Experimental Investigations upon Ultrasound Influence on Calefaction of AdBlue in Selective Catalytic Reduction Systems (SCR)
Abstract
1. Introduction
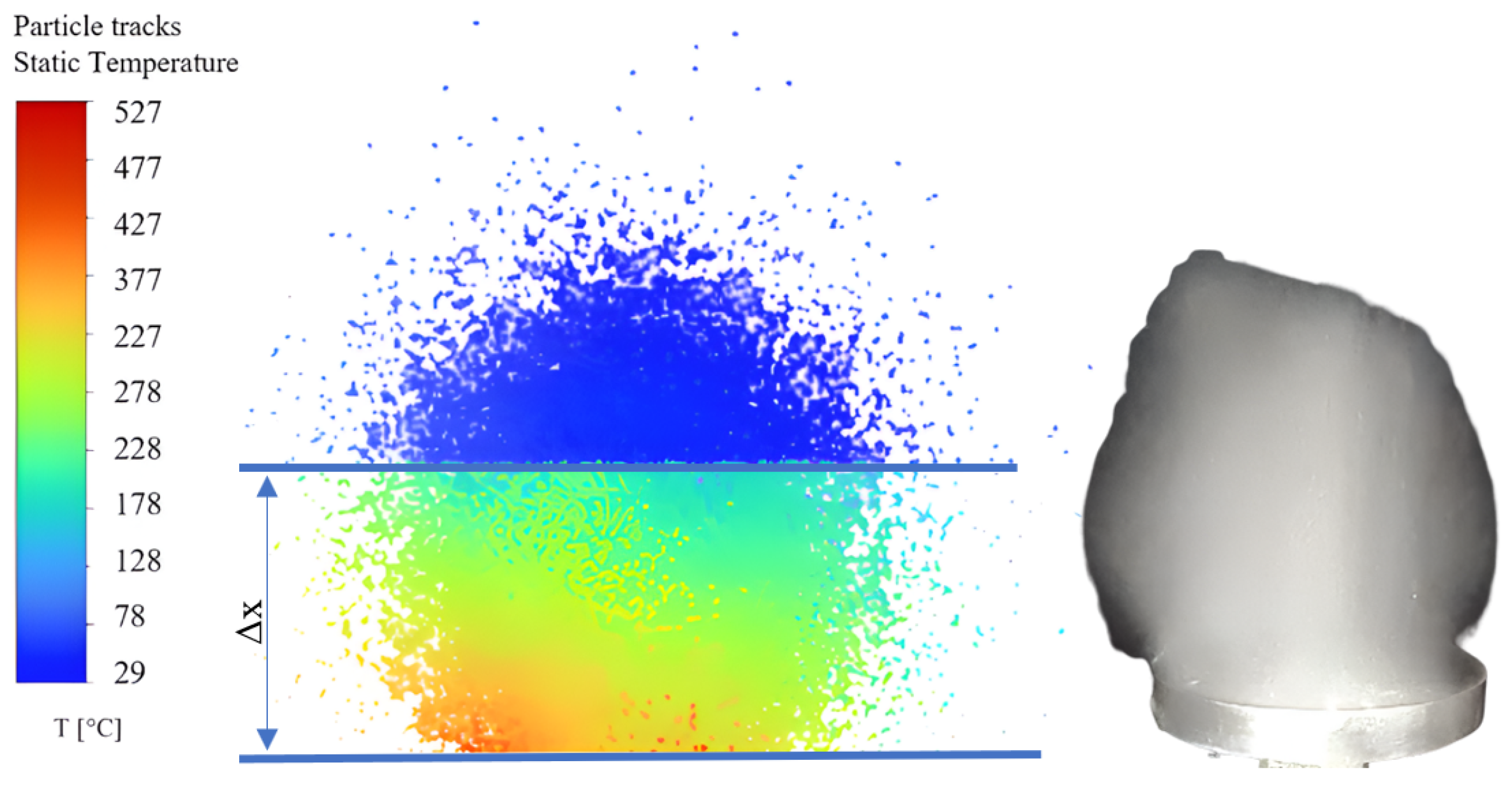
2. Determination of the Temperature Distribution of the Ultrasonic Generator Disc and the Droplets on Its Surface
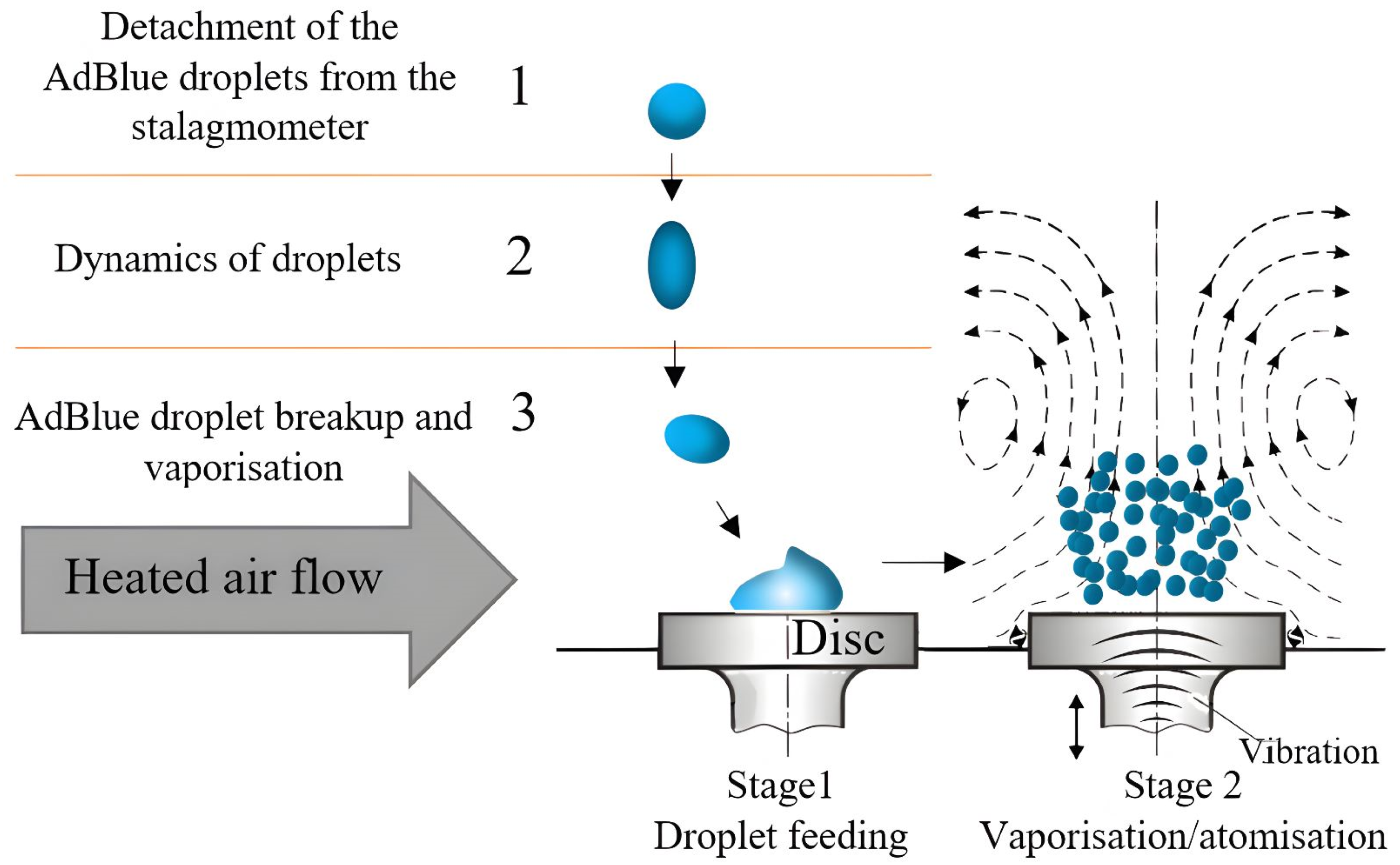
2.1. A Description of the Processes That Take Place When Injecting Adblue onto a Disc with and without Vibration
2.2. A Description of the Processes That Take Place When Injecting AdBlue onto a Disc with and without Vibration
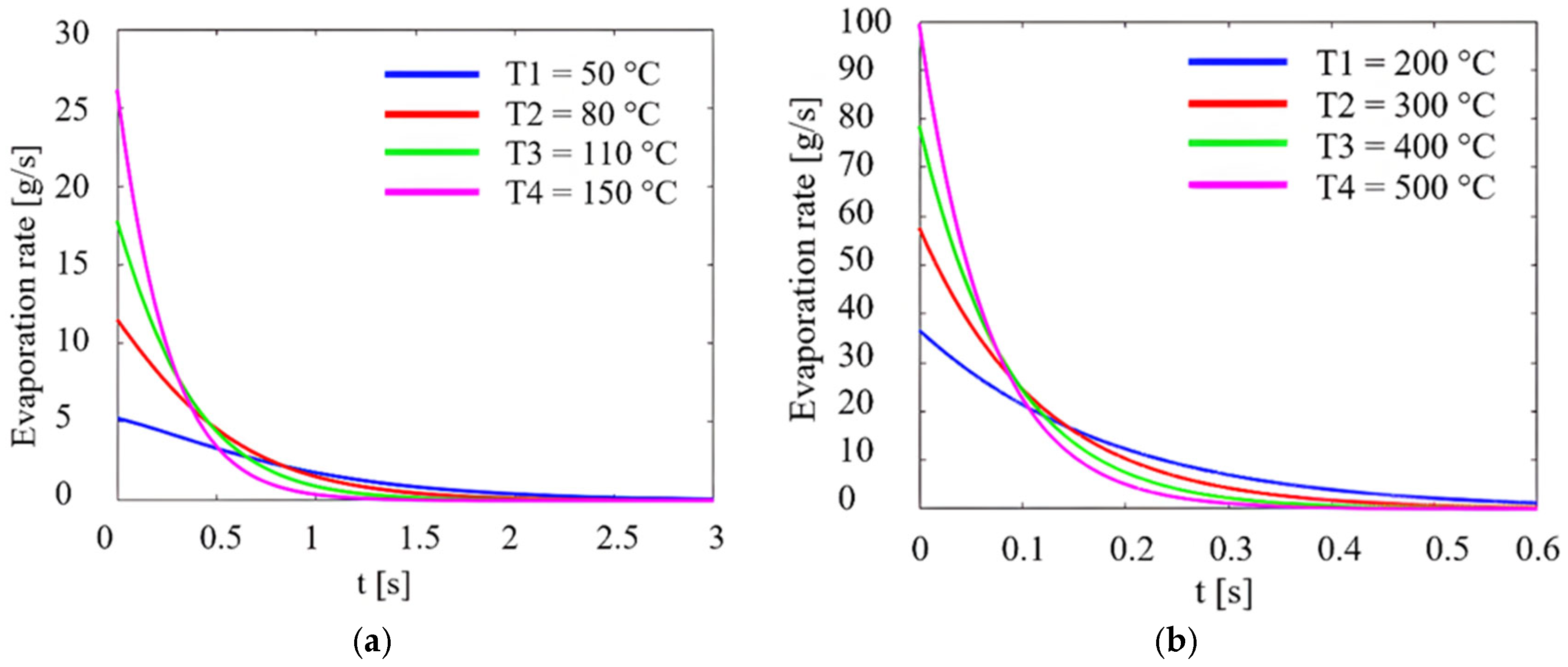
2.3. Determination of the Evaporation Rate of AdBlue Droplets and Modelling This Phenomenon in ANSYS
3. Experimental Results and Interpretation
3.1. Description of the Experimental Laboratory Test Bench
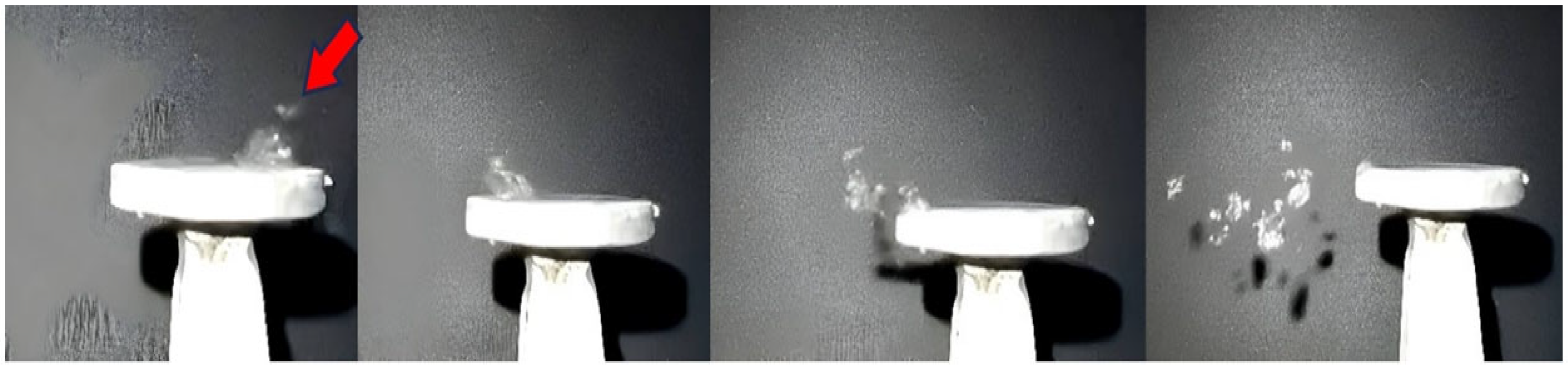
3.2. Use of High-Speed Recording to Highlight the Evolution of AdBlue Droplets in the Calefaction Process
