A User-Centric 3D-Printed Modular Peristaltic Pump for Microfluidic Perfusion Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Electronic Control Module
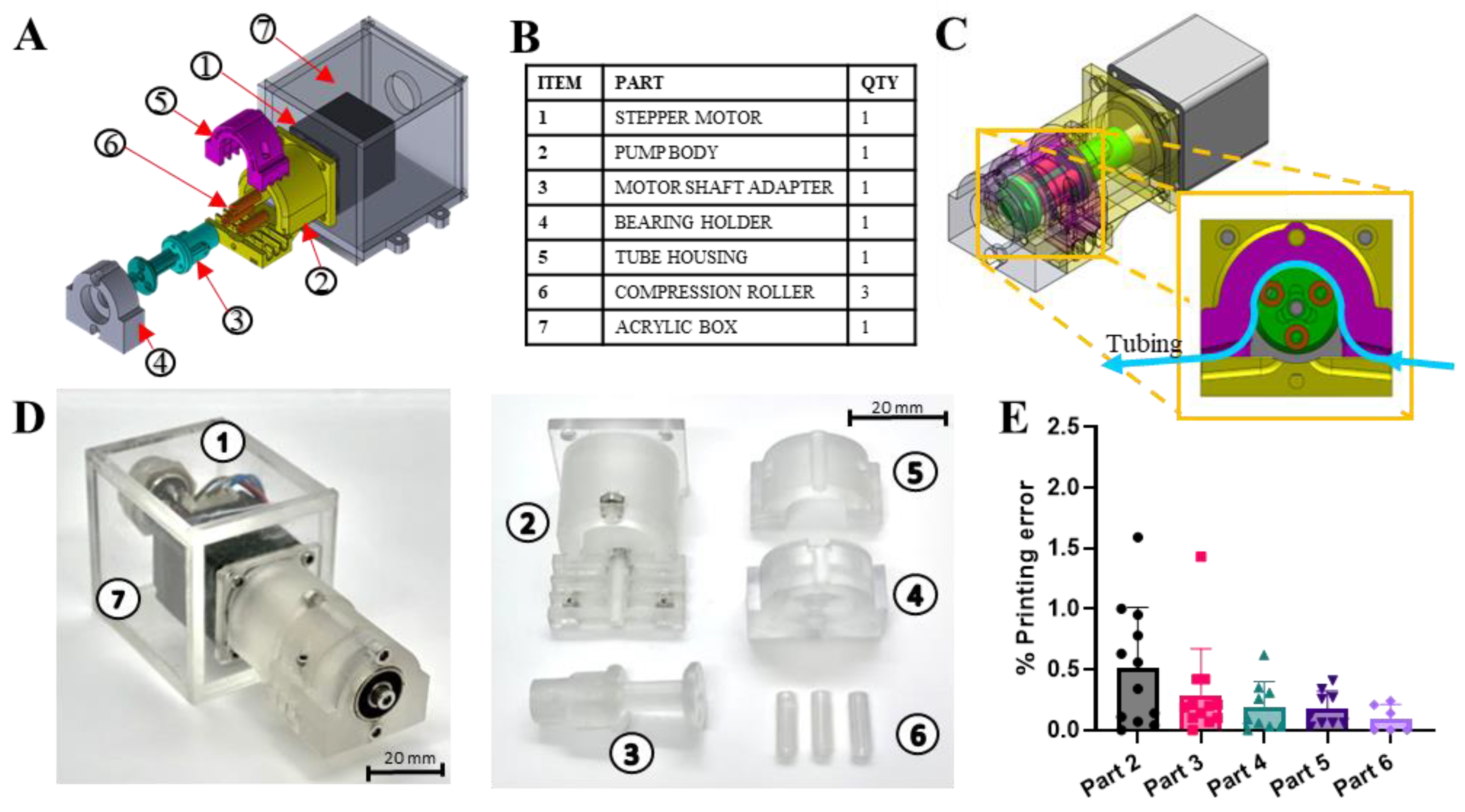
2.2. Fabrication of Peristaltic Pump Module
2.3. Programming Interface between Electronic Control and Peristaltic Pump Modules
2.4. Validation of Programmed Stepper Motor Rotation
2.5. Characterization of Pump Flow Rates and Multiplexing Operation
2.6. Fabrication of 3D-Printed Microfluidic Vasculature Channel Device
2.7. Cell Culture
2.8. DAPI and F-Actin Staining
2.9. Statistical Analysis
3. Results
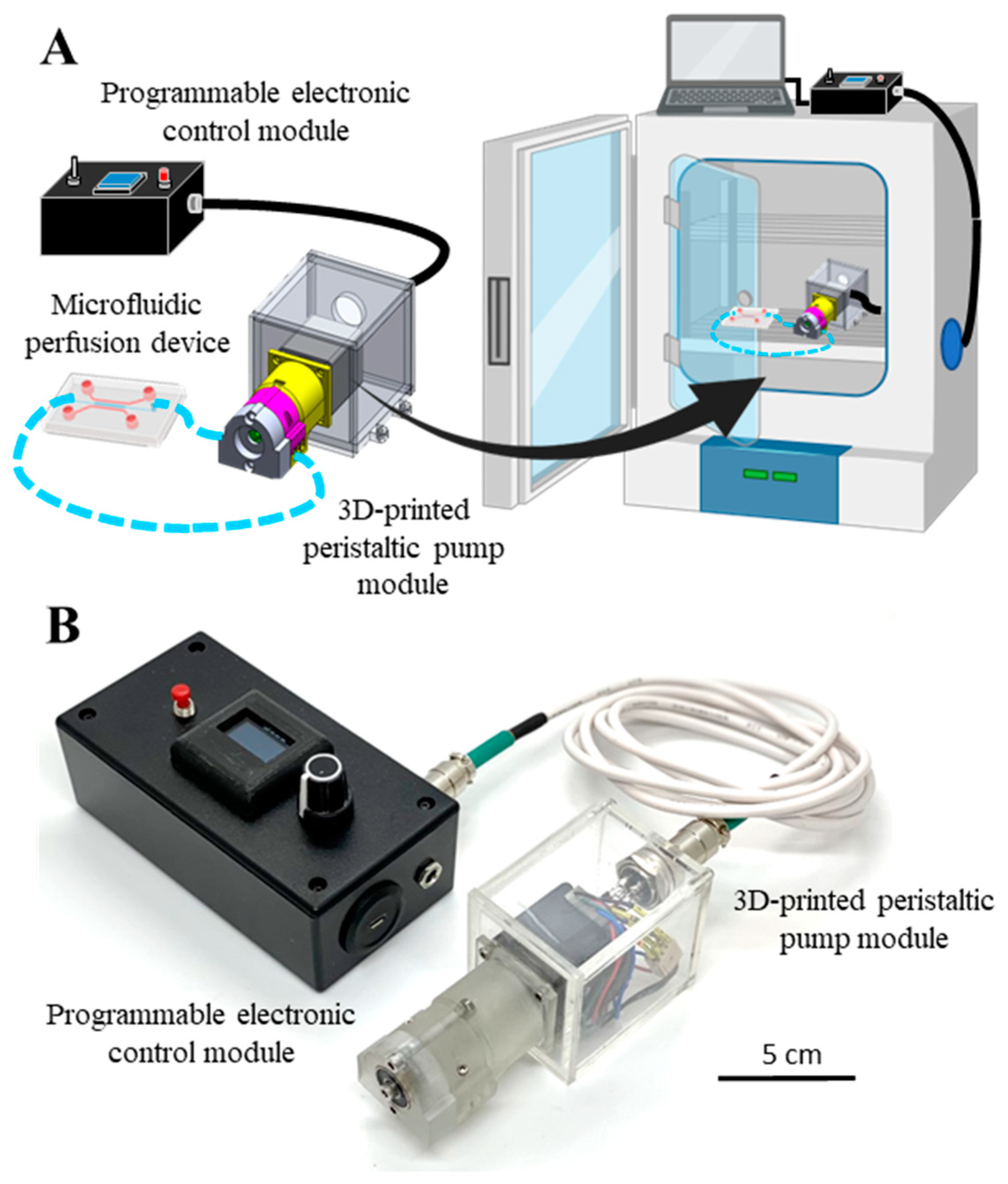
3.1. Overview of the Modular and Customizable Mini Peristaltic Pump
3.2. Construction of the Electronic Control Module
3.3. Programming and Validating the Electronic Control Module
3.4. Construction of the Mechanical Pump Module
3.5. Characterization of Pump Performance and Flow Rate Range
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H. Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2020, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.C.H.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. Vitr. Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Kaarj, K.; Yoon, J.-Y. Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip. Micromachines 2019, 10, 700. [Google Scholar] [CrossRef]
- Przybyla, L.; Voldman, J. Probing embryonic stem cell autocrine and paracrine signaling using microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 293–315. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Wasson, E.M.; Dubbin, K.; Moya, M.L. Go with the flow: Modeling unique biological flows in engineered in vitro platforms. Lab Chip 2021, 21, 2095–2120. [Google Scholar] [CrossRef]
- Winkelman, M.A.; Kim, D.Y.; Kakarla, S.; Grath, A.; Silvia, N.; Dai, G. Interstitial flow enhances the formation, connectivity, and function of 3D brain microvascular networks generated within a microfluidic device. Lab Chip 2021, 22, 170–192. [Google Scholar] [CrossRef]
- Morsi, Y.S.; Yang, W.; Owida, A.; Wong, C.S. Development of a novel pulsatile bioreactor for tissue culture. J. Artif. Organs 2007, 10, 109–114. [Google Scholar] [CrossRef]
