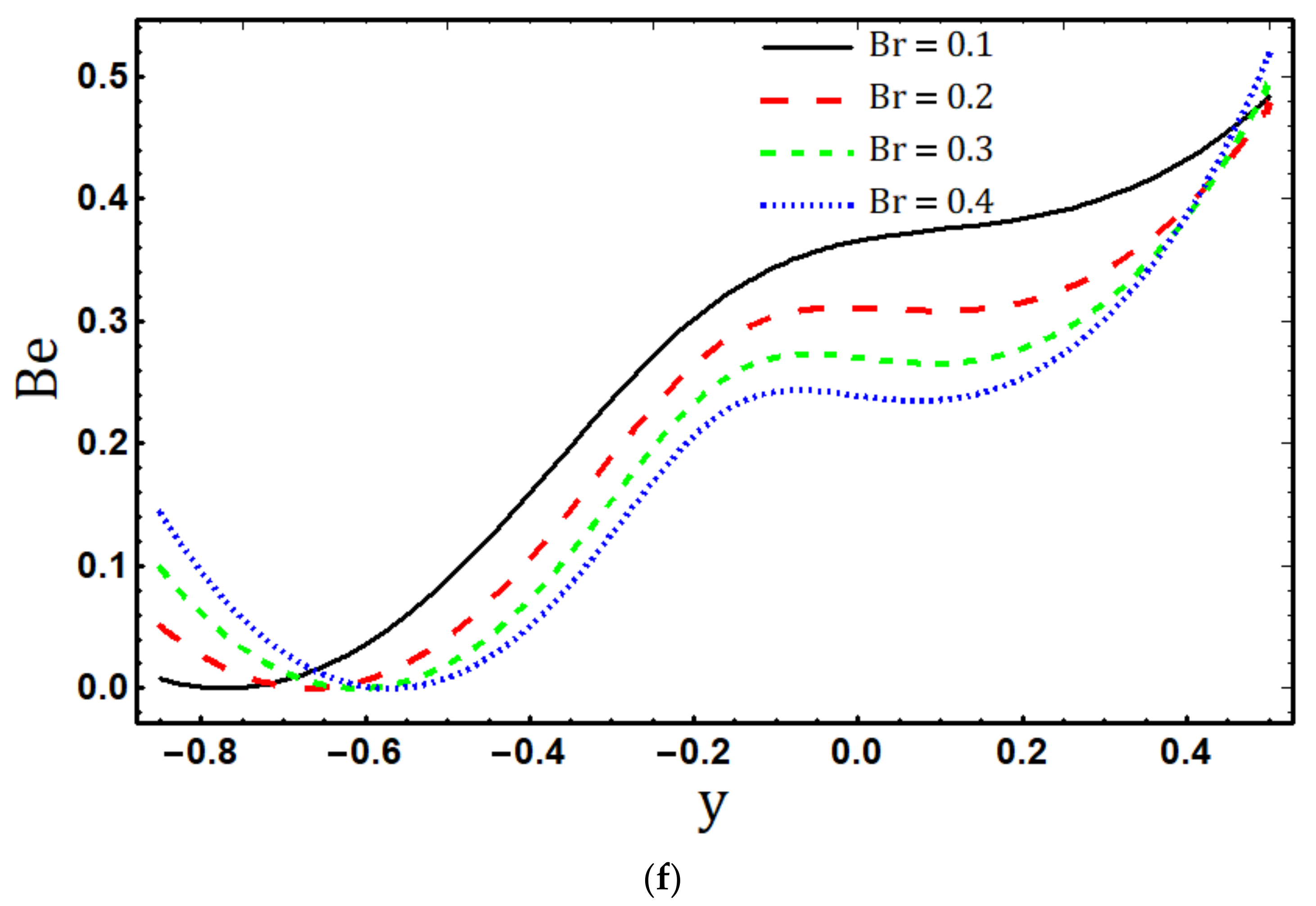
5.4. Entropy Generation
6. Concluding Remarks
- The axial velocity distribution displays divergent behaviour in the centre of the channel and near to its boundaries as the values of the material fluid parameters and increase;
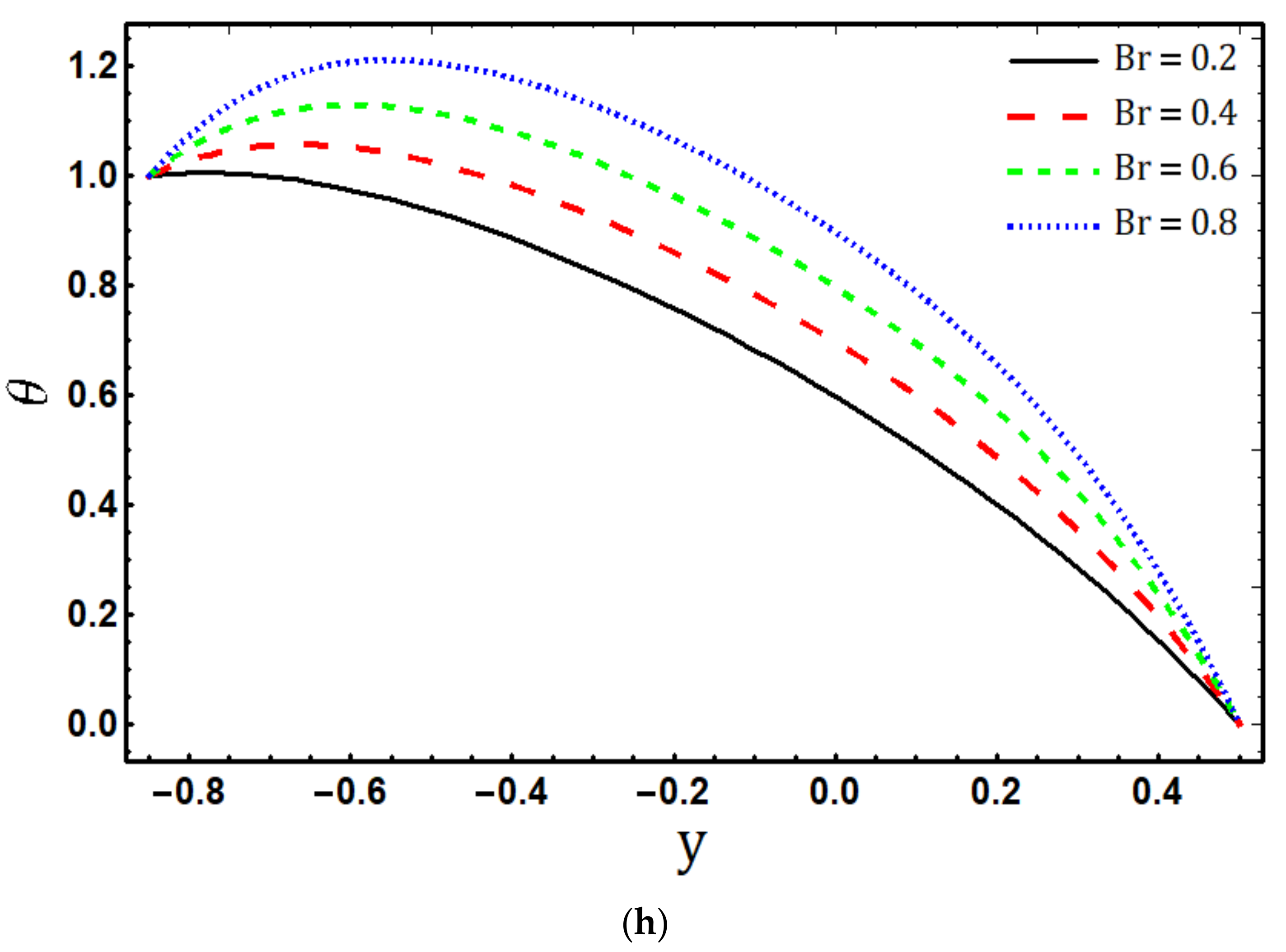
- The fluid temperature increases by increasing , and , while it decreases for and ;
- Temperature is the increasing function of viscous dissipation parameter and Joule heating parameter ;
- The concentration field declines as the Schmidt number rises;
- Heterogeneous reaction parameter enhances the concentration field while the homogenous reaction parameter reduces the concentration ;
- For and , the current research may be condensed to viscous fluid flow.
