Bismuth-Rich Co/Ni Bimetallic Metal–Organic Frameworks as Photocatalysts toward Efficient Removal of Organic Contaminants under Environmental Conditions
Abstract
1. Introduction
2. Experimental
2.1. Materials
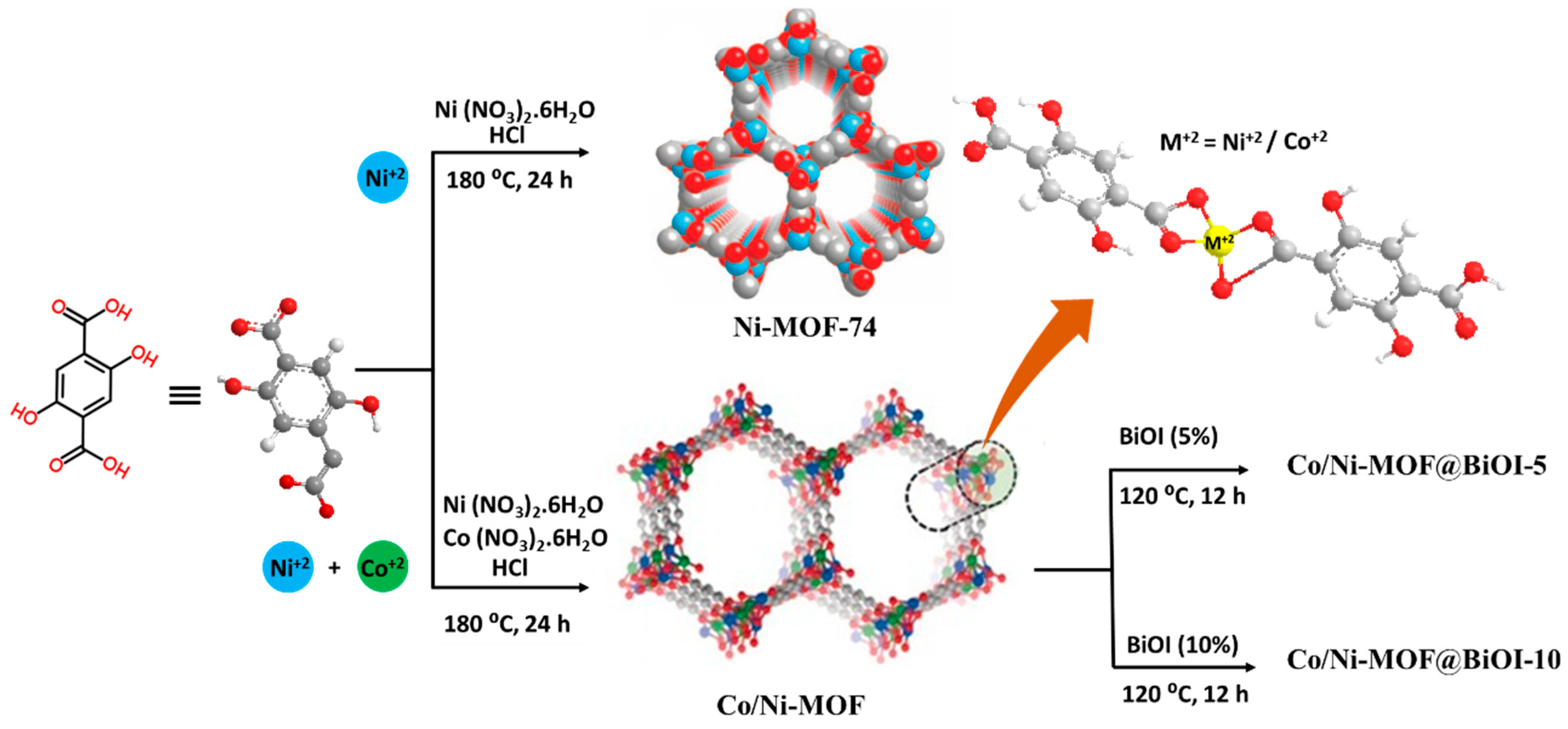
2.2. Synthesis of Ni-MOF
2.3. Synthesis of Co/Ni-MOF
2.4. Synthesis of Co/Ni-MOF@BiOI
3. Characterization
Photocatalytic Degradation Experiment
4. Results and Discussion
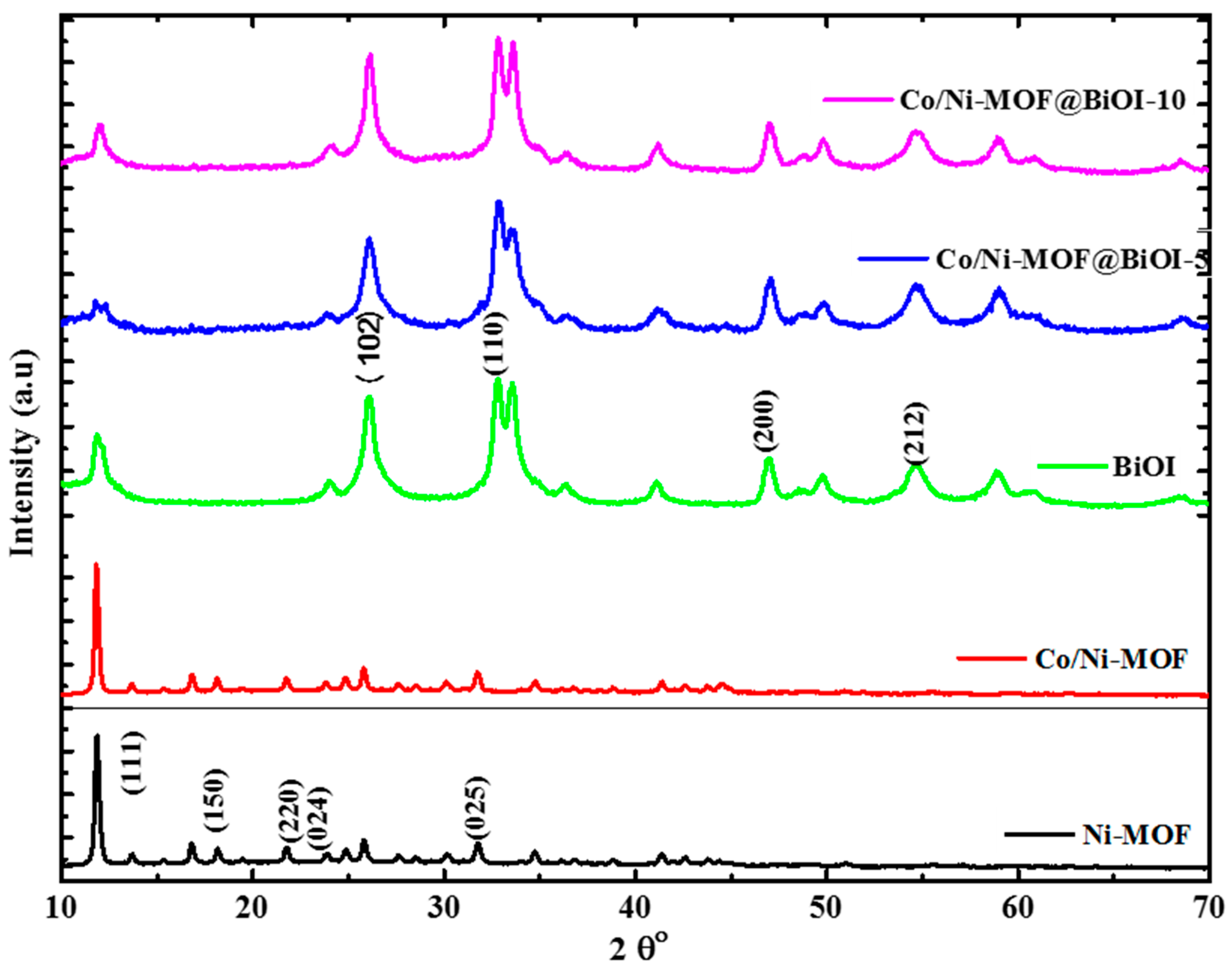
4.1. XRD Analysis
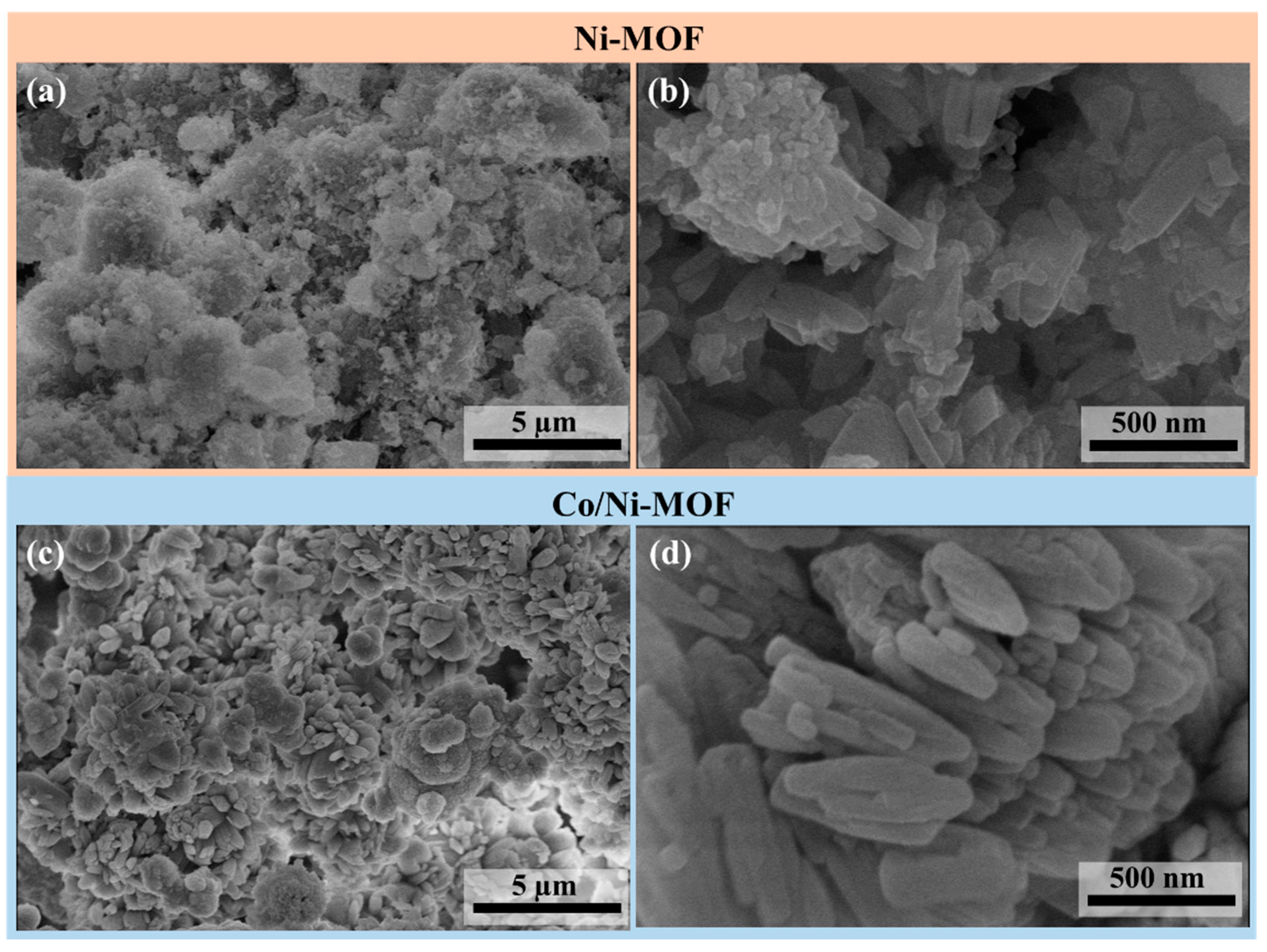
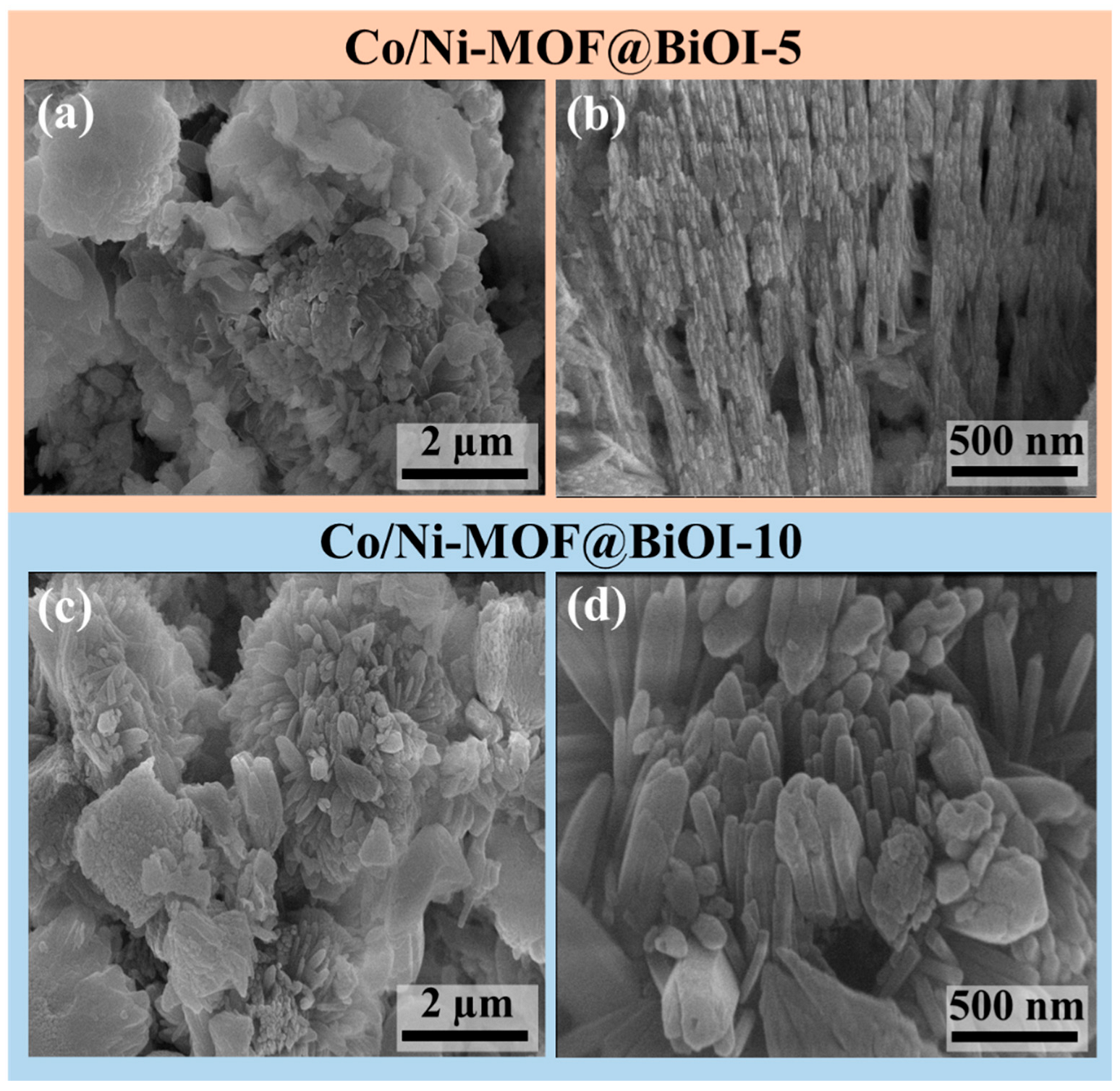
4.2. SEM Analysis
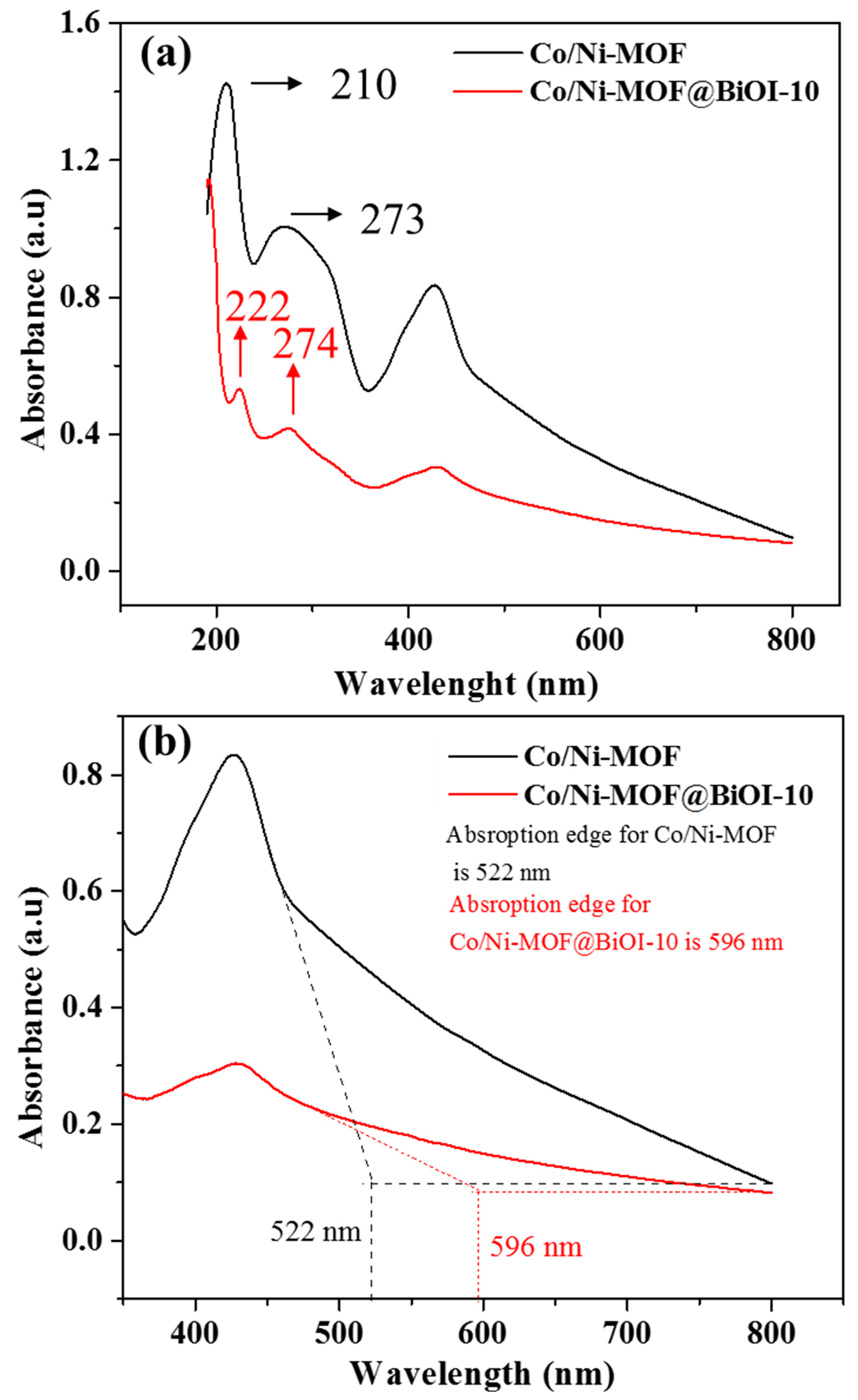
4.3. UV–Visible Spectroscopy
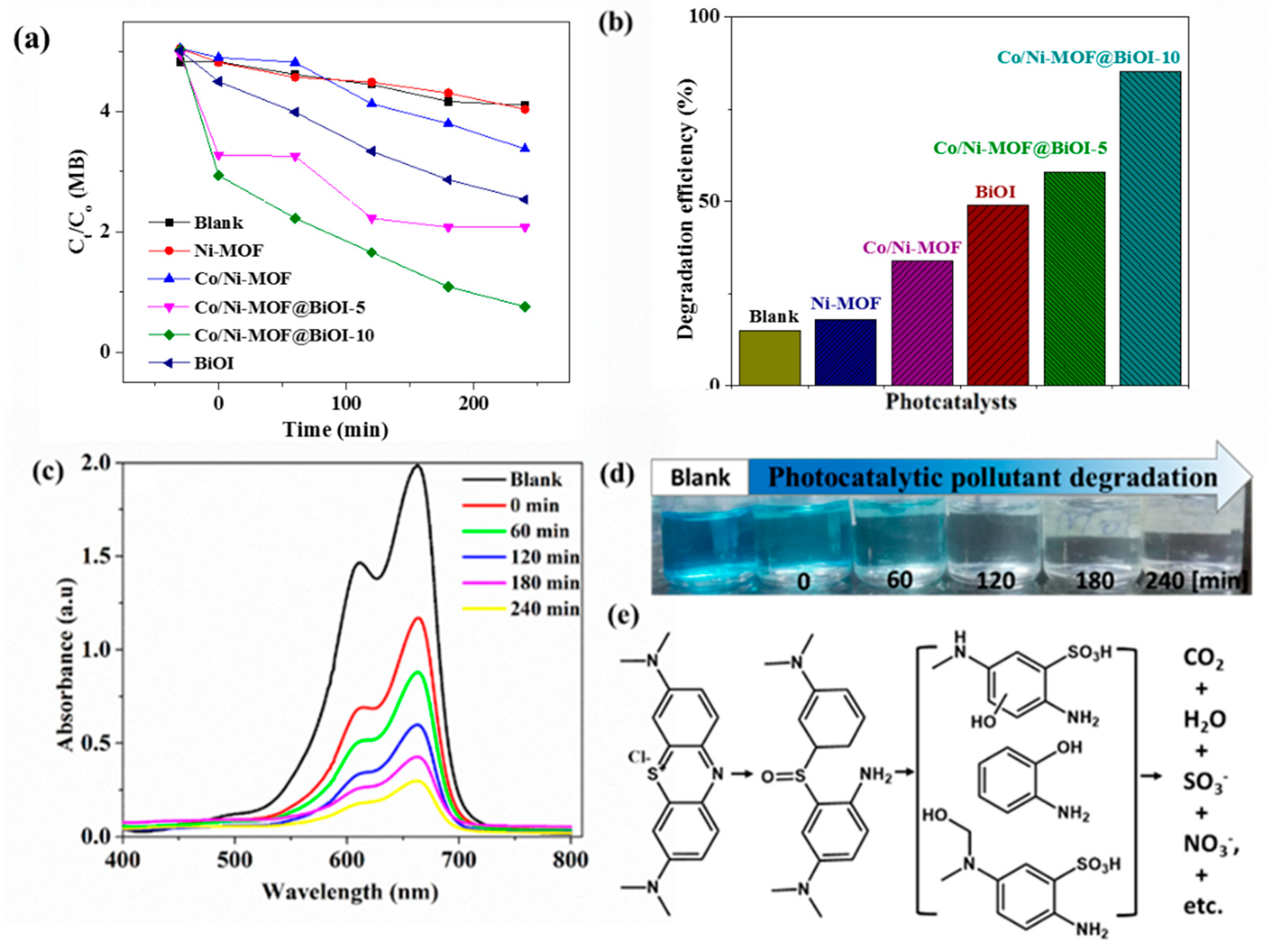
4.4. Photocatalytic Activity
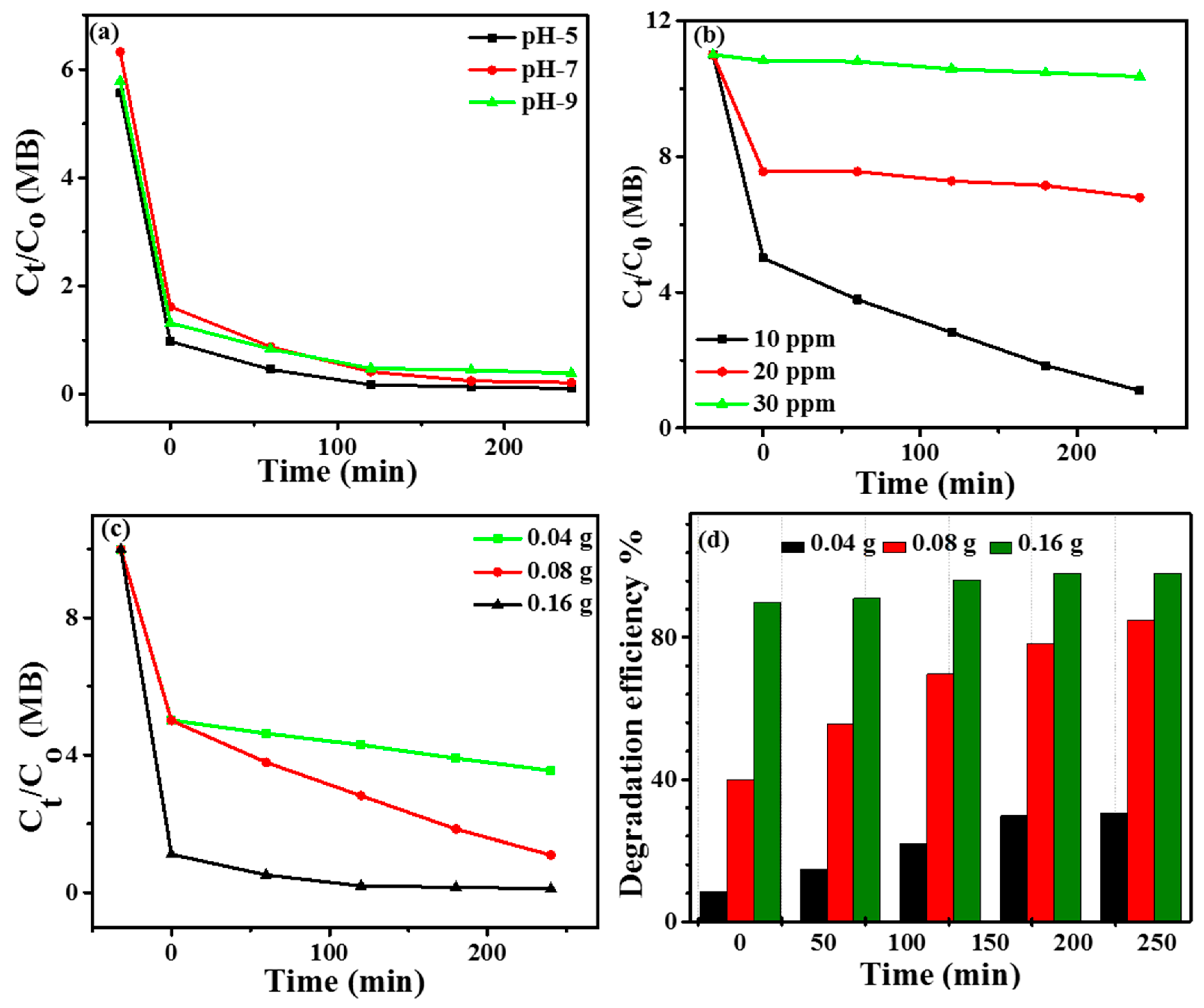
4.5. Effect of Various Parameters on MB Degradation
4.6. Effect of pH
4.7. Effect of Dye Concentration
4.8. Effect of Catalyst Dosage
4.9. Possible Mechanistic Explanation of MB Photodegradation
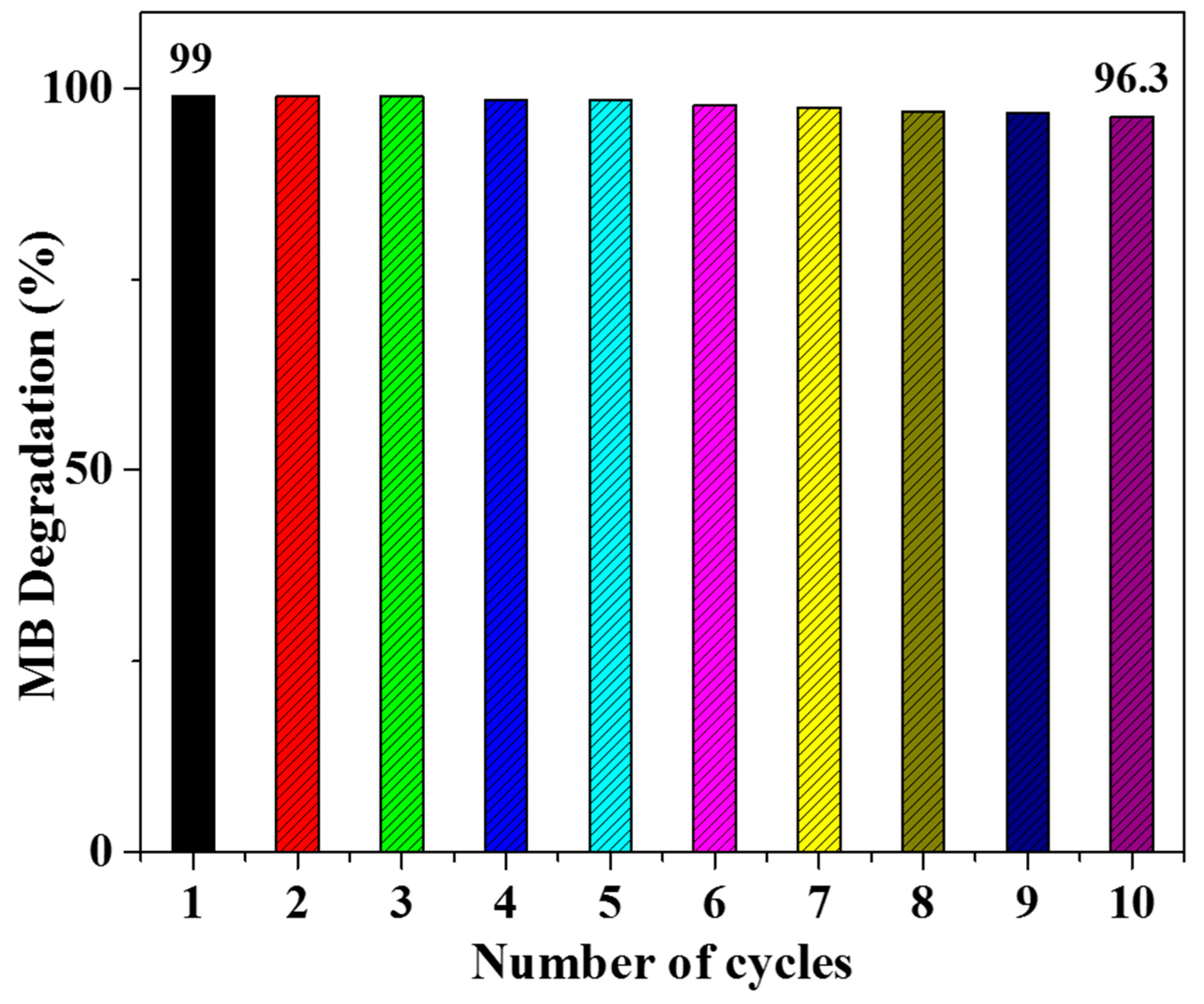
4.10. Recyclability of Photocatalyst
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Sun, X.; Liu, Y.; Ma, L.; Li, Z. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O.; Gazi, M. Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of azo dye via Fenton-like reactions. Appl. Catal. B Environ. 2019, 243, 243–252. [Google Scholar] [CrossRef]