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; Ijzerman, M. Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov. Today 2019, 24, 1720–1724. [Google Scholar]
- Lee, Y.-S.; Bhattacharjee, N.; Folch, A. 3D-printed Quake-style microvalves and micropumps. Lab Chip 2018, 18, 1207–1214. [Google Scholar] [CrossRef]
- Shin, S.; Kim, B.; Kim, Y.-J.; Choi, S. Integrated microfluidic pneumatic circuit for point-of-care molecular diagnostics. Biosens. Bioelectron. 2019, 133, 169–176. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lee, G.-B. Automatic bio-sampling chips integrated with micro-pumps and micro-valves for disease detection. Biosens. Bioelectron. 2005, 21, 419–425. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Chong, L.H.; Jin, L.; Singh, P.K.; Lee, P.S.; Yu, H.; Ananthanarayanan, A.; Leo, H.L.; Toh, Y.-C. A pump-free microfluidic 3D perfusion platform for the efficient differentiation of human hepatocyte-like cells. Biotechnol. Bioeng. 2017, 114, 2360–2370. [Google Scholar] [CrossRef]
- Cole, M.C.; Desai, A.V.; Kenis, P.J. Two-layer multiplexed peristaltic pumps for high-density integrated microfluidics. Sens. Actuators B Chem. 2011, 151, 384–393. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Ching, T.; Chong, L.H.; Arora, S.; Li, H.; Hashimoto, M.; DasGupta, R.; Yuen, P.K.; Toh, Y.-C. Self-aligning Tetris-Like (TILE) modular microfluidic platform for mimicking multi-organ interactions. Lab Chip 2019, 19, 2178–2191. [Google Scholar] [CrossRef]
- Fenech, M.; Girod, V.; Claveria, V.; Meance, S.; Abkarian, M.; Charlot, B. Microfluidic blood vasculature replicas using backside lithography. Lab Chip 2019, 19, 2096–2106. [Google Scholar] [CrossRef]
- Bellan, L.M.; Kniazeva, T.; Kim, E.S.; Epshteyn, A.A.; Cropek, D.M.; Langer, R.; Borenstein, J.T. Fabrication of a Hybrid Microfluidic System Incorporating both Lithographically Patterned Microchannels and a 3D Fiber-Formed Microfluidic Network. Adv. Healthc. Mater. 2012, 1, 164–167. [Google Scholar] [CrossRef]
- Kiseliovas, V.; Milosevic, M.; Kojic, M.; Mazutis, L.; Kai, M.; Liu, Y.T.; Yokoi, K.; Ferrari, M.; Ziemys, A. Tumor progression effects on drug vector access to tumor-associated capillary bed. J. Control. Release 2017, 261, 216–222. [Google Scholar] [CrossRef]
