Development of an Implantable Capacitive Pressure Sensor for Biomedical Applications
Abstract
:1. Introduction
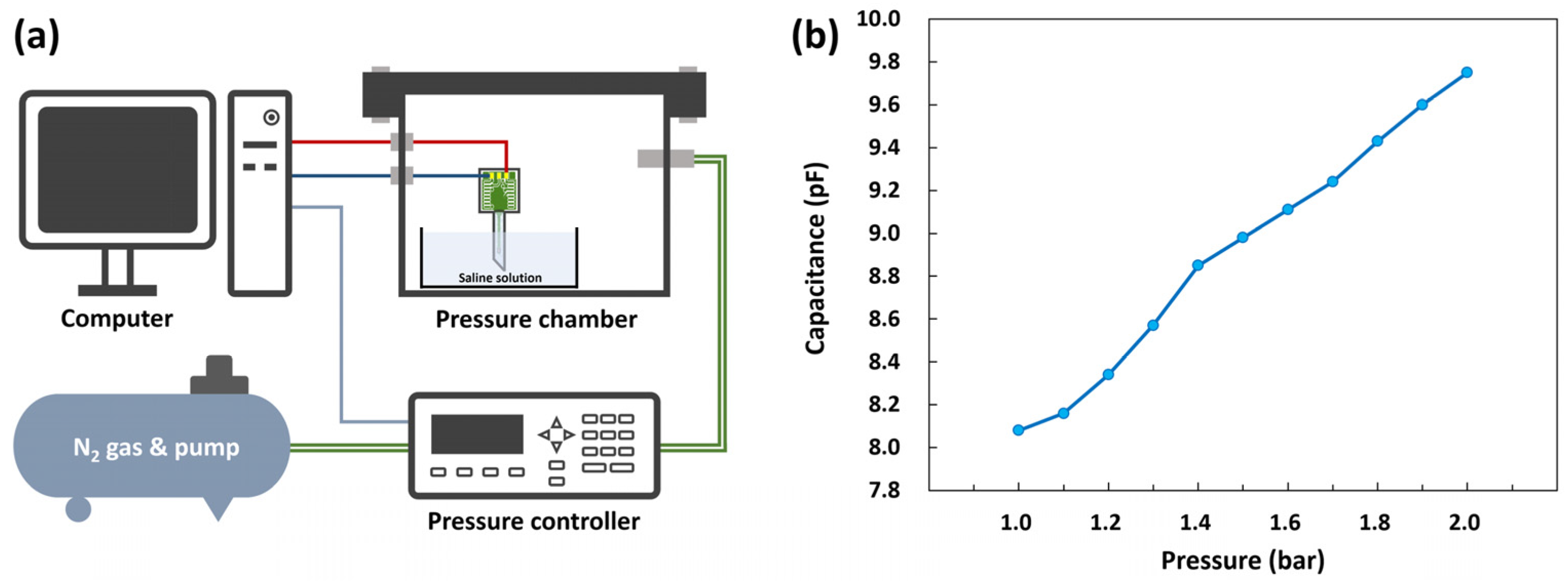
2. Experimental Details
2.1. Design and Fabrication
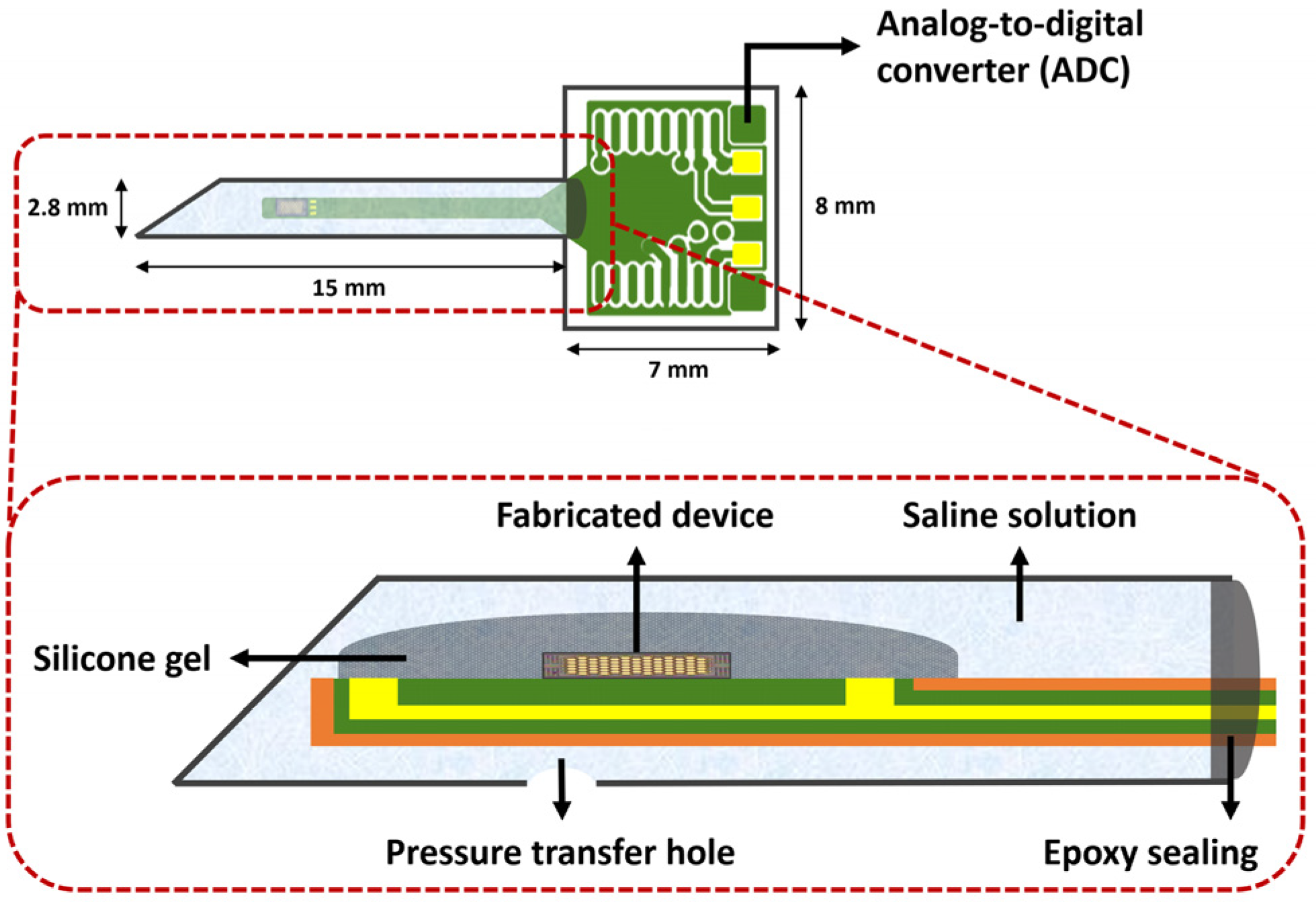
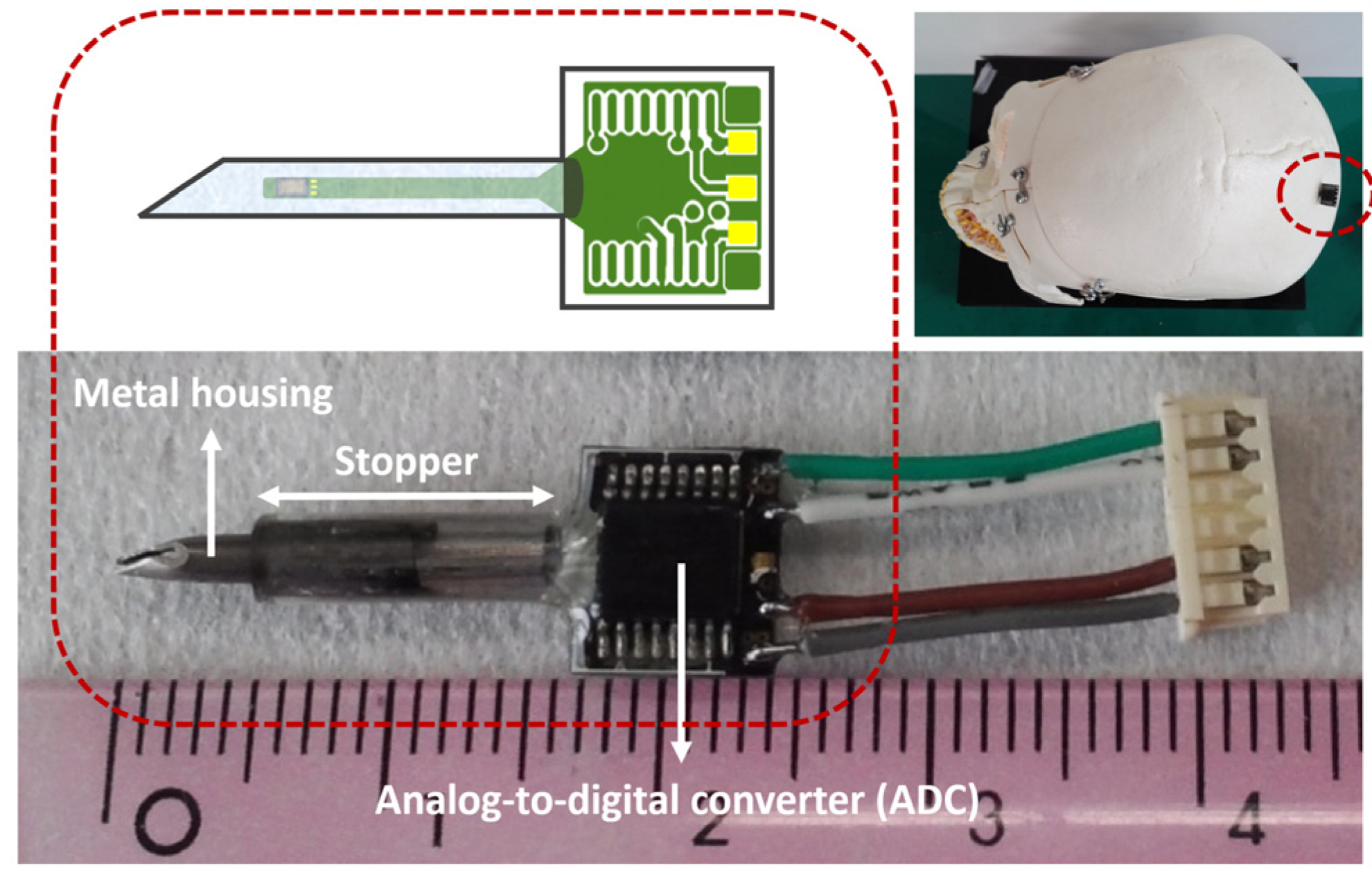
2.2. Packaging
3. Results and Discussion
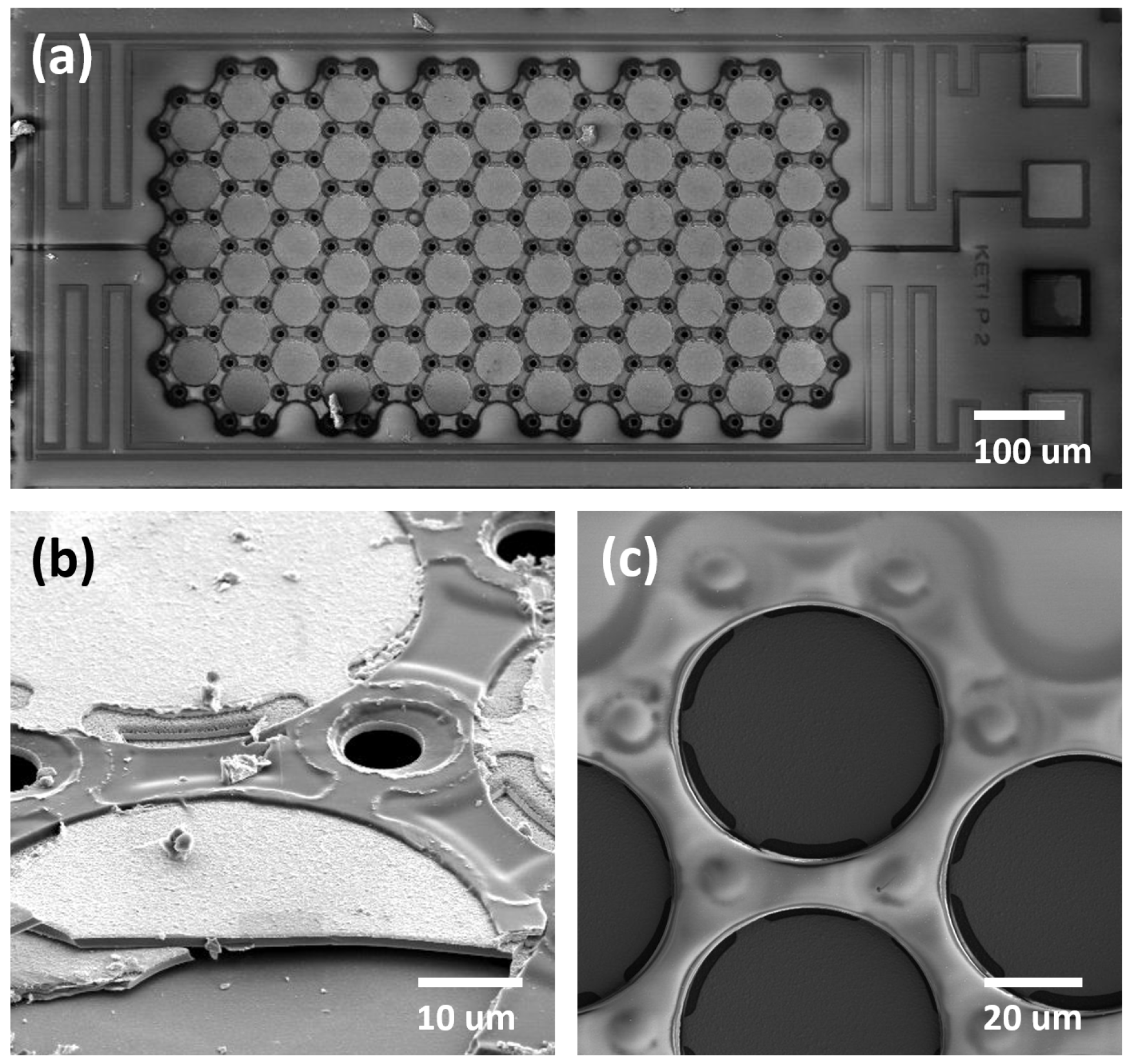
3.1. Imaging
3.2. Characterizations
3.2.1. Output Characteristics before Packaging
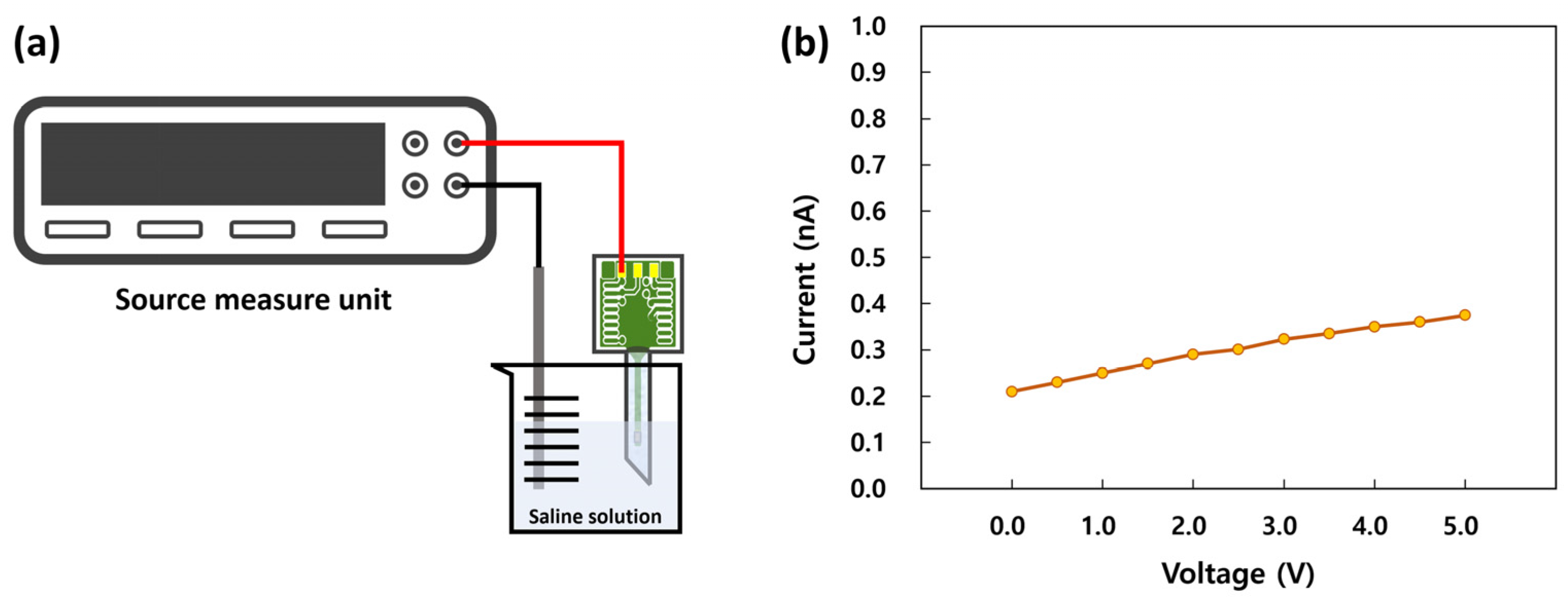
3.2.2. Leakage Current
3.2.3. Performance of the Pressure Sensor
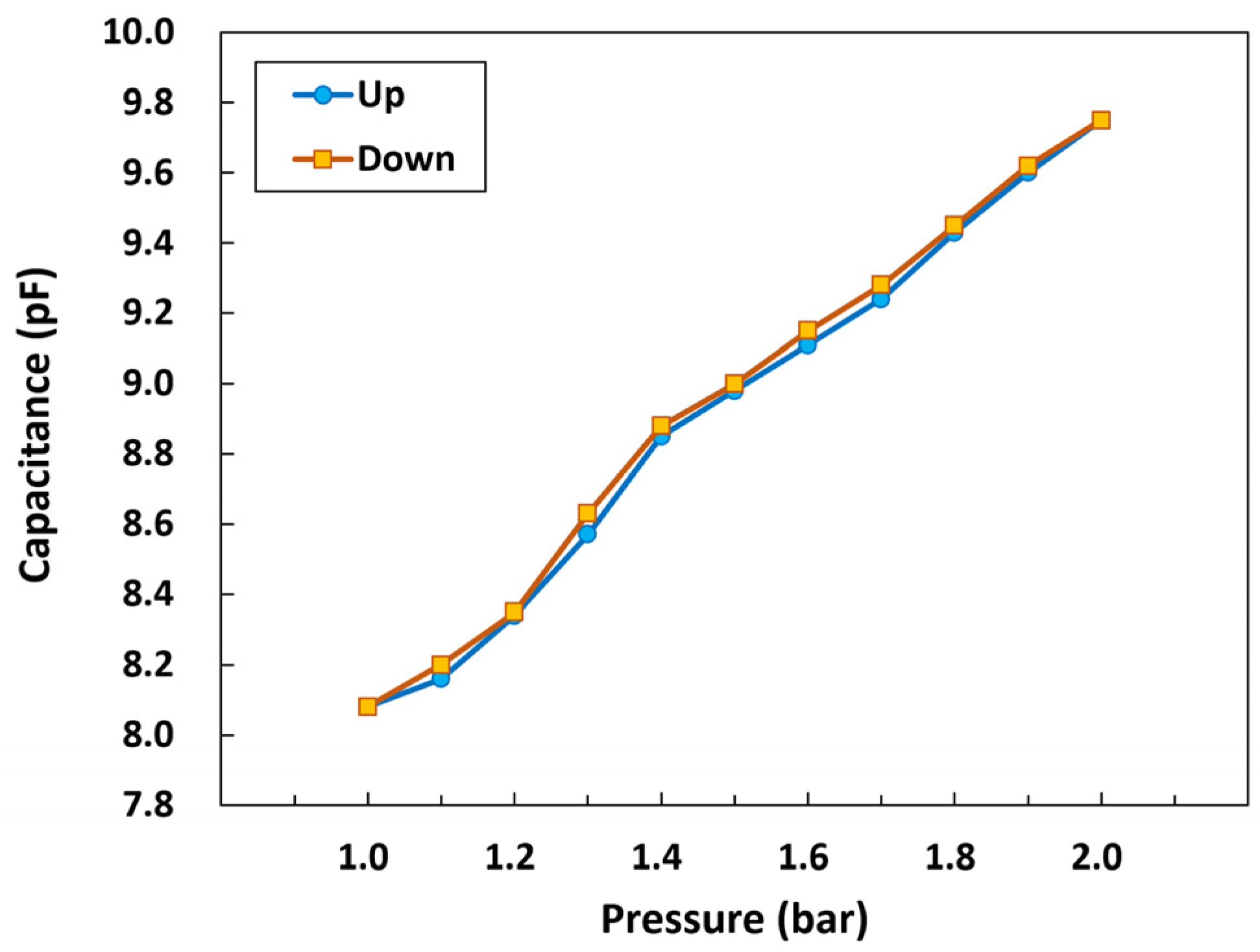
3.2.4. Hysteresis of the Pressure Sensor
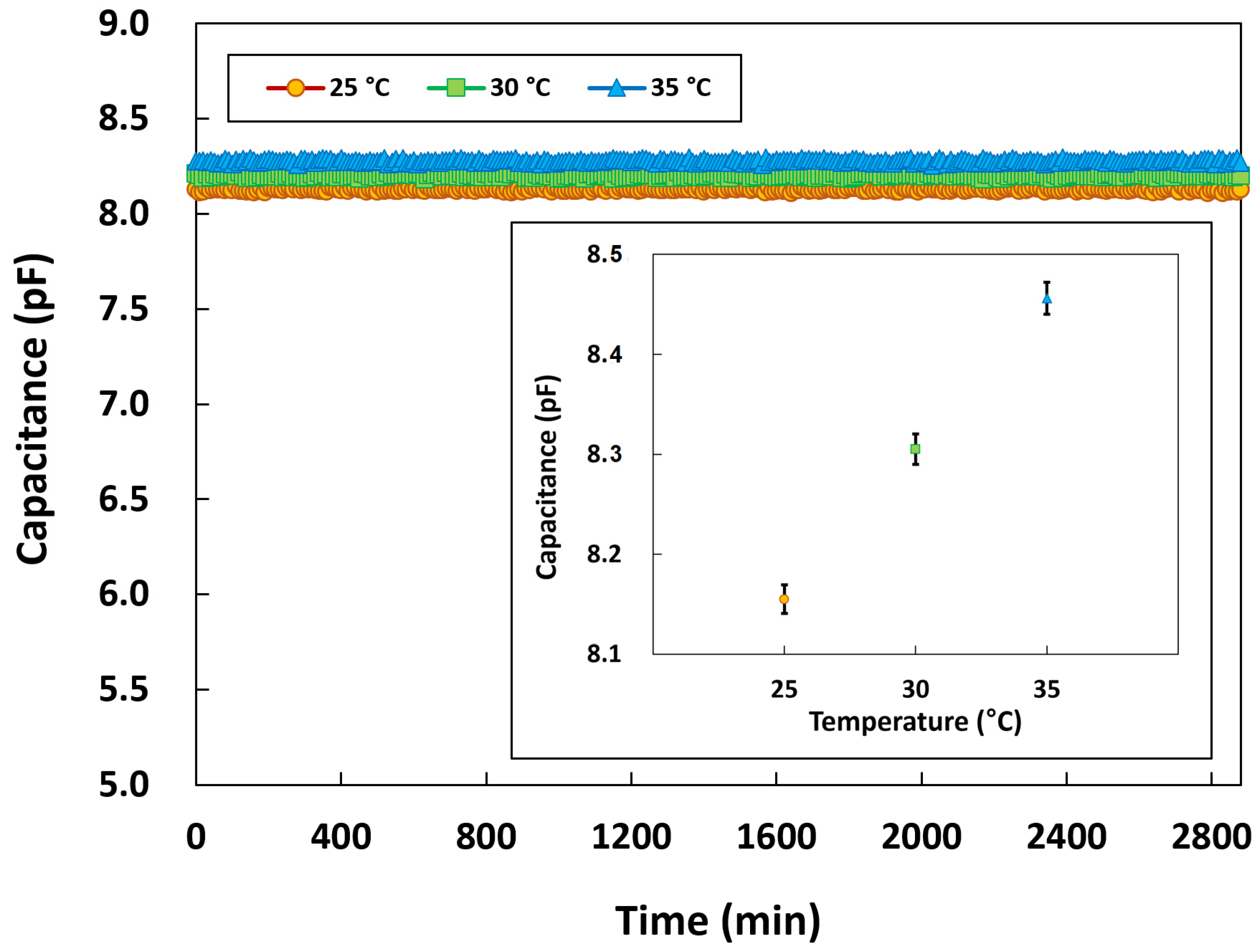
3.2.5. Long-Term Stability of the Pressure Sensor
3.2.6. Performance of the Temperature Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruth, S.R.A.; Feig, V.R.; Tran, H.; Bao, Z. Microengineering Pressure Sensor Active Layers for Improved Performance. Adv. Funct. Mater. 2020, 30, 2003491. [Google Scholar] [CrossRef]