- Pan, Y.; Ding, Q.; Xu, H.; Shi, C.; Singh, A.; Kumar, A.; Liu, J. A new Zn (ii)-based 3D metal–organic framework with uncommon sev topology and its photocatalytic properties for the degradation of organic dyes. CrystEngComm 2019, 21, 4578–4585. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Alharbi, N.S.; Chen, C. Fabrication of a novel Co/Ni-MOFs@ BiOI composite with boosting photocatalytic degradation of methylene blue under visible light. J. Environ. Chem. Eng. 2021, 9, 106194. [Google Scholar] [CrossRef]
- Vinu, R.; Madras, G. Environmental remediation by photocatalysis. J. Indian Inst. Sci. 2010, 90, 189–230. [Google Scholar]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Fang, K.; Liu, Y.; Chen, W.; Fan, J.; Wang, X.; Ren, Y.; Song, Y. Z-scheme In2O3/WO3 heterogeneous photocatalysts with enhanced visible-light-driven photocatalytic activity toward degradation of organic dyes. J. Mater. Sci. 2020, 55, 11919–11937. [Google Scholar] [CrossRef]
- Sharma, K.; Raizada, P.; Hasija, V.; Singh, P.; Bajpai, A.; Nguyen, V.-H.; Rangabhashiyam, S.; Kumar, P.; Nadda, A.K.; Kim, S.Y. ZnS-based quantum dots as photocatalysts for water purification. J. Water Process Eng. 2021, 43, 102217. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Ali, M.U.; Ali, A.; Shabbir, H.; Nazeer, U.B.; Saleem, M.S. Solar light triggered catalytic performance of graphene-CuO nanocomposite for waste water treatment. Ceram. Int. 2017, 43, 10654–10660. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Li, T.; Cai, J.; Luo, M.; Zhao, L. Photocatalytic degradation of methylene blue on CaBi6O10/Bi2O3 composites under visible light. Chem. Eng. J. 2012, 189, 473–481. [Google Scholar] [CrossRef]
