Fabrication of Silver Nanobowl Arrays on Patterned Sapphire Substrate for Surface-Enhanced Raman Scattering
Abstract
1. Introduction
2. Materials and Methods
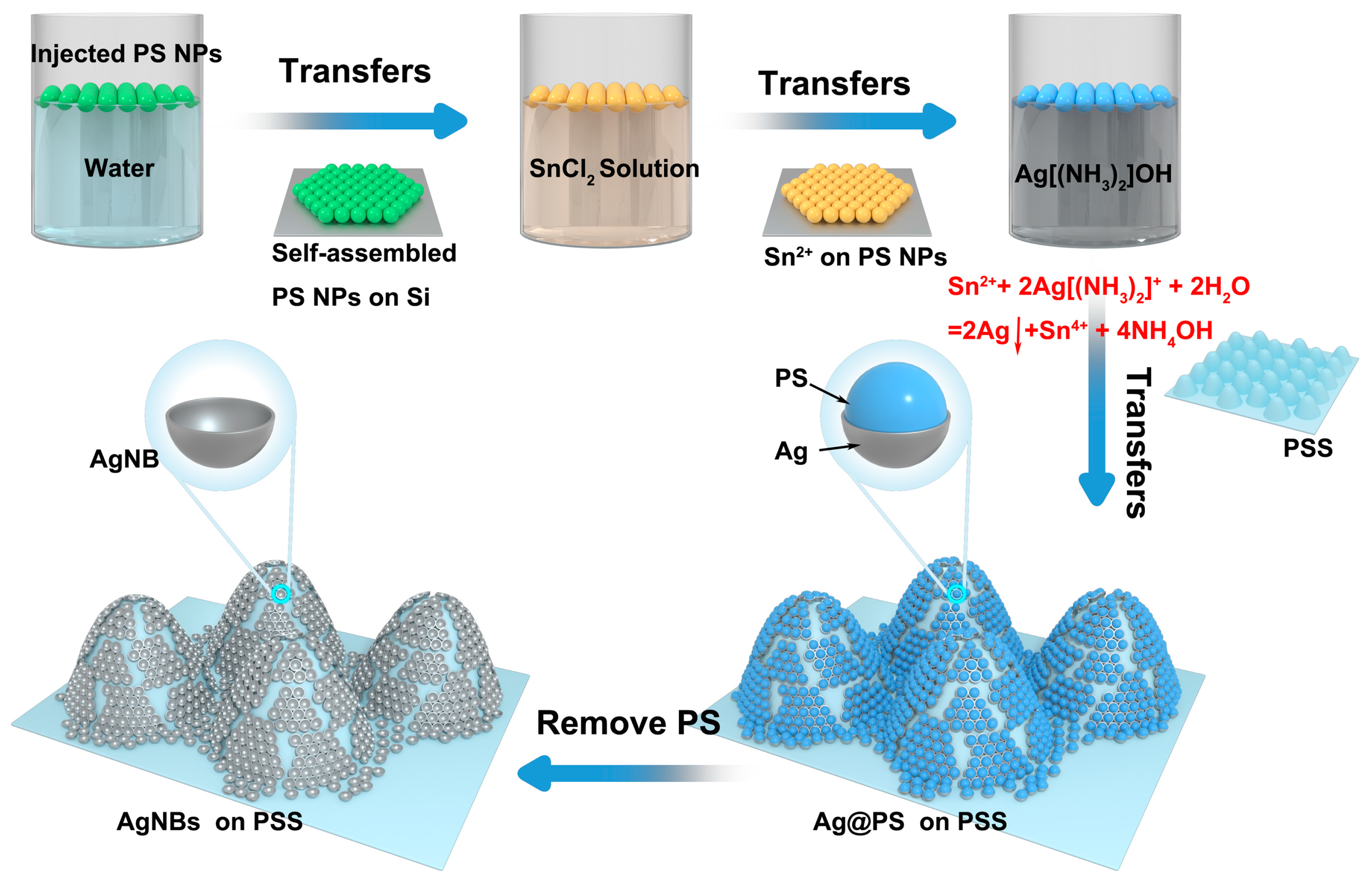
2.1. Preparation of PSS-Loaded Ag Nanobowl Structures
2.2. SERS Testing and Structural Characterization of the Substrate
2.3. Time-Domain Finite-Difference Simulation
3. Results and Discussion
3.1. Structure and Optical Properties of PSS
3.2. Preparation of AgNBs Structure
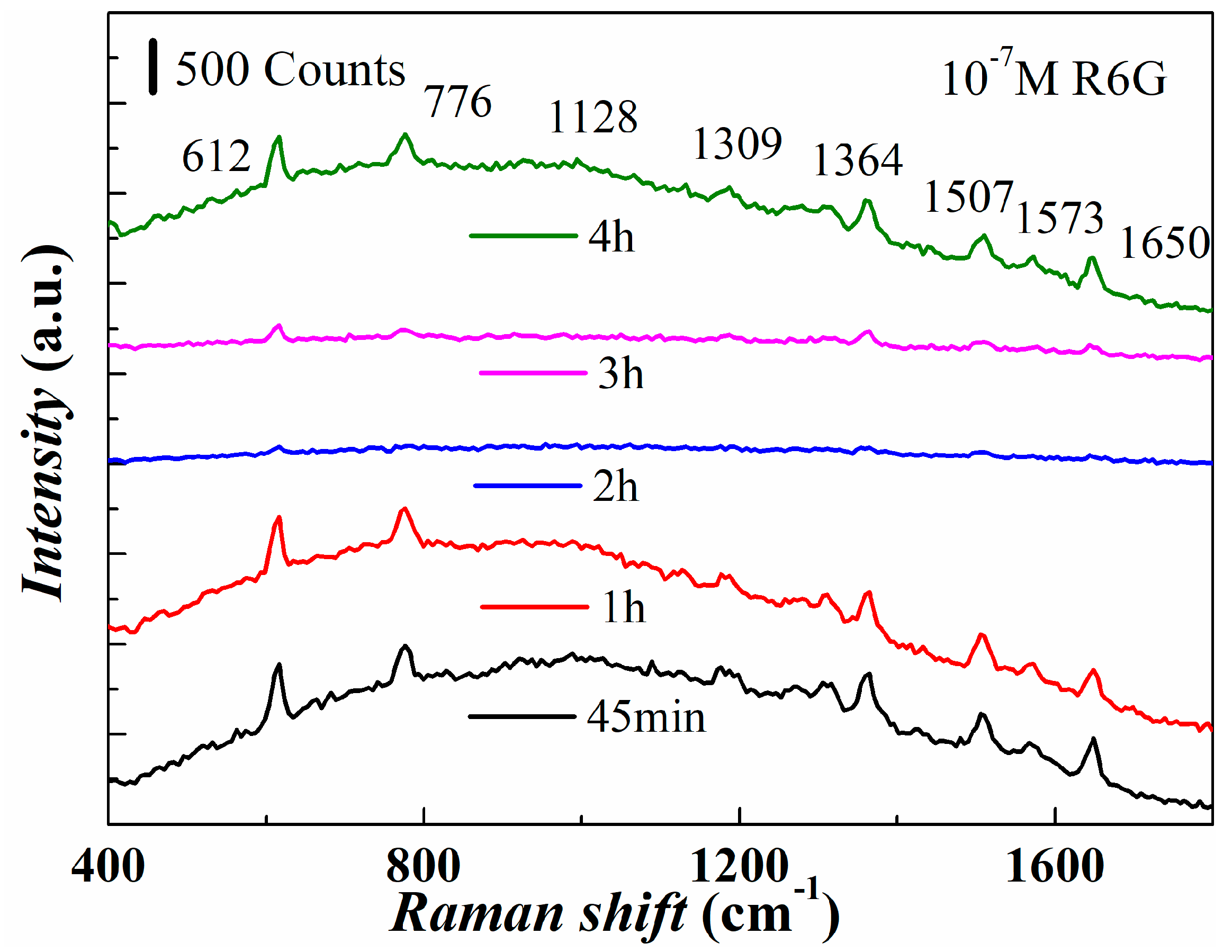
3.3. Effect of Reaction Time on the Structure and SERS Performance of the Substrate
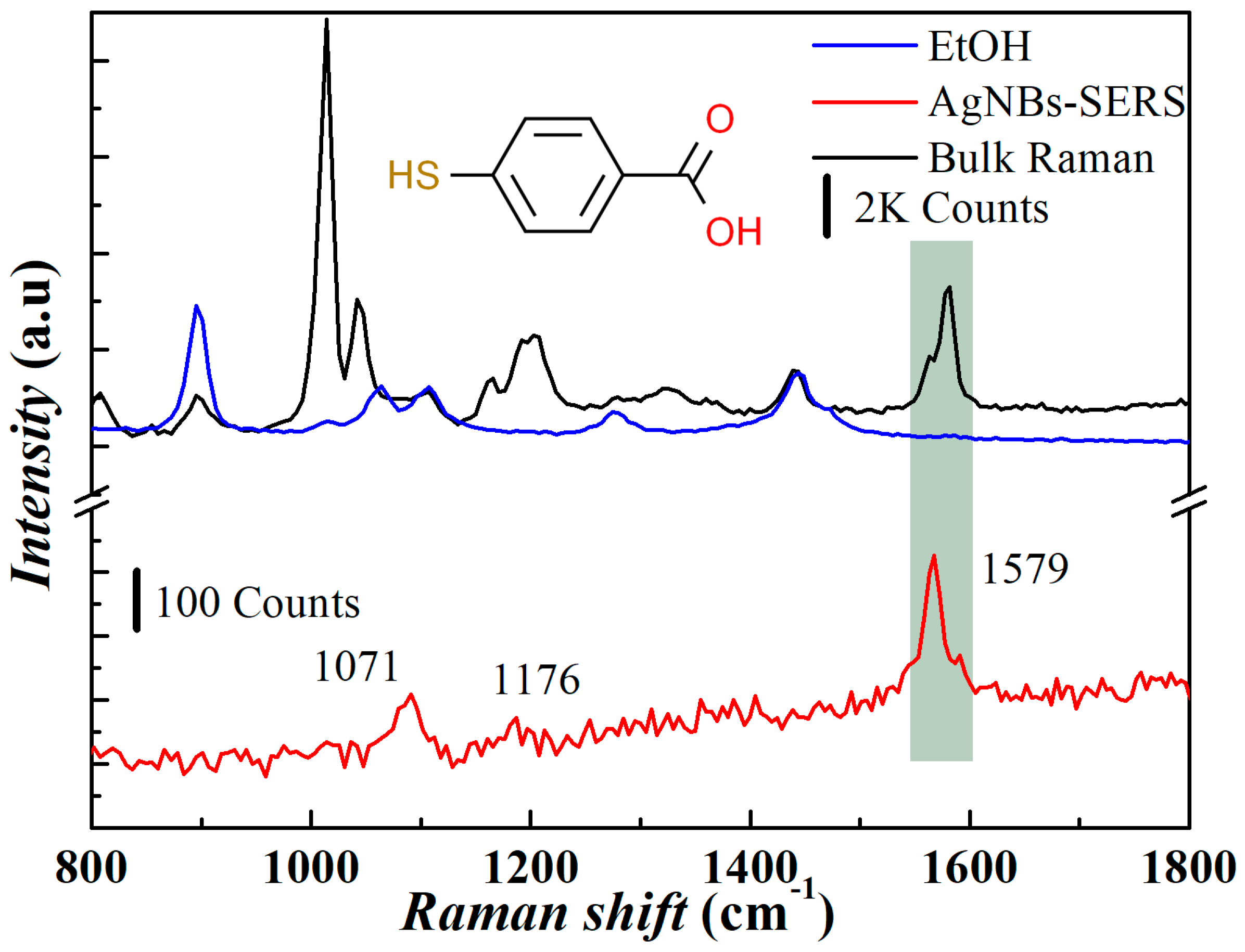
3.4. SERS Enhancement Factor of AgNBs-PSS Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Yang, L.; Liu, J. Three-dimensional SERS hot spots for chemical sensing: Towards developing a practical analyzer. TrAC Trends Anal. Chem. 2016, 80, 364–372. [Google Scholar] [CrossRef]
- Granger, J.H.; Schlotter, N.E.; Crawford, A.C.; Porter, M.D. Prospects for point-of-care pathogen diagnostics using surface-enhanced Raman scattering (SERS). Chem. Soc. Rev. 2016, 45, 3865–3882. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Thomas, M.R.; Bergholt, M.S.; Pence, I.J.; Seong, H.; Charchar, P.; Todorova, N.; Nagelkerke, A.; Belessiotis-Richards, A.; Payne, D.J. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef]
- Schatz, G.C.; Young, M.A.; Van Duyne, R.P. Electromagnetic mechanism of SERS. In Surface-Enhanced Raman Scattering: Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2006; pp. 19–45. [Google Scholar]