- Li, J.; Bao, R.; Tao, J.; Peng, Y.; Pan, C. Recent Progress in Flexible Pressure Sensor Arrays: From Design to Applications. J. Mater. Chem. C 2018, 6, 11878–11892. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.; Bi, Y.; Cao, S.; Xia, X.; Yang, A.; Li, S.; Xiao, X. Research Progress of Flexible Capacitive Pressure Sensor for Sensitivity Enhancement Approaches. Sens. Actuators A Phys. 2021, 321, 112425. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent Developments for Flexible Pressure Sensors: A Review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef]
- Song, P.; Ma, Z.; Ma, J.; Yang, L.; Wei, J.; Zhao, Y.; Zhang, M.; Yang, F.; Wang, X. Recent Progress of Miniature MEMS Pressure Sensors. Micromachines 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Honjol, Y.; Rajkumar, V.S.; Parent-Harvey, C.; Selvasandran, K.; Kordlouie, S.; Comeau-Gauthier, M.; Harvey, E.; Merle, G. Current View and Prospect: Implantable Pressure Sensors for Health and Surgical Care. Med. Devices Sens. 2020, 3, e10068. [Google Scholar] [CrossRef]
- Clausen, I.; Glott, T. Development of Clinically Relevant Implantable Pressure Sensors: Perspectives and Challenges. Sensors 2014, 14, 17686–17702. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kim, B.J.; Meng, E. Chronically Implanted Pressure Sensors: Challenges and State of the Field. Sensors 2014, 14, 20620–20644. [Google Scholar] [CrossRef]
- Pang, C.; Lee, C.; Suh, K. Recent Advances in Flexible Sensors for Wearable and Implantable Devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Li, L.; Zheng, J.; Chen, J.; Luo, Z.; Su, Y.; Tang, W.; Gao, X.; Li, Y.; Cao, C.; Liu, Q. Flexible