- Luan, J.; Pan, B.; Paz, Y.; Li, Y.; Wu, X.; Zou, Z. Structural, photophysical and photocatalytic properties of new Bi 2 SbVO 7 under visible light irradiation. Phys. Chem. Chem. Phys. 2009, 11, 6289–6298. [Google Scholar] [CrossRef]
- Yan, S.C.; Ouyang, S.X.; Gao, J.; Yang, M.; Feng, J.Y.; Fan, X.X.; Wan, L.J.; Li, Z.S.; Ye, J.H.; Zhou, Y. A room-temperature reactive-template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2. Angew. Chem. 2010, 122, 6544–6548. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible-light irradiation. Angew. Chem. 2004, 116, 4563–4566. [Google Scholar] [CrossRef]
- Song, T.; Yu, X.; Tian, N.; Huang, H.-w. Preparation, structure and application of g-C3N4/BiOX composite photocatalyst. Int. J. Hydrogen Energy 2021, 46, 1857–1878. [Google Scholar] [CrossRef]
- Kato, H.; Kobayashi, H.; Kudo, A. Role of Ag+ in the band structures and photocatalytic properties of AgMO3 (M: Ta and Nb) with the perovskite structure. J. Phys. Chem. B 2002, 106, 12441–12447. [Google Scholar] [CrossRef]
- Florez-Rios, J.; Santana-Aranda, M.; Quiñones-Galván, J.; Escobedo-Morales, A.; Chávez-Chávez, A.; Pérez-Centeno, A. Alternative Bi precursor effects on the structural, optical, morphological and photocatalytic properties of BiOI nanostructures. Mater. Res. Express 2020, 7, 015912. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, W.; Wang, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. pn Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J. Hazard. Mater. 2020, 399, 123109. [Google Scholar] [CrossRef]