- Sinha, S.S.; Jones, S.; Pramanik, A.; Ray, P.C. Nanoarchitecture based SERS for biomolecular fingerprinting and label-free disease markers diagnosis. Acc. Chem. Res. 2016, 49, 2725–2735. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Xia, L.; Yang, J.; Su, R.; Zhou, W.; Zhang, Y.; Zhong, Y.; Huang, S.; Chen, Y.; Li, G. Recent progress in fast sample preparation techniques. Anal. Chem. 2019, 92, 34–48. [Google Scholar] [CrossRef]
- Zhang, J.; Winget, S.A.; Wu, Y.; Su, D.; Sun, X.; Xie, Z.-X.; Qin, D. Ag@ Au concave cuboctahedra: A unique probe for monitoring Au-catalyzed reduction and oxidation reactions by surface-enhanced Raman spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Manoharan, R.; Hanlon, E.B.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Extremely large enhancement factors in surface-enhanced Raman scattering for molecules on colloidal gold clusters. Appl. Spectrosc. 1998, 52, 1493–1497. [Google Scholar] [CrossRef]
- Hu, W.; Xia, L.; Hu, Y.; Li, G. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchem. J. 2022, 172, 106908. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, Q.; Feng, L.; Li, X.; Zhu, H.; Miao, H.; Zeng, Z.; Wang, Y.; Li, Y.; Wang, L. Light-trapping SERS substrate with regular bioinspired arrays for detecting trace dyes. ACS Appl. Mater. Inter. 2021, 13, 11535–11542. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.; Zhou, M.; Duan, P.; Liu, W.; Zhang, W.; Zha, Z.; Gao, J.; Wali, S.; Jiang, S.; Man, B. Highly sensitive and recyclable surface-enhanced Raman scattering (SERS) substrates based on photocatalytic activity of ZnSe nanowires. Sens. Actuators B Chem. 2022, 356, 131360. [Google Scholar] [CrossRef]
- Kim, J.; Cheekati, S.; Sarwar, T.; Ku, P.-C. Designing an Ultrathin Film Spectrometer Based on III-Nitride Light-Absorbing Nanostructures. Micromachines 2021, 12, 760. [Google Scholar] [CrossRef]