Pressure Sensors for Biomedical Applications: From Ex Vivo to in Vivo. Adv. Mater. Interfaces 2020, 7, 2000743. [Google Scholar] [CrossRef]
- Raboel, P.H.; Bartek, J.; Andresen, M.; Bellander, B.M.; Romner, B. Intracranial Pressure Monitoring: Invasive Versus Non-Invasive Methods—A Review. Crit. Care Res. Pract. 2012, 2012, 950393. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, A.; Huang, J.H.; Papadakos, P.J. Intracranial Monitoring in Traumatic Brain Injury. Curr. Opin. Anesthesiol. 2011, 24, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Kahle, K.T.; Simard, J.M. Novel Treatment Targets for Cerebral Edema. Neurotherapeutics 2012, 9, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, S.; Moron, A.F.; Hisaba, W.; Dastoli, P.; Silva, N.S. Fetal Brain Tumors. Child’s Nerv. Syst. 2003, 19, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, A.; Gerber, L.M.; Chiu, Y.; Carney, N.; Härtl, R.; Ghajar, J. Increased Mortality in Patients with Severe Traumatic Brain Injury Treated without Intracranial Pressure Monitoring. J. Neurosurg. 2012, 117, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Dujovny, M.; Park, H.K.; Perez, E.; Perlin, A.R.; Diaz, F.G. Advances in ICP Monitoring Techniques. Neurol. Res. 2003, 25, 339–350. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wise, K.D. A Batch-Fabricated Silicon Capacitive Pressure Transducer with Low Temperature Sensitivity. IEEE Trans. Electron Devices 1982, 29, 42–48. [Google Scholar] [CrossRef]
- Patra, J.C. An Artificial Neural Network-Based Smart Capacitive Pressure Sensor. Measurement 1997, 22, 113–121. [Google Scholar] [CrossRef]