- Li, X.; Niu, C.; Huang, D.; Wang, X.; Zhang, X.; Zeng, G.; Niu, Q. Preparation of magnetically separable Fe3O4/BiOI nanocomposites and its visible photocatalytic activity. Appl. Surf. Sci. 2013, 286, 40–46. [Google Scholar] [CrossRef]
- Niu, J.; Dai, P.; Zhang, Q.; Yao, B.; Yu, X. Microwave-assisted solvothermal synthesis of novel hierarchical BiOI/rGO composites for efficient photocatalytic degardation of organic pollutants. Appl. Surf. Sci. 2018, 430, 165–175. [Google Scholar] [CrossRef]
- Zhang, D. Heterostructural BiOI/TiO2 composite with highly enhanced visible light photocatalytic performance. Russ. J. Phys. Chem. A 2014, 88, 2476–2485. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, Y.; Zheng, M.; Yang, P.; Asiri, A.M.; Wang, X. Metal–organic frameworks for solar energy conversion by photoredox catalysis. Coord. Chem. Rev. 2018, 373, 83–115. [Google Scholar] [CrossRef]
- Liu, K.; Deng, L.; Li, H.; Bao, Y.; Xiao, Z.; Li, B.; Zhou, Q.; Geng, Y.; Wang, L. Two isostructural Co/Ni fluorine-containing metal-organic frameworks for dye adsorption and supercapacitor. J. Solid State Chem. 2019, 275, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zeng, T.; Shang, Q.; Zhou, H.; Pan, Z.; Cheng, Q. Two pure MOF-photocatalysts readily prepared for the degradation of methylene blue dye under visible light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-metal MOFs: Unique opportunities in metal–organic framework (MOF) functionality and design. Angew. Chem. 2019, 131, 15330–15347. [Google Scholar] [CrossRef]