- Xie, S.; Si, H.; Liu, C.; Liu, W.; Shafi, M.; Jiang, S.; Yue, W. LSP-SPP Coupling Structure Based on Three-Dimensional Patterned Sapphire Substrate for Surface Enhanced Raman Scattering Sensing. Nanomaterials 2023, 13, 1518. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, J.; Kim, Y.; Lee, J.-H. Stress reduction and enhanced extraction efficiency of GaN-based LED grown on cone-shape-patterned sapphire. IEEE Photonics Technol. Lett. 2008, 20, 1563–1565. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, X.; Du, P.; Zhang, Z.; Liu, X.; Liu, S.; Guo, L.J. Application of patterned sapphire substrate for III-nitride light-emitting diodes. Nanoscale 2022, 14, 4887–4907. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, D.-Y.; Oh, B.-W.; Lee, J.-H. Comparison of InGaN-based LEDs grown on conventional sapphire and cone-shape-patterned sapphire substrate. IEEE Trans. Electron. Devices 2009, 57, 157–163. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; Chang, C.-C.; Su, W.-N.; Uen, Y.-H.; Pan, C.-J.; Liu, J.-Y.; Rick, J.; Lin, K.-Y.; Hwang, B.-J. Self-focusing Au@ SiO2 nanorods with rhodamine 6G as highly sensitive SERS substrate for carcinoembryonic antigen detection. J. Mater. Chem. B 2014, 2, 629–636. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Li, Z.; Zhang, Y.; Li, H.; Yi, X.; Wang, J.; Wang, G.; Li, J. Nanostructure nitride light emitting diodes via the Talbot effect using improved colloidal photolithography. Nanoscale 2017, 9, 7021–7026. [Google Scholar] [CrossRef]
- Cao, X.; Gu, Y.; Li, Z.; Ge, S.; Mao, Y.; Gu, Y.; Lu, D. A SERS microfluidic chip for ultrasensitive and simultaneous detection of SCCA and CYFRA21-1 in serum based on Au nanobowl arrays and hybridization chain reaction. Sens. Actuators B Chem. 2023, 375, 132894. [Google Scholar] [CrossRef]