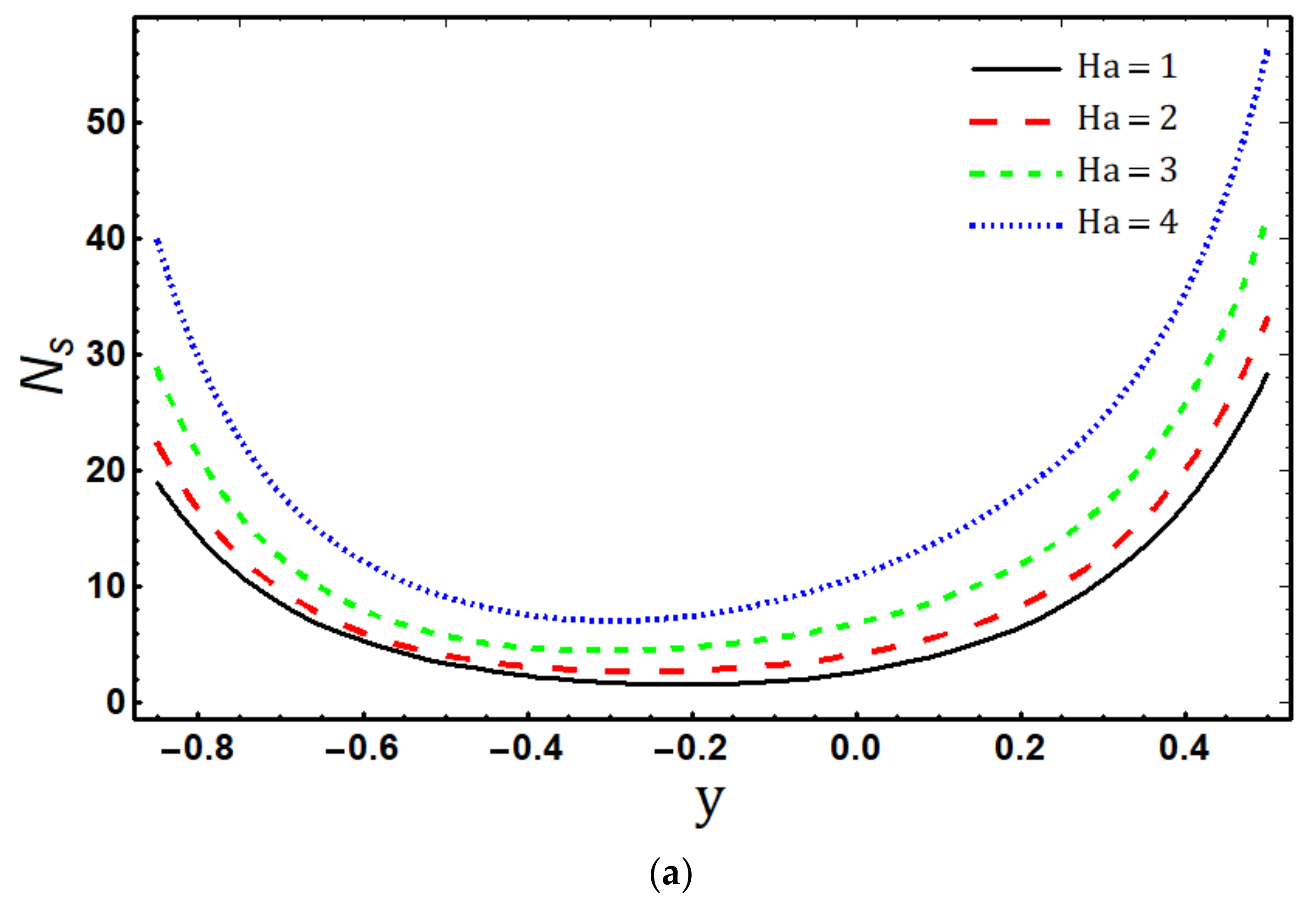
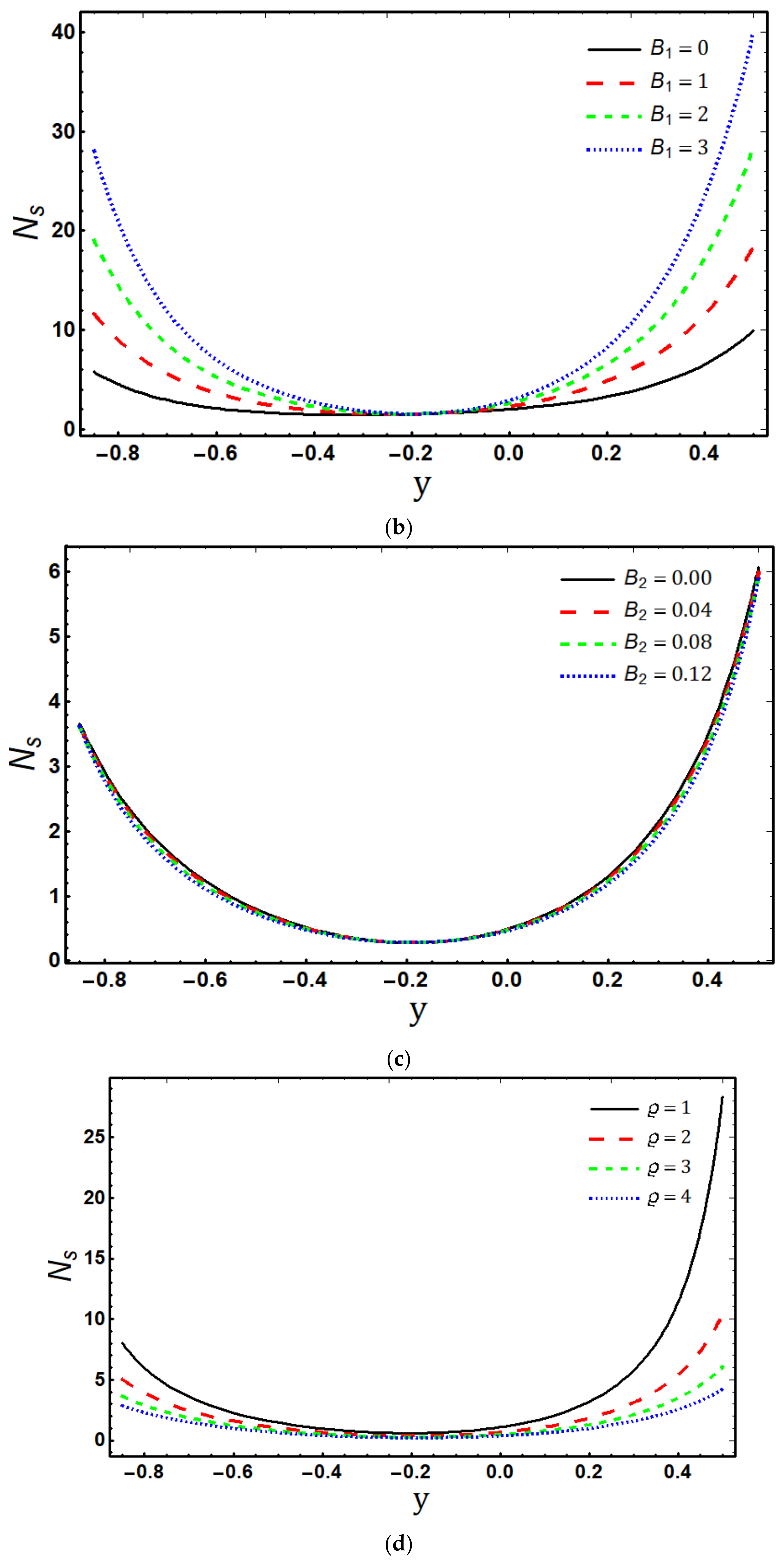
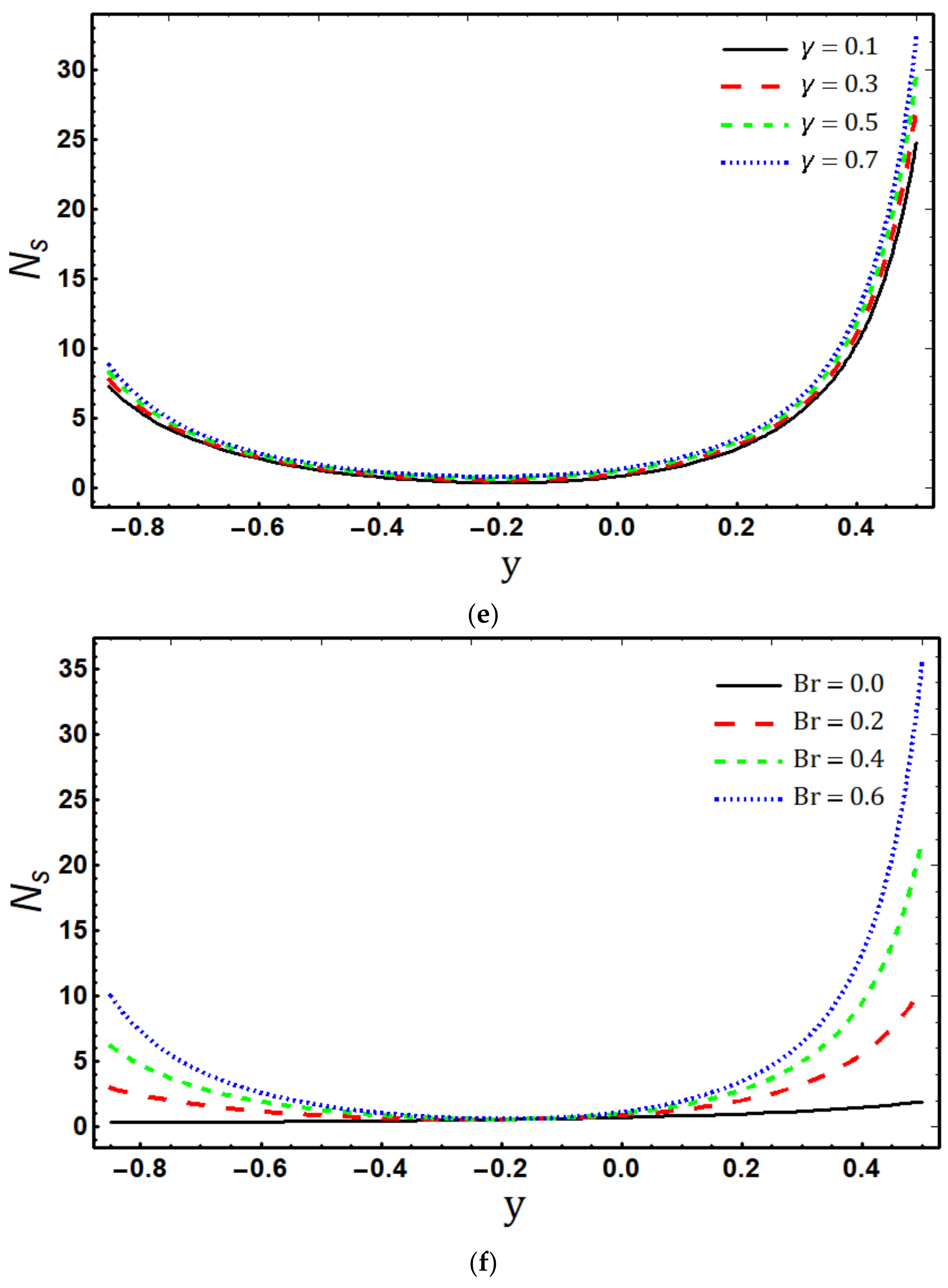
- The entropy generation rate increases with increasing , and and decreases with increasing and ;
- Maximum entropy production occurs in the vicinity of the channel walls;
- The dominance of the electric field, fluid friction, and magnetic field irreversibility is represented by near the lower wall in the Bejan number profile.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Meaning | Dimensions |
| Dimensional lower and upper walls in the fixed frame | ||
| Dimensional lower and upper walls in the wave frame | ||
| Dimensional amplitude of the wavy walls in the fixed frame | ||
| Dimensionless amplitude of the wavy walls in the wave frame | ||
| Speed of wave | ||
| Width of the channel | ||
| Dimensional time in fixed frame | ||
| Wavelength | ||
| Dimensional coordinates in the fixed frame | ||
| Dimensional coordinates in the wave frame | ||
| Dimensionless coordinates | ||
| Dimensional velocity components in the fixed frame | ||
| Dimensional velocity components in the wave frame | ||
| Dimensionless velocity components | ||
| Angle of inclination | ||
| Phase difference | ||
| Dimensional upper and lower wall temperature | ||
| Dimensionless temperature | ||
| Chemical species | ||
| Concentration of chemical | ||
| Uniform concentration of reactant | ||
| Dimensionless concentration of chemical species | ||
| Mass diffusivity of species | ||
| Homogeneous reaction constant for cubic autocatalysis | ||
| Reaction rate constant for the first order heterogeneous reaction | ||