- Ye, G.; Luo, P.; Zhao, Y.; Qiu, G.; Hu, Y.; Preis, S.; Wei, C. Three-dimensional Co/Ni bimetallic organic frameworks for high-efficient catalytic ozonation of atrazine: Mechanism, effect parameters, and degradation pathways analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Yu, W. Iron-nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: Kinetics and mechanistic studies. J. Hazard. Mater. 2021, 401, 123261. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Zuo, J.; Lin, J.; Liu, Z. Hybrid 0D/2D heterostructures: In-situ growth of 0D g-C3N4 on 2D BiOI for efficient photocatalyst. Adv. Compos. Hybrid Mater. 2021, 4, 1122–1136. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Lu, S.; Zhang, X.; Alsaedi, A.; Hayat, T. MOFs-induced encapsulation of ultrafine Ni nanoparticles into 3D N-doped graphene-CNT frameworks as a recyclable catalyst for Cr (VI) reduction with formic acid. Carbon 2019, 148, 52–63. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, D.; Yang, X.; Liu, C.; Yin, J.; Cai, K. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta 2018, 278, 114–123. [Google Scholar] [CrossRef]
- Radhika, M.; Gopalakrishna, B.; Chaitra, K.; Bhatta, L.K.G.; Venkatesh, K.; Kamath, M.S.; Kathyayini, N. Electrochemical studies on Ni, Co & Ni/Co-MOFs for high-performance hybrid supercapacitors. Mater. Res. Express 2020, 7, 054003. [Google Scholar]