- Woessner, Z.J.; Lewis, G.R.; Bueno, S.L.; Ringe, E.; Skrabalak, S.E. Asymmetric seed passivation for regioselective overgrowth and formation of plasmonic nanobowls. Nanoscale 2022, 14, 16918–16928. [Google Scholar] [CrossRef]
- Deng, F.; Xiang, J.; Wang, S.; Wang, X.; Hu, H.; Liu, Z.; Ding, T. Dynamic and Reversible Tuning of Particle-in-Bowl Shaped Plasmonic Resonators for Switchable Surface Enhanced Raman Scattering. Adv. Mater. Interfaces 2022, 9, 2101837. [Google Scholar] [CrossRef]
- Wu, L.; Dias, A.; Diéguez, L. Surface enhanced Raman spectroscopy for tumor nucleic acid: Towards cancer diagnosis and precision medicine. Biosens. Bioelectron. 2022, 204, 114075. [Google Scholar] [CrossRef]
- Ye, J.; Van Dorpe, P.; Van Roy, W.; Borghs, G.; Maes, G. Fabrication, characterization, and optical properties of gold nanobowl submonolayer structures. Langmuir 2009, 25, 1822–1827. [Google Scholar] [CrossRef]
- Ye, J.; Lagae, L.; Maes, G.; Borghs, G.; Van Dorpe, P. Symmetry breaking induced optical properties of gold open shell nanostructures. Opt. Express 2009, 17, 23765–23771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Liu, Y.; Yang, S. Template-confined site-specific electrodeposition of nanoparticle cluster-in-bowl arrays as surface enhanced Raman spectroscopy substrates. ACS Sens. 2018, 3, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, F.; Ostendorp, S.; Wilde, G.; Zhao, H.; Lei, Y. Template-Confined Dewetting Process to Surface Nanopatterns: Fabrication, Structural Tunability, and Structure-Related Properties. Adv. Funct. Mater. 2011, 21, 2446–2455. [Google Scholar] [CrossRef]