| Thermal expansion coefficient | ||
| Concentration expansion coefficient | ||
| Acceleration due to gravity | ||
| Dimensional pressure in the fixed frame | ||
| Dimensionless pressure | ||
| Dimensional zeta potential of lower and upper walls | ||
| Dimensionless zeta potential of lower and upper walls | ||
| Dimensional electric potential | ||
| Dimensionless electric potential | ||
| Charge balance | ||
| Electric charge | ||
| Mean temperature | ||
| Boltzmann constant | ||
| Positive, negative ions | ||
| Number density | ||
| Plastic viscosity | ||
| Eyring–Powell fluid constant | ||
| Eyring–Powell fluid constant | ||
| Stress tensor | ||
| Eyring-Powel fluid parameters | ||
| Electric field | ||
| Magnetic field | ||
| Debye length | ||
| Helmholtz–Smoluchowski velocity | ||
| Dimensionless Helmholtz–Smoluchowski velocity | ||
| Density of fluid | ||
| Net charge density | ||
| Relative permittivity of fluid | ||
| Permittivity of free space | ||
| Thermal conductivity | ||
| Wave number | ||
| Specific heat | ||
| Electroosmotic parameter | ||
| Peclet number | ||
| Reynolds number | ||
| Prandtl number | ||
| Brinkman number | ||
| Joule heating parameter | ||
| Schmidt number | ||
| Temperature Grashoff number | ||
| Concentration Grashoff number | ||
| Homogeneous reaction parameter | ||
| Heterogeneous reaction parameter |
References
- Chaudhary, M.A.; Merkin, J.H. A simple isothermal model for homogeneous-heterogeneous reactions in boundary-layer flow. I Equal diffusivities. Fluid Dyn. Res. 1995, 16, 311–333. [Google Scholar] [CrossRef]
- Merkin, J.H. A model for isothermal homogeneous-heterogeneous reactions in boundary-layer flow. Math. Comput. Model. 1996, 24, 125–136. [Google Scholar] [CrossRef]