- Sun, D.; Sun, F.; Deng, X.; Li, Z. Mixed-metal strategy on metal–organic frameworks (MOFs) for functionalities expansion: Co substitution induces aerobic oxidation of cyclohexene over inactive Ni-MOF-74. Inorg. Chem. 2015, 54, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kim, H.K.; Reddy, D.A.; Kim, Y.; Ma, R.; Baek, H.; Kim, J.; Kim, T.K. Hierarchical BiOI nanostructures supported on a metal organic framework as efficient photocatalysts for degradation of organic pollutants in water. Dalton Trans. 2017, 46, 6013–6023. [Google Scholar] [CrossRef]
- Lv, Z.; Zhong, Q.; Bu, Y.; Wu, J. 3D flower-like hierarchical Ag@ nickel-cobalt hydroxide microsphere with enhanced electrochemical properties. Electron. Mater. Lett. 2016, 12, 824–829. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.H. Structure distortion of Zn4O13C24H12 framework (MOF-5). Mater. Sci. Eng. B 2011, 176, 573–578. [Google Scholar] [CrossRef]
- Pantos, E.; Philis, J.; Bolovinos, A. The extinction coefficient of benzene vapor in the region 4.6 to 36 eV. J. Mol. Spectrosc. 1978, 72, 36–43. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Xin, X.; Yang, Z.; Gao, Y.; Shi, Y.; Zhao, Z.; An, K.; Wang, W.; Tan, J. Multi-stepwise charge transfer via MOF@ MOF/TiO 2 dual-heterojunction photocatalysts towards hydrogen evolution. J. Mater. Chem. A 2022, 10, 9717–9725. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal–organic frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, S.; Shao, C.; Zhu, G.; Ni, Z.; Sun, J.; Huang, X. Visible light-driven BiOI/ZIF-8 heterostructure and photocatalytic adsorption synergistic degradation of BPA. Res. Chem. Intermed. 2020, 46, 2951–2967. [Google Scholar] [CrossRef]