- Edington, C.D.; Chen, W.L.K.; Geishecker, E.; Kassis, T.; Soenksen, L.R.; Bhushan, B.M.; Freake, D.; Kirschner, J.; Maass, C.; Tsamandouras, N.; et al. Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci. Rep. 2018, 8, 4530. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2017, 18, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Oh, S.; Hoang, H.-H.; Nguyen, D.T.T.; Lim, W.; Shin, T.H.; Lee, G.; Park, S. Recapitulation of cancer stem cell niches in glioblastoma on 3D microfluidic cell culture devices under gravity-driven perfusion. J. Ind. Eng. Chem. 2018, 62, 352–361. [Google Scholar] [CrossRef]
- Wang, Y.; Shuler, M.L. UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems. Lab Chip 2018, 18, 2563–2574. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, F.; Zhang, T.; Chen, D.; Jia, X.; Wang, J.; Guo, W.; Chen, J. A Tubing-Free Microfluidic Wound Healing Assay Enabling the Quantification of Vascular Smooth Muscle Cell Migration. Sci. Rep. 2015, 5, 14049. [Google Scholar] [CrossRef]
- Xiang, X.; Ye, Q.; Shang, Y.; Li, F.; Zhou, B.; Shao, Y.; Wang, C.; Zhang, J.; Xue, L.; Chen, M.; et al. Quantitative detection of aflatoxin B1 using quantum dots-based immunoassay in a recyclable gravity-driven microfluidic chip. Biosens. Bioelectron. 2021, 190, 113394. [Google Scholar] [CrossRef]
- Oleaga, C.; Riu, A.; Rothemund, S.; Lavado, A.; McAleer, C.W.; Long, C.J.; Persaud, K.; Narasimhan, N.S.; Tran, M.; Roles, J.; et al. Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system. Biomaterials 2018, 182, 176–190. [Google Scholar] [CrossRef]
- Lee, N.W.; Choi, N.; Sung, J.H. A microfluidic chip with gravity-induced unidirectional flow for perfusion cell culture. Biotechnol. Prog. 2018, 35, e2701. [Google Scholar] [CrossRef]
- van Dijk, C.G.; Brandt, M.M.; Poulis, N.; Anten, J.; van der Moolen, M.; Kramer, L.; Homburg, E.F.; Louzao-Martinez, L.; Pei, J.; Krebber, M.M.; et al. A new microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix. Lab Chip 2020, 20, 1827–1844. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.-W.; Seol, Y.-J.; Zhang, Y.S.; Shin, S.-R.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef]