- Denkov, N.; Velev, O.; Kralchevski, P.; Ivanov, I.; Yoshimura, H.; Nagayama, K. Mechanism of formation of two-dimensional crystals from latex particles on substrates. Langmuir 1992, 8, 3183–3190. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Li, J.; Li, X.; Chen, J.; Wang, L.; Qi, D.; Liu, X.; Shi, G. Fabrication of an insect-like compound-eye SERS substrate with 3D Ag nano-bowls and its application in optical sensor. Sens. Actuators B Chem. 2021, 330, 129357. [Google Scholar] [CrossRef]
- Oran, J.M.; Hinde, R.J.; Abu Hatab, N.; Retterer, S.T.; Sepaniak, M.J. Nanofabricated periodic arrays of silver elliptical discs as SERS substrates. J. Raman Spectrosc. Int. J. Orig. Work. All Asp. Raman Spectrosc. Incl. High. Order Process. Also Brillouin Rayleigh Scatt. 2008, 39, 1811–1820. [Google Scholar] [CrossRef]
- Lee, S.H.; Bantz, K.C.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Self-assembled plasmonic nanohole arrays. Langmuir 2009, 25, 13685–13693. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Q.; Lu, Y.; Du, W.; Li, B.; Cui, Y.; Yuan, C.; Zhan, P.; Ge, H.; Wang, Z. A high-performance and low cost SERS substrate of plasmonic nanopillars on plastic film fabricated by nanoimprint lithography with AAO template. AIP Adv. 2017, 7, 065205. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.-W.; Qin, D. Galvanic replacement-free deposition of Au on Ag for core–shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Yilmaz, M.; Ozdemir, M.; Erdogan, H.; Tamer, U.; Sen, U.; Facchetti, A.; Usta, H.; Demirel, G. Micro-/nanostructured highly crystalline organic semiconductor films for surface-enhanced Raman spectroscopy applications. Adv. Funct. Mater. 2015, 25, 5669–5676. [Google Scholar] [CrossRef]
- Zhong, L.-B.; Yin, J.; Zheng, Y.-M.; Liu, Q.; Cheng, X.-X.; Luo, F.-H. Self-assembly of Au nanoparticles on PMMA template as flexible, transparent, and highly active SERS substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, X.; Liu, L.; Zhou, Q.; Jin, M.; Zhou, G.; Gao, X.; Lu, X.; Zhang, Z.; Liu, J. High-density ordered Ag@ Al2O3 nanobowl arrays in applications of surface-enhanced Raman spectroscopy. Nanotechnology 2016, 27, 165304. [Google Scholar] [CrossRef]