- Budi, C.S.; Deka, J.R.; Hsu, W.-C.; Saikia, D.; Chen, K.-T.; Kao, H.-M.; Yang, Y.-C. Bimetallic Co/Zn zeolitic imidazolate framework ZIF-67 supported Cu nanoparticles: An excellent catalyst for reduction of synthetic dyes and nitroarenes. J. Hazard. Mater. 2021, 407, 124392. [Google Scholar] [CrossRef]
- Guo, H.; Lin, F.; Chen, J.; Li, F.; Weng, W. Metal–organic framework MIL-125 (Ti) for efficient adsorptive removal of Rhodamine B from aqueous solution. Appl. Organomet. Chem. 2015, 29, 12–19. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zeng, L.; Zhang, Q.; Ge, S.; Zhao, X.; Lin, H.; He, Y. In situ preparation of g-C3N4/Bi4O5I2 complex and its elevated photoactivity in Methyl Orange degradation under visible light. J. Environ. Sci. 2020, 87, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Zhang, X.; Tang, L.; Wang, L.; Wang, Y.; Wu, M. Ultrathin graphene oxide encapsulated in uniform MIL-88A (Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
| Photocatalysts | Time (min) | MB Degradation (%) | References |
|---|---|---|---|
| Fe2TiO5 nanoparticles | 240 | ~53 | Ref [42] of sana |
| Ag3PO4/MnFe2O4 NCs | 82 | 98 | [43] |
| AC-Bi/TiO2 NCs | 100 | 97 | [44] |
| MOF-5@rGO | 20 | 93 | [45] |
| SnO2/TiO2 nanocomposites | 50 | ~90 | [46] |
| Ag.Co3O4 nanosheets | 90 | 88.4 | [47] |