- Kameswaran, P.K.; Shaw, S.; Sibanda, P.V.S.N.; Murthy, P.V.S.N. Homogeneous–heterogeneous reactions in a nanofluid flow due to a porous stretching sheet. Int. J. Heat Mass Transf. 2013, 57, 465–472. [Google Scholar] [CrossRef]
- Shaw, S.; Kameswaran, P.K.; Sibanda, P. Homogeneous-heterogeneous reactions in micropolar fluid flow from a permeable stretching or shrinking sheet in a porous medium. Bound. Value Probl. 2013, 2013, 77. [Google Scholar] [CrossRef]
- Hayat, T.; Imtiaz, M.; Alsaedi, A. Impact of magnetohydrodynamics in bidirectional flow of nanofluid subject to second order slip velocity and homogeneous–heterogeneous reactions. J. Magn. Magn. Mater. 2015, 395, 294–302. [Google Scholar] [CrossRef]
- Powell, R.E.; Eyring, H. Mechanisms for the relaxation theory of viscosity. Nature 1944, 154, 427–428. [Google Scholar] [CrossRef]
- Khan, N.A.; Aziz, S.; Khan, N.A. MHD flow of Powell–Eyring fluid over a rotating disk. J. Taiwan Inst. Chem. Eng. 2014, 45, 2859–2867. [Google Scholar] [CrossRef]
- Nadeem, S.; Saleem, S. Mixed convection flow of Eyring–Powell fluid along a rotating cone. Results Phys. 2014, 4, 54–62. [Google Scholar] [CrossRef]
- Hayat, T.; Waqas, M.; Shehzad, S.A.; Alsaedi, A. Mixed convection stagnation-point flow of Powell-Eyring fluid with Newtonian heating, thermal radiation, and heat generation/absorption. J. Aerosp. Eng. 2017, 30, 04016077. [Google Scholar] [CrossRef]
- Adesanya, S.O.; Ogunseye, H.A.; Jangili, S. Unsteady squeezing flow of a radiative Eyring-Powell fluid channel flow with chemical reactions. Int. J. Therm. Sci. 2018, 125, 440–447. [Google Scholar] [CrossRef]