- Han, M.; Chen, L.; Aras, K.; Liang, C.; Chen, X.; Zhao, H.; Li, K.; Faye, N.R.; Sun, B.; Kim, J. Catheter-Integrated Soft Multilayer Electronic Arrays for Multiplexed Sensing and Actuation during Cardiac Surgery. Nat. Biomed. Eng. 2020, 4, 997–1009. [Google Scholar] [CrossRef]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.; Jang, K.; Liu, Z.; Ying, M. 3D Multifunctional Integumentary Membranes for Spatiotemporal Cardiac Measurements and Stimulation Across the Entire Epicardium. Nat. Commun. 2014, 5, 3329. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Chen, B.; Zhang, J.; Li, Y. 3D Printed Graphene/Polyurethane Wearable Pressure Sensor for Motion Fitness Monitoring. Nanotechnology 2021, 32, 395503. [Google Scholar] [CrossRef]
- Aryafar, M.; Hamedi, M.; Ganjeh, M.M. A Novel Temperature Compensated Piezoresistive Pressure Sensor. Measurement 2015, 63, 25–29. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Todoroki, A. Wireless Monitoring of Automobile Tires for Intelligent Tires. Sensors 2008, 8, 8123–8138. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gianchandani, Y.B. A 100 Μm Diameter Capacitive Pressure Sensor with 50 MPa Dynamic Range. J. Micromech. Microeng. 2016, 26, 045009. [Google Scholar] [CrossRef]
- Guo, S.; Guo, J.; Ko, W.H. A Monolithically Integrated Surface Micromachined Touch Mode Capacitive Pressure Sensor. Sens. Actuators A Phys. 2000, 80, 224–232. [Google Scholar] [CrossRef]
- Wang, Q.; Ko, W.H. Modeling of Touch Mode Capacitive Sensors and Diaphragms. Sens. Actuators A Phys. 1999, 75, 230–241. [Google Scholar] [CrossRef]
- Kuo, J.T.; Yu, L.; Meng, E. Micromachined Thermal Flow sensors—A Review. Micromachines 2012, 3, 550–573. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Zhang, Q.; Zheng, K.; Xu, J.; Xu, W.; Shang, E.; Jiang, J.; Zhang, J.; Liu, Y. 3D-Printed Graphene/Polydimethylsiloxane Composites for Stretchable and Strain-Insensitive Temperature Sensors. ACS Appl. Mater. Interfaces 2018, 11, 1344–1352. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, J.-H.; Shin, K.-S.; Song, T.-H.; Kim, J.; Lee, D.-S. Development of an Implantable Capacitive Pressure Sensor for Biomedical Applications. Micromachines 2023, 14, 975. https://doi.org/10.3390/mi14050975
Roh J-H, Shin K-S, Song T-H, Kim J, Lee D-S. Development of an Implantable Capacitive Pressure Sensor for Biomedical Applications. Micromachines. 2023; 14(5):975. https://doi.org/10.3390/mi14050975
Chicago/Turabian StyleRoh, Ji-Hyoung, Kyu-Sik Shin, Tae-Ha Song, Jihong Kim, and Dae-Sung Lee. 2023. "Development of an Implantable Capacitive Pressure Sensor for Biomedical Applications" Micromachines 14, no. 5: 975. https://doi.org/10.3390/mi14050975