- Deinhardt-Emmer, S.; Rennert, K.; Schicke, E.; Cseresnyés, Z.; Windolph, M.; Nietzsche, S.; Heller, R.; Siwczak, F.; Haupt, K.F.; Carlstedt, S.; et al. Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication 2020, 12, 025012. [Google Scholar] [CrossRef]
- Qian, T.; Gil, D.A.; Guzman, E.C.; Gastfriend, B.D.; Tweed, K.E.; Palecek, S.P.; Skala, M.C. Adaptable pulsatile flow generated from stem cell-derived cardiomyocytes using quantitative imaging-based signal transduction. Lab Chip 2020, 20, 3744–3756. [Google Scholar] [CrossRef]
- Kurth, F.; Györvary, E.; Heub, S.; Ledroit, D.; Paoletti, S.; Renggli, K.; Revol, V.; Verhulsel, M.; Weder, G.; Loizeau, F. Chapter 3—Organs-on-a-chip engineering. In Organ-on-a-Chip; Hoeng, J., Bovard, D., Peitsch, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 47–130. [Google Scholar]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Kondaveeti, H.K.; Kumaravelu, N.K.; Vanambathina, S.D.; Mathe, S.E.; Vappangi, S. A systematic literature review on prototyping with Arduino: Applications, challenges, advantages, and limitations. Comput. Sci. Rev. 2021, 40, 100364. [Google Scholar] [CrossRef]
- Rosa, T.R.; Betim, F.S.; Ferreira, R.D.Q. Development and application of a labmade apparatus using open-source “arduino” hardware for the electrochemical pretreatment of boron-doped diamond electrodes. Electrochim. Acta 2017, 231, 185–189. [Google Scholar] [CrossRef]
- Jönsson, A.; Toppi, A.; Dufva, M. The FAST Pump, a low-cost, easy to fabricate, SLA-3D-printed peristaltic pump for multi-channel systems in any lab. Hardwarex 2020, 8, e00115. [Google Scholar] [CrossRef]
- Alam, M.N.H.Z.; Hossain, F.; Vale, A.; Kouzani, A. Design and fabrication of a 3D printed miniature pump for integrated microfluidic applications. Int. J. Precis. Eng. Manuf. 2017, 18, 1287–1296. [Google Scholar] [CrossRef]
- Behrens, M.R.; Fuller, H.C.; Swist, E.R.; Wu, J.; Islam, M.; Long, Z.; Ruder, W.C.; Steward, R., Jr. Open-source, 3D-printed Peristaltic Pumps for Small Volume Point-of-Care Liquid Handling. Sci. Rep. 2020, 10, 1543. [Google Scholar] [CrossRef]