- Latham, T.W. Fluid Motions in a Peristaltic Pump. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1966. [Google Scholar]
- Shapiro, A.H.; Jaffrin, M.Y.; Weinberg, S.L. Peristaltic pumping with long wavelengths at low Reynolds number. J. Fluid Mech. 1969, 37, 799–825. [Google Scholar] [CrossRef]
- Jaffrin, M.Y.; Shapiro, A.H. Peristaltic pumping. Annu. Rev. Fluid Mech. 1971, 3, 13–37. [Google Scholar] [CrossRef]
- Noreen, S.; Qasim, M. Peristaltic flow of MHD Eyring-Powell fluid in a channel. Eur. Phys. J. Plus 2013, 128, 91. [Google Scholar] [CrossRef]
- Noreen, S. Soret-Dufour effects in electroosmotic biorheological flow of Jeffrey fluid. Heat Transf. 2020, 49, 2355–2374. [Google Scholar] [CrossRef]
- Hayat, T.; Asghar, S.; Tanveer, A.; Alsaedi, A. Homogeneous–heterogeneous reactions in peristaltic flow of Prandtl fluid with thermal radiation. J. Mol. Liq. 2017, 240, 504–513. [Google Scholar] [CrossRef]
- Tanveer, A.; Hayat, T.; Alsaadi, F.; Alsaedi, A. Mixed convection peristaltic flow of Eyring-Powell nanofluid in a curved channel with compliant walls. Comput. Biol. Med. 2017, 82, 71–79. [Google Scholar] [CrossRef]
- Noreen, S. Magneto-thermo hydrodynamic peristaltic flow of Eyring-Powell nanofluid in asymmetric channel. Nonlinear Eng. 2018, 7, 83–90. [Google Scholar] [CrossRef]
- Asha, S.K.; Sunitha, G. Mixed convection peristaltic flow of a Eyring–Powell nanofluid with magnetic field in a non-uniform channel. JAMA 2018, 2, 332–334. [Google Scholar] [CrossRef]
- Chakraborty, S. Augmentation of peristaltic microflows through electro-osmotic mechanisms. J. Phys. D Appl. Phys. 2006, 39, 5356. [Google Scholar] [CrossRef]