3.3. Application of the IR Technique to the Calefaction of AdBlue Droplets
3.4. Evidence of Crystal Deposition in the Case of Calefaction Phenomenon
4. Conclusions
- injecting the AdBlue solution into an ultrasonic field
- gas temperature control with an external induction heating system (not part of this study).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmid, J.; Zarikos, I.; Terzis, A.; Roth, N.; Weigand, B. Crystallization of urea from an evaporative aqueous solution sessile droplet at sub-boiling temperatures and surfaces with different wettability. Exp. Therm. Fluid Sci. 2018, 91, 80–88. [Google Scholar] [CrossRef]
- Eakle, S.; Kroll, S.; Yau, A.; Gomez, J.; Henry, C. Investigation of Urea Derived Deposits Composition in SCR Systems and Their Potential Effect on Overall PM Emissions (No. 2016-01-0989); SAE Technical Paper; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Weeks, C.L.; Ibeling, D.R.; Han, S.; Ludwig, L.; Ayyappan, P. Analytical Investigation of Urea Deposits in SCR System. SAE Int. J. Engines 2015, 8, 1219–1239. [Google Scholar] [CrossRef]
- Smith, H.; Lauer, T.; Mayer, M.; Pierson, S. Optical and numerical investigations on the mechanisms of deposit formation in SCR systems. SAE Int. J. Fuels Lubr. 2014, 7, 525–542. [Google Scholar] [CrossRef]
- Munnannur, A.; Chiruta, M.; Liu, Z.G. Thermal and Fluid Dynamic Considerations in Aftertreatment System Design for SCR Solid Deposit Mitigation (No. 2012-01-1287); SAE Technical Paper; SAE International: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Zheng, G.; Fila, A.; Kotrba, A.; Floyd, R. Investigation of urea deposits in urea SCR systems for medium and heavy-duty trucks (No. 2010-01-1941). In Proceedings of the SAE 2010 Commercial Vehicle Engineering Congress, Chicago, IL, USA, 5 October 2010; Volume 1. [Google Scholar]