- Zhu, A.; Gao, R.; Zhao, X.; Zhang, F.; Zhang, X.; Yang, J.; Zhang, Y.; Chen, L.; Wang, Y. Site-selective growth of Ag nanoparticles controlled by localized surface plasmon resonance of nanobowl arrays. Nanoscale 2019, 11, 6576–6583. [Google Scholar] [CrossRef]
- Jana, D.; Lehnhoff, E.; Bruzas, I.; Robinson, J.; Lum, W.; Sagle, L. Tunable Au–Ag nanobowl arrays for size-selective plasmonic biosensing. Analyst 2016, 141, 4870–4878. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, S.; Wang, Y.; Guo, Y.; Sun, L.; Li, K.; Cui, G. Effective plasmon coupling in conical cavities for sensitive surface enhanced Raman scattering with quantitative analysis ability. Nanoscale 2019, 11, 17913–17919. [Google Scholar] [CrossRef] [PubMed]




| Fabrication Process | Characteristic Parameters | Advantages | Limitations | Enhanced Capability of SERS | References |
|---|---|---|---|---|---|
| EBL/FIB *1 | The aspect ratio of ellipses | Ordered 2D structure, good structural precision | Complex equipment, expensive preparation costs, low efficiency | 5 × 106 | [32] |
| NSL *2 | Nanopore | Ordered 2D structure, simple equipment, and low cost | Medium structural accuracy, deformable support | 3 × 106 | [33] |
| NIL *3 | Nanopore | Simple equipment and low cost | Depends on the template, deformable support | 108 | [34] |
| Deposition | Nanoparticles | Simple equipment and low cost | Poor structural accuracy, disordered structure | 9.7 × 105 | [35,36] |
| Self-assembly | Nanoparticles | Ordered 2D structure, simple equipment, and low cost | Medium structural accuracy | 2 × 107 | [37] |