- Gervasi, A.; Cardol, P.; Meyer, P.E. Open-hardware wireless controller and 3D-printed pumps for efficient liquid manipulation. Hardwarex 2021, 9, e00199. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.T.; Shi, B.; Wang, M.; Catsamas, S. BoSL FAL pump: A small, low-cost, easily constructed, 3D-printed peristaltic pump for sampling of waters. Hardwarex 2021, 10, e00214. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Vasudevan, J.; Tan, H.Y.; Lim, C.T.; Fernandez, J.; Toh, Y.-C.; Hashimoto, M. Highly-customizable 3D-printed peristaltic pump kit. Hardwarex 2021, 10, e00202. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Vasudevan, J.; Chang, S.; Tan, H.Y.; Ranganath, A.S.; Lim, C.T.; Fernandez, J.G.; Ng, J.J.; Toh, Y.; Hashimoto, M. Biomimetic Vasculatures by 3D-Printed Porous Molds. Small 2022, 18, 2203426. [Google Scholar] [CrossRef]
- Cook, S.R.; Musgrove, H.B.; Throckmorton, A.L.; Pompano, R.R. Microscale impeller pump for recirculating flow in organs-on-chip and microreactors. Lab Chip 2022, 22, 605–620. [Google Scholar] [CrossRef]
- Schneider, S.; Bubeck, M.; Rogal, J.; Weener, H.J.; Rojas, C.; Weiss, M.; Heymann, M.; van der Meer, A.D.; Loskill, P. Peristaltic on-chip pump for tunable media circulation and whole blood perfusion in PDMS-free organ-on-chip and Organ-Disc systems. Lab Chip 2021, 21, 3963–3978. [Google Scholar] [CrossRef]
- Pan, T.; McDonald, S.J.; Kai, E.M.; Ziaie, B. A magnetically driven PDMS micropump with ball check-valves. J. Micromech. Microeng. 2005, 15, 1021–1026. [Google Scholar] [CrossRef]
- Du, M.; Ye, X.; Wu, K.; Zhou, Z. A Peristaltic Micro Pump Driven by a Rotating Motor with Magnetically Attracted Steel Balls. Sensors 2009, 9, 2611–2620. [Google Scholar] [CrossRef]
- Dincau, B.; Dressaire, E.; Sauret, A. Pulsatile Flow in Microfluidic Systems. Small 2019, 16, e1904032. [Google Scholar] [CrossRef]
- Jacobs, C.R.; Yellowley, C.E.; Davis, B.R.; Zhou, Z.; Cimbala, J.M.; Donahue, H.J. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 1998, 31, 969–976. [Google Scholar] [CrossRef]