- Park, K.; Hong, C.H.; Oh, S.; Moon, S. Numerical prediction on the influence of mixer on the performance of urea-SCR system. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2014, 8, 998–1004. [Google Scholar]
- Habchi, C.; Nicolle, A.; Gillet, N. Numerical study of deposits formation in SCR systems using urea-water solution injection. J. Mater. Sci. Nanotechnol. 2018, 6, 201. [Google Scholar]
- Jeong, S.; Kim, H.; Kim, H.; Kwon, O.; Park, E.; Kang, J. Optimization of the urea injection angle and direction: Maximizing the uniformity index of a selective catalytic reduction system. Energies 2020, 14, 157. [Google Scholar] [CrossRef]
- Mehdi, G.; Zhou, S.; Zhu, Y.; Shah, A.H.; Chand, K. Numerical investigation of SCR mixer design optimization for improved performance. Processes 2019, 7, 168. [Google Scholar] [CrossRef]
- Bouaziz, M.; Daïf, A. Etude numérique et expérimentale de l’évaporation simultanée de plusieurs gouttes de carburants. Influence de la pression, des convections et de interaction mécanique. Perpignan Via Domitia Perpignan 1998. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=192736 (accessed on 15 May 2023).
- Li, S.; Zhao, Z.; Li, J.; Liu, H.; Liu, M.; Zhang, Y.; Su, L.; Pérez-Jiménez, A.I.; Guo, Y.; Yang, F.; et al. Mechanically induced highly efficient hydrogen evolution from water over piezoelectric SnSe nanosheets. Small 2022, 18, 2202507. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Liu, M.; Liu, X.; Huang, W.; Sun, S.; Jiang, Y.; Liu, Y.; Zhang, J.; Zhang, Z. Remarkably enhanced photocatalytic performance of Au/AgNbO3 heterostructures by coupling piezotronic with plasmonic effects. Nano Energy 2022, 95, 107031. [Google Scholar] [CrossRef]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A