- Mallick, B.; Misra, J.C. Peristaltic flow of Eyring-Powell nanofluid under the action of an electromagnetic field. Eng. Sci. Technol. Int. J. 2019, 22, 266–281. [Google Scholar] [CrossRef]
- Rafiq, M.; Sajid, M.; Alhazmi, S.E.; Khan, M.I.; El-Zahar, E.R. MHD electroosmotic peristaltic flow of Jeffrey nanofluid with slip conditions and chemical reaction. Alex. Eng. J. 2022, 61, 9977–9992. [Google Scholar] [CrossRef]
- Bejan, A. A study of entropy generation in fundamental convective heat transfer. J. Heat Transfer. 1979, 101, 718–725. [Google Scholar] [CrossRef]
- Bejan, A. Entropy generation minimization: The new thermodynamics of finite-size devices and finite-time processes. J. Appl. Phys. 1996, 79, 1191–1218. [Google Scholar] [CrossRef]
- Bejan, A.; Dincer, I.; Lorente, S.; Miguel, A.; Reis, H. Porous and Complex Flow Structures in Modern Technologies; Springer Science & Business Media: Berlin/Heidelberfg, Germany, 2004. [Google Scholar]
- Madhu, M.; Shashikumar, N.S.; Gireesha, B.J.; Kishan, N. Second law analysis of Powell–Eyring fluid flow through an inclined microchannel with thermal radiation. Phys. Scr. 2019, 94, 125205. [Google Scholar] [CrossRef]
- Alsaedi, A.; Hayat, T.; Qayyum, S.; Yaqoob, R. Eyring–Powell nanofluid flow with nonlinear mixed convection: Entropy generation minimization. Comput. Methods Programs Biomed. 2020, 186, 105183. [Google Scholar] [CrossRef] [PubMed]
- Mabood, F.; Farooq, W.; Abbasi, A. Entropy generation analysis in the electro-osmosis-modulated peristaltic flow of Eyring–Powell fluid. J. Therm. Anal. Calorim. 2022, 147, 3815–3830. [Google Scholar] [CrossRef]