- Du, D.; Furukawa, K.S.; Ushida, T. 3D culture of osteoblast-like cells by unidirectional or oscillatory flow for bone tissue engineering. Biotechnol. Bioeng. 2008, 102, 1670–1678. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiol. Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar] [CrossRef]
- Guo, D.; Chien, S.; Shyy, J.Y.-J. Regulation of Endothelial Cell Cycle by Laminar Versus Oscillatory Flow. Circ. Res. 2007, 100, 564–571. [Google Scholar] [CrossRef]
- Byun, C.K.; Abi-Samra, K.; Cho, Y.-K.; Takayama, S. Pumps for microfluidic cell culture. Electrophoresis 2013, 35, 245–257. [Google Scholar] [CrossRef]
- Ma, T.; Sun, S.; Li, B.; Chu, J. Piezoelectric peristaltic micropump integrated on a microfluidic chip. Sens. Actuators A Phys. 2019, 292, 90–96. [Google Scholar] [CrossRef]
- Yobas, L.; Tang, K.-C.; Yong, S.-E.; Ong, E.K.-Z. A disposable planar peristaltic pump for lab-on-a-chip. Lab Chip 2008, 8, 660–662. [Google Scholar] [CrossRef]
- Xiang, J.; Cai, Z.; Zhang, Y.; Wang, W. A micro-cam actuated linear peristaltic pump for microfluidic applications. Sens. Actuators A Phys. 2016, 251, 20–25. [Google Scholar] [CrossRef]




| % Error Relative to the Average Flow Rate of Three Tubings | ||||
|---|---|---|---|---|
| RPM | Tubing ID (mm) | |||
| 0.5 | 1 | 1.5 | 2 | |
| 1 | 2.6 | 2.4 | 3.7 | 1.8 |
| 5 | 0.9 | 2.5 | 3.8 | 1.3 |
| 10 | 1.8 | 4.2 | 1.2 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Cataño, J.; Farthing, S.; Mascarenhas, Z.; Lake, N.; Yarlagadda, P.K.D.V.; Li, Z.; Toh, Y.-C. A User-Centric 3D-Printed Modular Peristaltic Pump for Microfluidic Perfusion Applications. Micromachines 2023, 14, 930. https://doi.org/10.3390/mi14050930
A. Cataño J, Farthing S, Mascarenhas Z, Lake N, Yarlagadda PKDV, Li Z, Toh Y-C. A User-Centric 3D-Printed Modular Peristaltic Pump for Microfluidic Perfusion Applications. Micromachines. 2023; 14(5):930. https://doi.org/10.3390/mi14050930
Chicago/Turabian StyleA. Cataño, Jorge, Steven Farthing, Zeus Mascarenhas, Nathaniel Lake, Prasad K. D. V. Yarlagadda, Zhiyong Li, and Yi-Chin Toh. 2023. "A User-Centric 3D-Printed Modular Peristaltic Pump for Microfluidic Perfusion Applications" Micromachines 14, no. 5: 930. https://doi.org/10.3390/mi14050930