Potential Sustainable Pathway for the Valorization of Lignocellulosic Biomass and Derivatives. Top. Curr. Chem. (Z) 2017, 375, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, B.; Huang, F.; Lin, Z. Enabling PIEZOpotential in PIEZOelectric Semiconductors for Enhanced Catalytic Activities. Angew. Chem. Int. Ed. Engl. 2019, 58, 7526–7536. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.V.; Santos, J.R.N.D.; Januario, M.A.P.; Corrêa, A.G. Greener organic synthetic methods: Sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrason. Sonochem. 2021, 78, 105704. [Google Scholar] [CrossRef] [PubMed]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; Wiley: New York, NY, USA, 1996; Volume 6, pp. 408–409. [Google Scholar]
- Raghavan, V. Numerical modelling of evaporation and combustion of isolated liquid fuel droplets: A review. J. Indian Inst. Sci. 2019, 99, 5–23. [Google Scholar] [CrossRef]
- Godsave, G.A.E. Studies of the combustion of drops in a fuel spray—The burning of single drops of fuel. Symp. (Int.) Combust. 1953, 4, 818–830. [Google Scholar] [CrossRef]
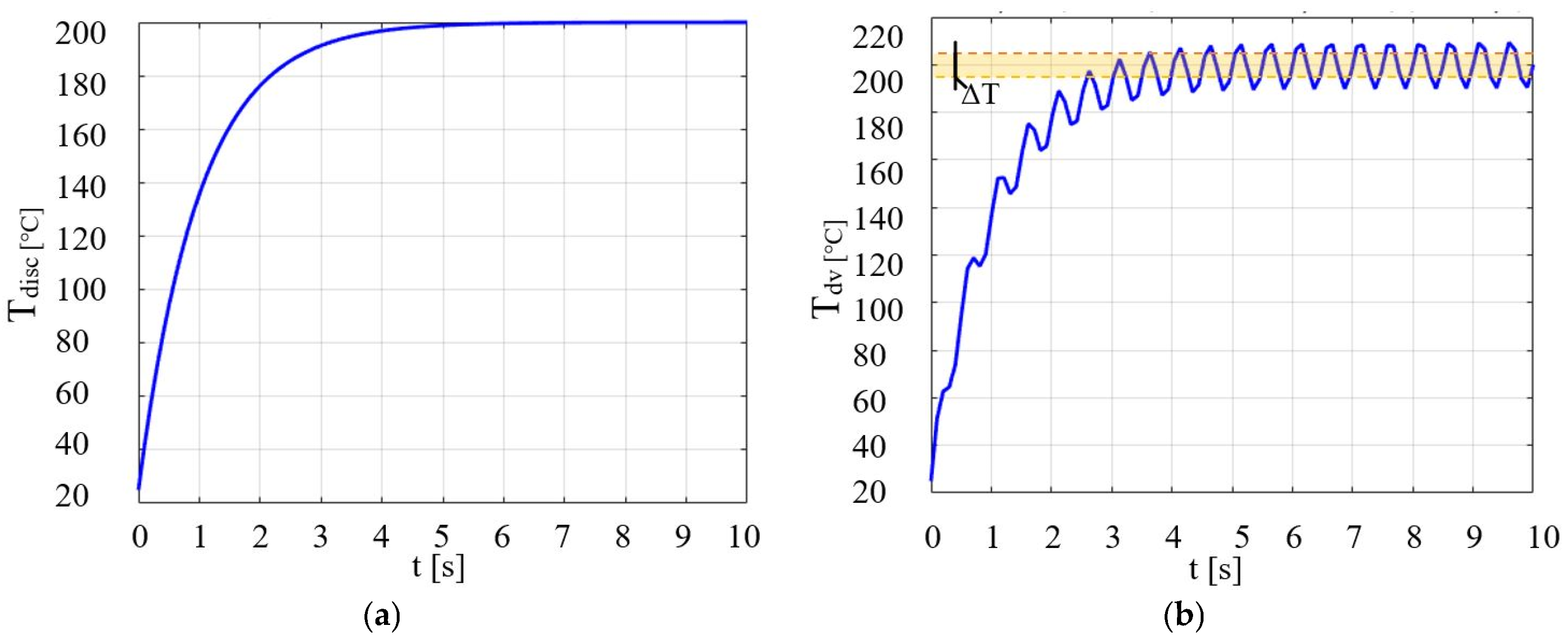
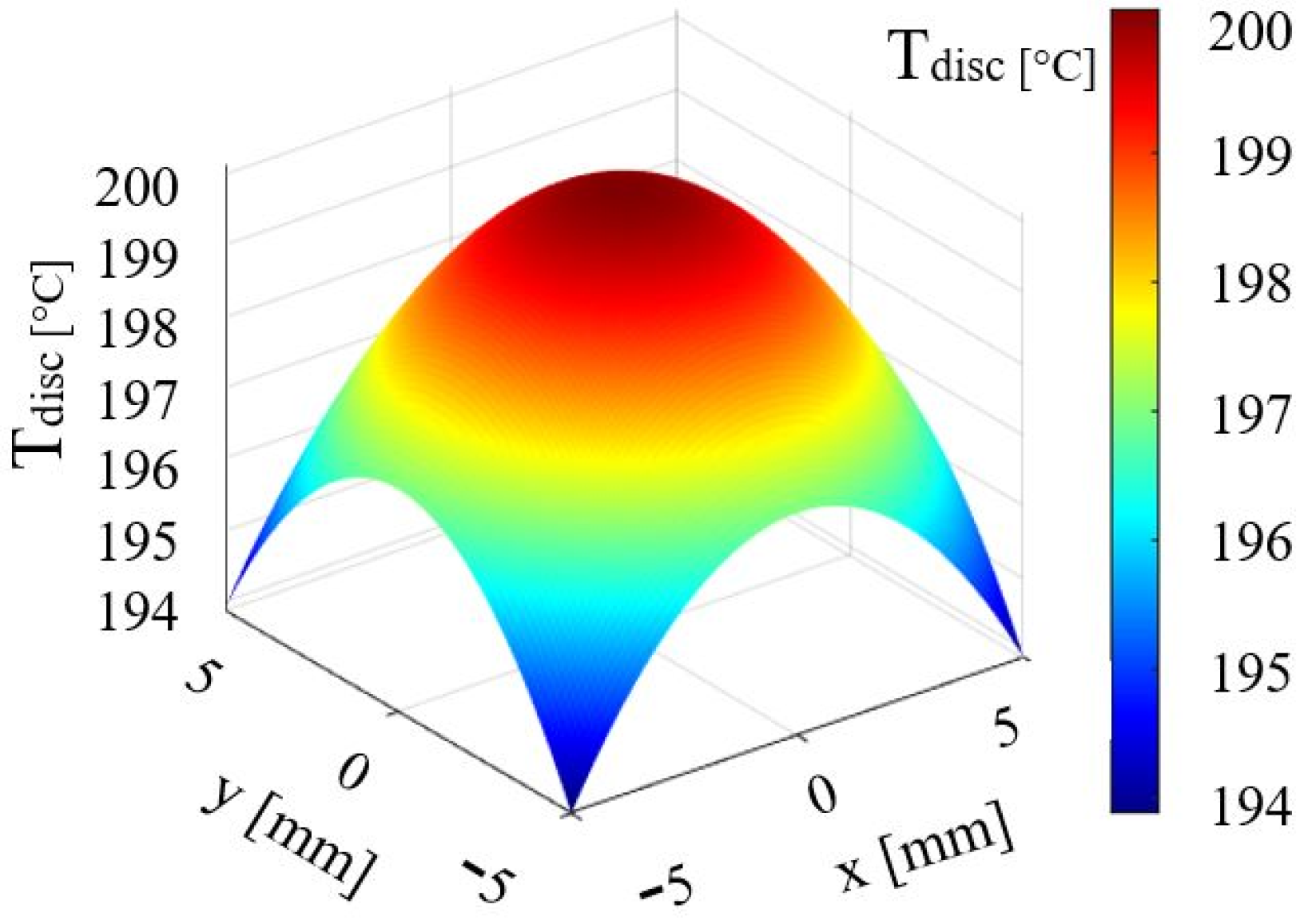
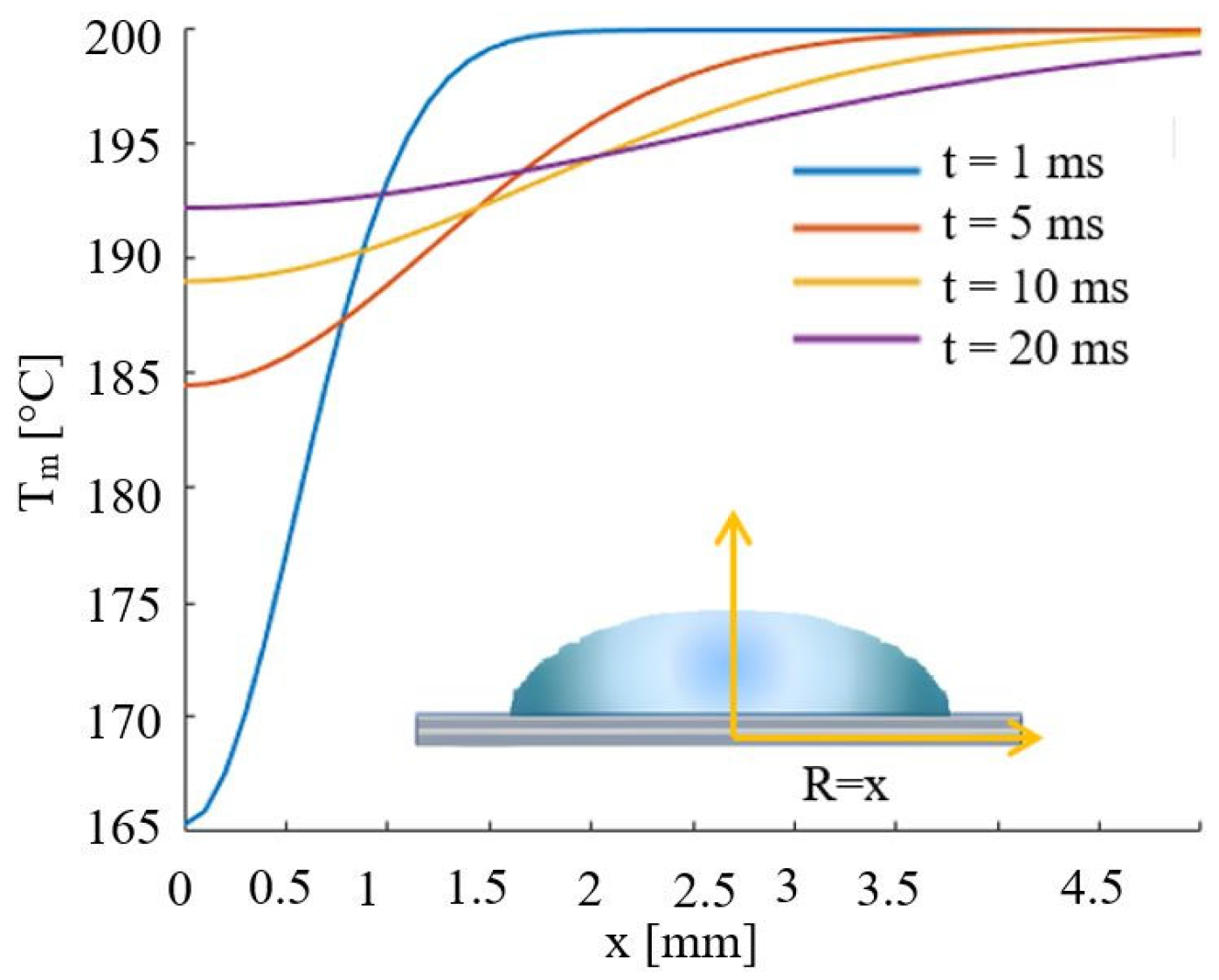

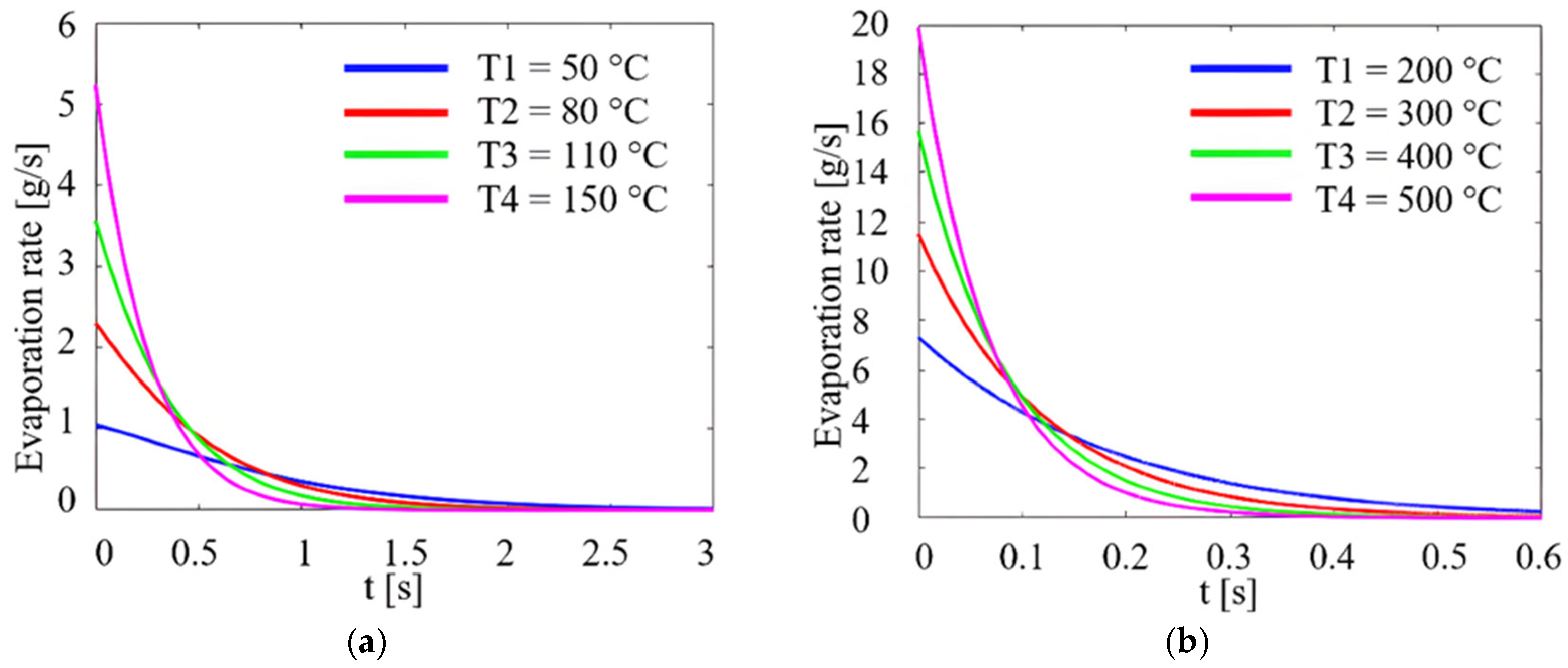



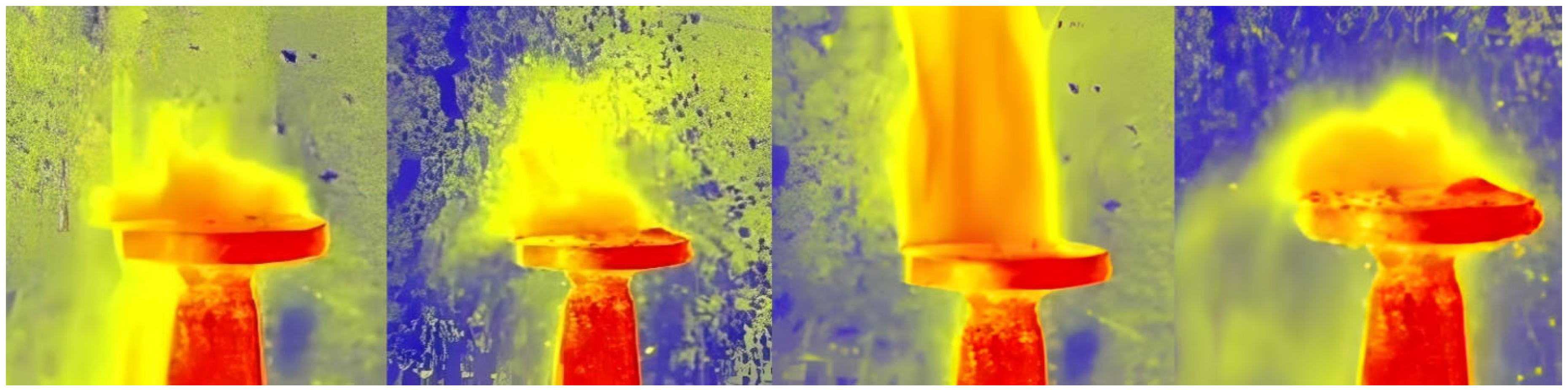
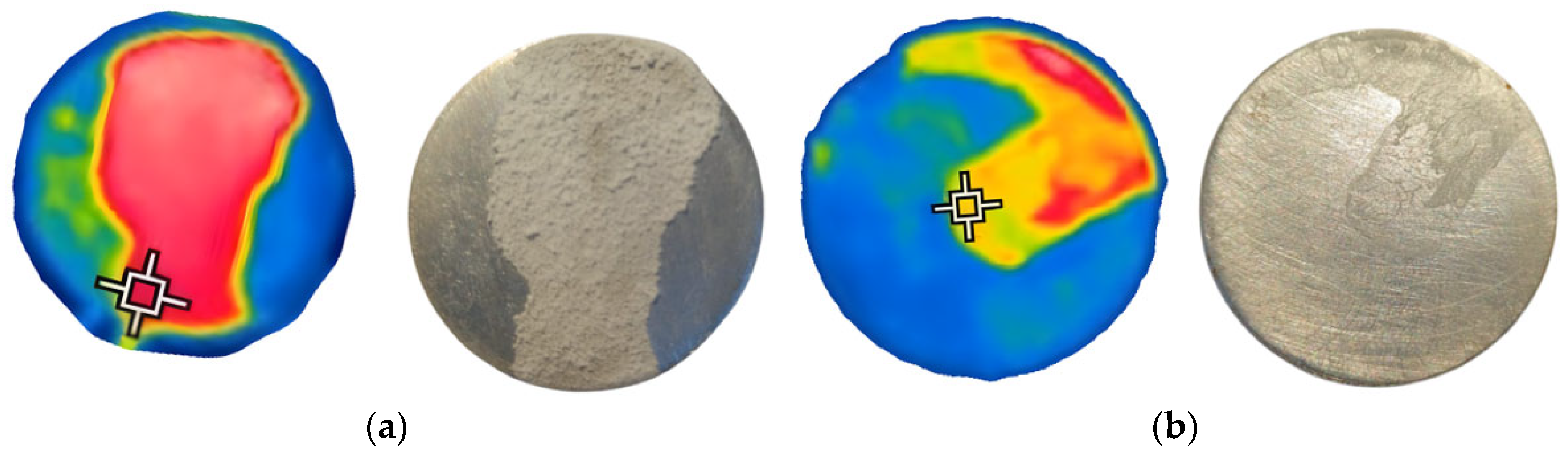
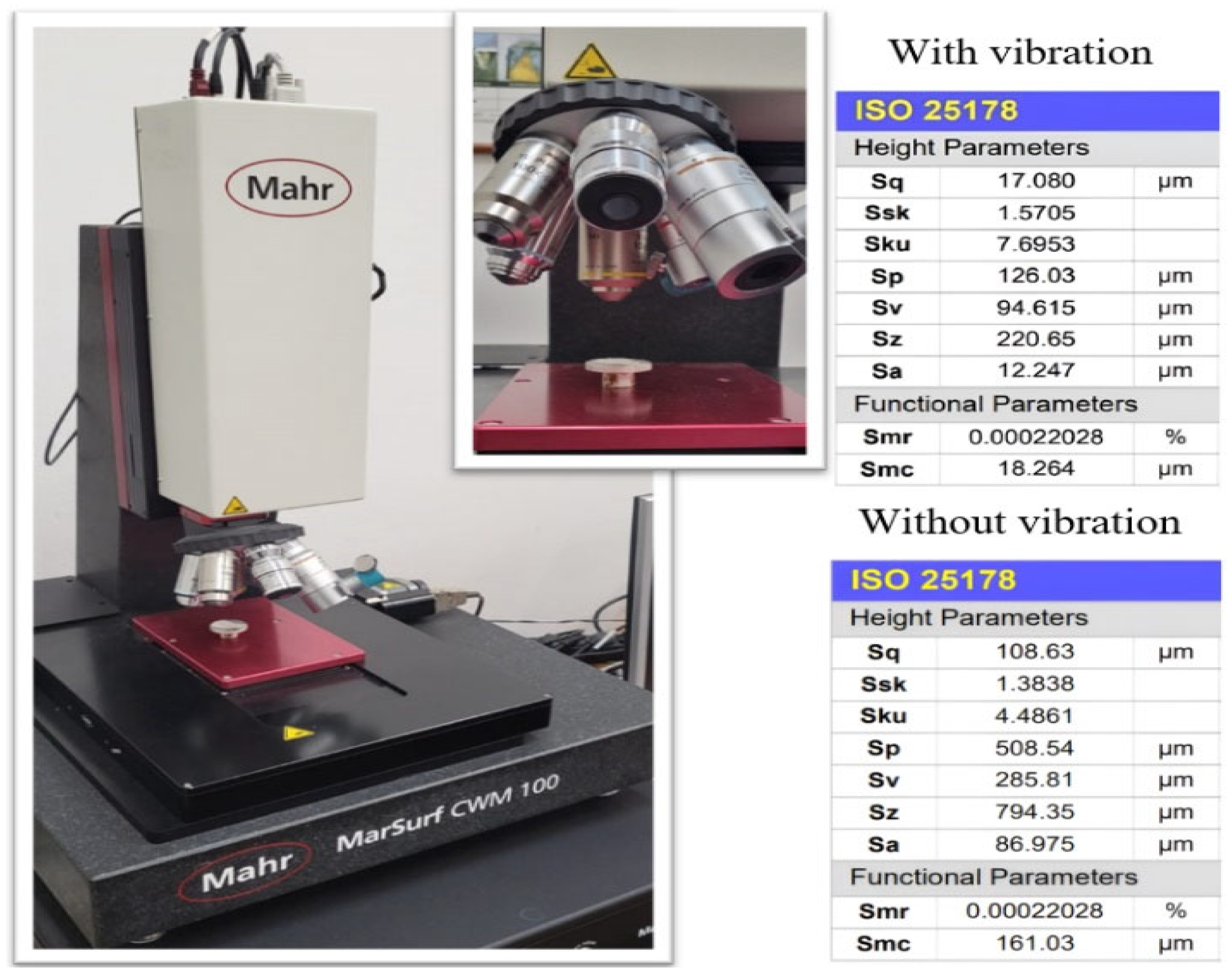
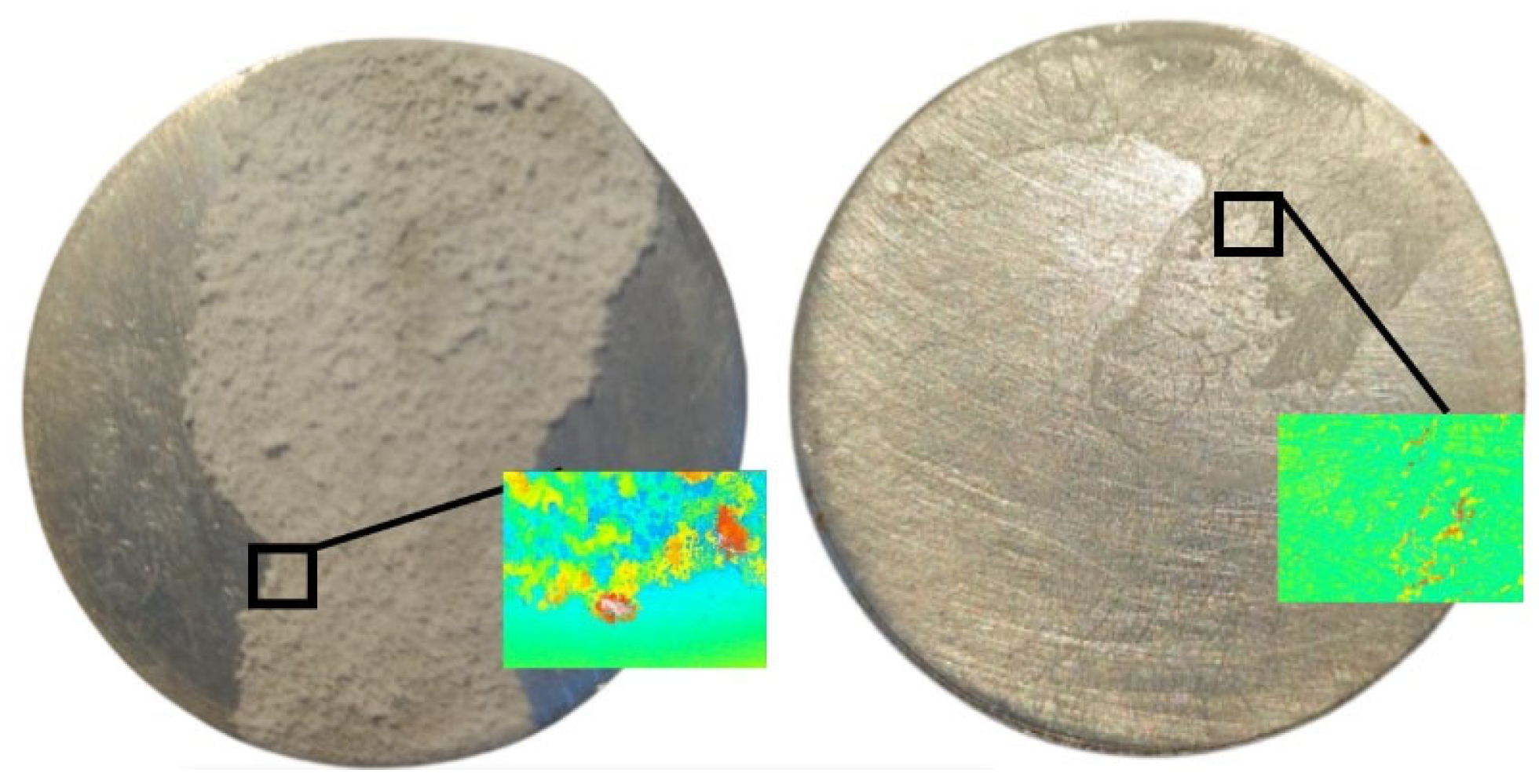
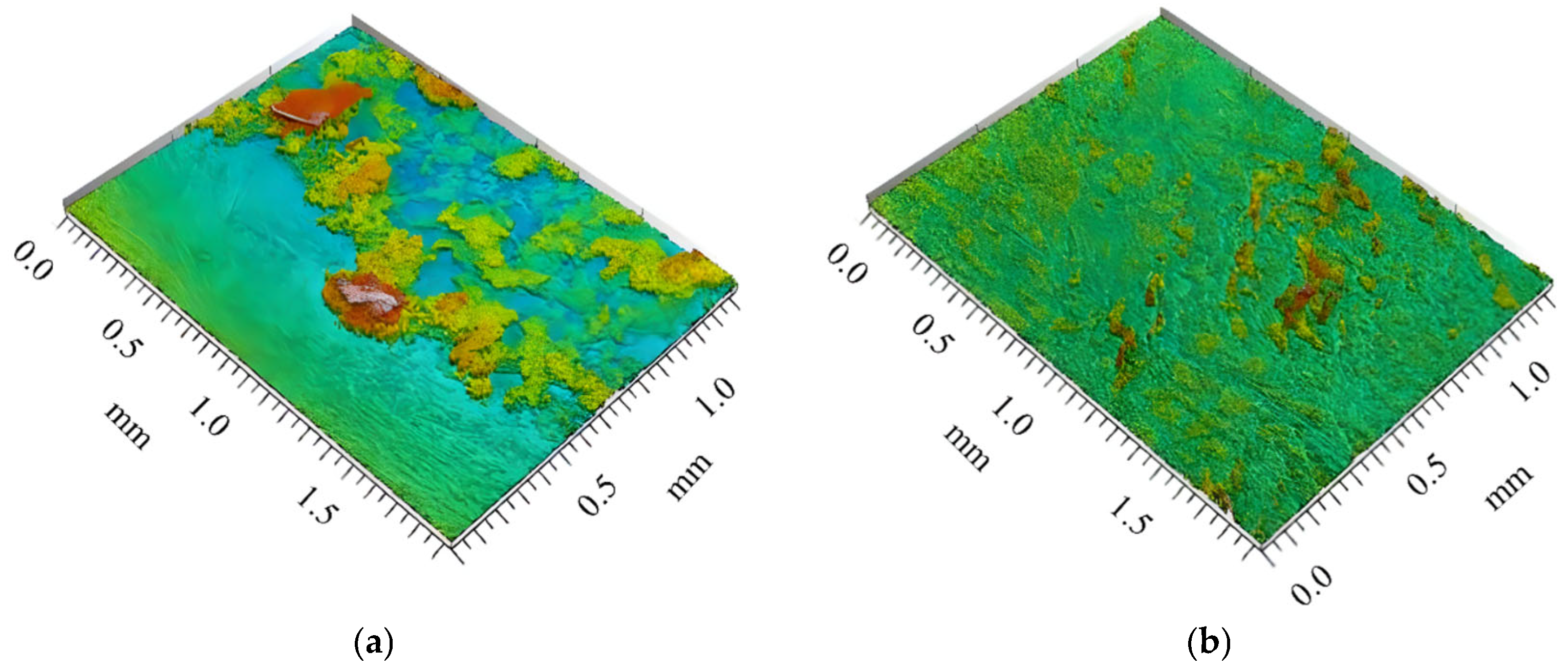
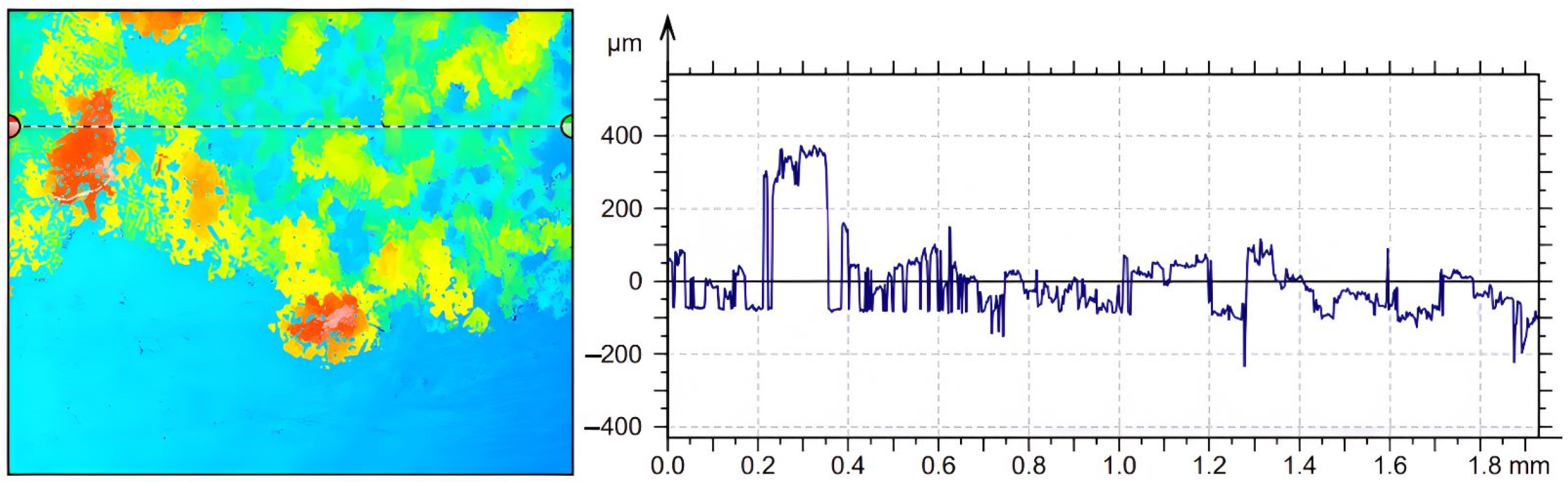
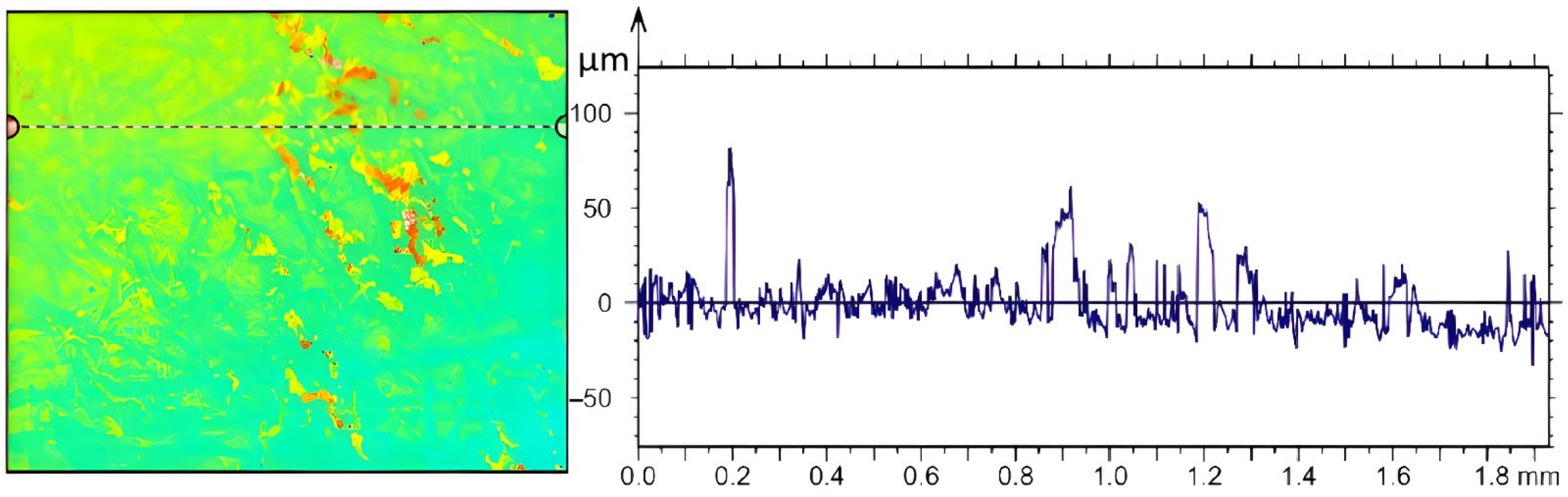

| Parameters | Values | Units |
|---|---|---|
| AdBlue droplet temperature (Ti) | 25 | [°C] |
| Disc temperature (Tdisc) | 200 | [°C] |
| Frequency (f) | 20,000 | [Hz] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picus, C.M.; Mihai, I.; Suciu, C. Experimental Investigations upon Ultrasound Influence on Calefaction of AdBlue in Selective Catalytic Reduction Systems (SCR). Micromachines 2023, 14, 1488. https://doi.org/10.3390/mi14081488
Picus CM, Mihai I, Suciu C. Experimental Investigations upon Ultrasound Influence on Calefaction of AdBlue in Selective Catalytic Reduction Systems (SCR). Micromachines. 2023; 14(8):1488. https://doi.org/10.3390/mi14081488
Chicago/Turabian StylePicus, Claudiu Marian, Ioan Mihai, and Cornel Suciu. 2023. "Experimental Investigations upon Ultrasound Influence on Calefaction of AdBlue in Selective Catalytic Reduction Systems (SCR)" Micromachines 14, no. 8: 1488. https://doi.org/10.3390/mi14081488
APA StylePicus, C. M., Mihai, I., & Suciu, C. (2023). Experimental Investigations upon Ultrasound Influence on Calefaction of AdBlue in Selective Catalytic Reduction Systems (SCR). Micromachines, 14(8), 1488. https://doi.org/10.3390/mi14081488