| Eyring–Powell Fluid | Viscous Fluid | ||||
|---|---|---|---|---|---|
| 1 | 0.4 | 0.6 | 1 | 0.619415 | 0.677288 |
| 2 | 0.4 | 0.6 | 1 | 1.06133 | 1.14184 |
| 3 | 0.4 | 0.6 | 1 | 1.64373 | 1.71052 |
| 1 | 0.0 | 0.6 | 1 | 0.524283 | 0.524283 |
| 1 | 0.2 | 0.6 | 1 | 0.565297 | 0.58877 |
| 1 | 0.4 | 0.6 | 1 | 0.602348 | 0.648808 |
| 1 | 0.4 | 0.0 | 1 | 0.388873 | 0.418463 |
| 1 | 0.4 | 0.4 | 1 | 0.545421 | 0.59462 |
| 1 | 0.4 | 0.8 | 1 | 0.690755 | 0.756632 |
| 1 | 0.4 | 0.6 | 1 | 0.619415 | 0.677288 |
| 1 | 0.4 | 0.6 | 2 | 0.405706 | 0.416037 |
| 1 | 0.4 | 0.6 | 3 | 0.301105 | 0.299335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, F.; Noreen, S.; Afridi, M.I.; Qasim, M. Analysis of Homogeneous/Heterogeneous Reactions in an Electrohydrodynamic Environment Utilizing the Second Law. Micromachines 2023, 14, 821. https://doi.org/10.3390/mi14040821
Aslam F, Noreen S, Afridi MI, Qasim M. Analysis of Homogeneous/Heterogeneous Reactions in an Electrohydrodynamic Environment Utilizing the Second Law. Micromachines. 2023; 14(4):821. https://doi.org/10.3390/mi14040821
Chicago/Turabian StyleAslam, Farida, Saima Noreen, Muhammad Idrees Afridi, and Muhammad Qasim. 2023. "Analysis of Homogeneous/Heterogeneous Reactions in an Electrohydrodynamic Environment Utilizing the Second Law" Micromachines 14, no. 4: 821. https://doi.org/10.3390/mi14040821
APA StyleAslam, F., Noreen, S., Afridi, M. I., & Qasim, M. (2023). Analysis of Homogeneous/Heterogeneous Reactions in an Electrohydrodynamic Environment Utilizing the Second Law. Micromachines, 14(4), 821. https://doi.org/10.3390/mi14040821