| Ni/Cu-BDC MOF@BiOI-15% | 240 | 99 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqa, A.; Akhter, T.; Faheem, M.; Razzaque, S.; Mahmood, A.; Al-Masry, W.; Nadeem, S.; Hassan, S.U.; Yang, H.; Park, C.H. Bismuth-Rich Co/Ni Bimetallic Metal–Organic Frameworks as Photocatalysts toward Efficient Removal of Organic Contaminants under Environmental Conditions. Micromachines 2023, 14, 899. https://doi.org/10.3390/mi14050899
Siddiqa A, Akhter T, Faheem M, Razzaque S, Mahmood A, Al-Masry W, Nadeem S, Hassan SU, Yang H, Park CH. Bismuth-Rich Co/Ni Bimetallic Metal–Organic Frameworks as Photocatalysts toward Efficient Removal of Organic Contaminants under Environmental Conditions. Micromachines. 2023; 14(5):899. https://doi.org/10.3390/mi14050899
Chicago/Turabian StyleSiddiqa, Ayesha, Toheed Akhter, Muhammad Faheem, Shumaila Razzaque, Asif Mahmood, Waheed Al-Masry, Sohail Nadeem, Sadaf Ul Hassan, Hyunseung Yang, and Chan Ho Park. 2023. "Bismuth-Rich Co/Ni Bimetallic Metal–Organic Frameworks as Photocatalysts toward Efficient Removal of Organic Contaminants under Environmental Conditions" Micromachines 14, no. 5: 899. https://doi.org/10.3390/mi14050899
APA StyleSiddiqa, A., Akhter, T., Faheem, M., Razzaque, S., Mahmood, A., Al-Masry, W., Nadeem, S., Hassan, S. U., Yang, H., & Park, C. H. (2023). Bismuth-Rich Co/Ni Bimetallic Metal–Organic Frameworks as Photocatalysts toward Efficient Removal of Organic Contaminants under Environmental Conditions. Micromachines, 14(5), 899. https://doi.org/10.3390/mi14050899