| Self-assembly on 3D PSS | AgNBs | Simple equipment and low cost | Medium structural accuracy | 105 | This work |
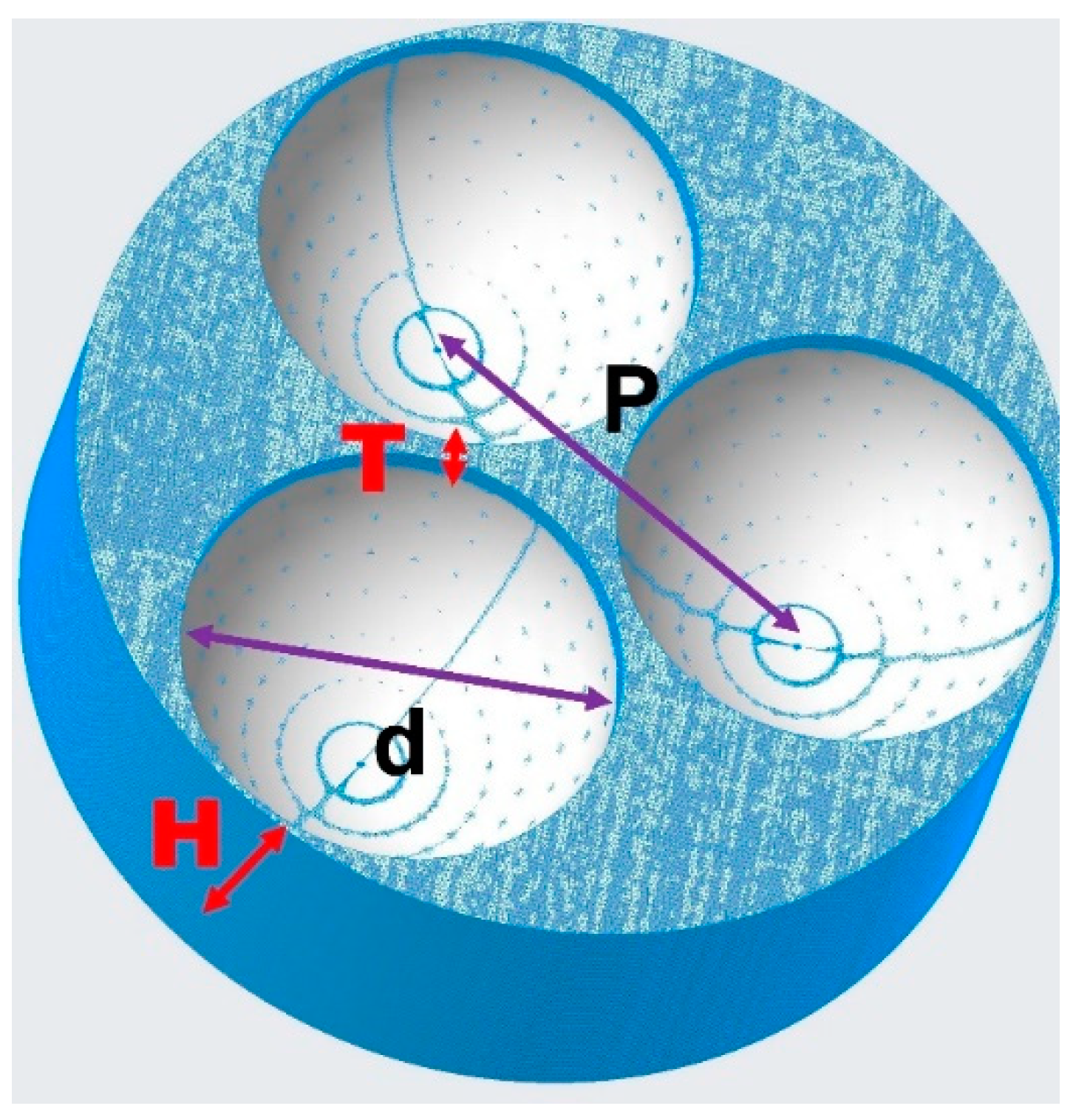
| Reaction Time | Inner Diameter d nm | Outer Diameter D nm | Wall Thickness T nm | Height H nm | Round Center Distance P nm |
|---|---|---|---|---|---|
| 45 min | 127 | 155 | 14 | 82 | 164 |
| 90 min | 114 | 156 | 21 | 90 | 165 |
| 2 h | 110 | 155 | 22.5 | 65 | 164 |
| 3 h | 99 | 155 | 28 | 115 | 165 |
| 4 h | 75 | 155 | 40 | 118 | 167 |
| Substrate Structure | Detection Limit | EF | References |
|---|---|---|---|
| Ag@Al2O3 NBs | 10−10 M P-Tc * | 6.5 × 106 | [39] |
| AgNBs/3D-Si | 10−9 M R6G | 3 × 107 | [31] |
| AgNPs on AuNBs | [40] | ||
| AuNPs in Au-AgNBs | H1N1 virus | [41] | |
| Ag conical cavity arrays | 10−9 M R6G | 7 × 106 | [42] |
| AgNBs-PSS | 10−7 M R6G | 9 × 104 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Jin, M. Fabrication of Silver Nanobowl Arrays on Patterned Sapphire Substrate for Surface-Enhanced Raman Scattering. Micromachines 2023, 14, 1197. https://doi.org/10.3390/mi14061197
Pang Y, Jin M. Fabrication of Silver Nanobowl Arrays on Patterned Sapphire Substrate for Surface-Enhanced Raman Scattering. Micromachines. 2023; 14(6):1197. https://doi.org/10.3390/mi14061197
Chicago/Turabian StylePang, Yanzhao, and Mingliang Jin. 2023. "Fabrication of Silver Nanobowl Arrays on Patterned Sapphire Substrate for Surface-Enhanced Raman Scattering" Micromachines 14, no. 6: 1197. https://doi.org/10.3390/mi14061197
APA StylePang, Y., & Jin, M. (2023). Fabrication of Silver Nanobowl Arrays on Patterned Sapphire Substrate for Surface-Enhanced Raman Scattering. Micromachines, 14(6), 1197. https://doi.org/10.3390/mi14061197






