Investigation on Wire Electrochemical Discharge Micro-Machining
Abstract
1. Introduction
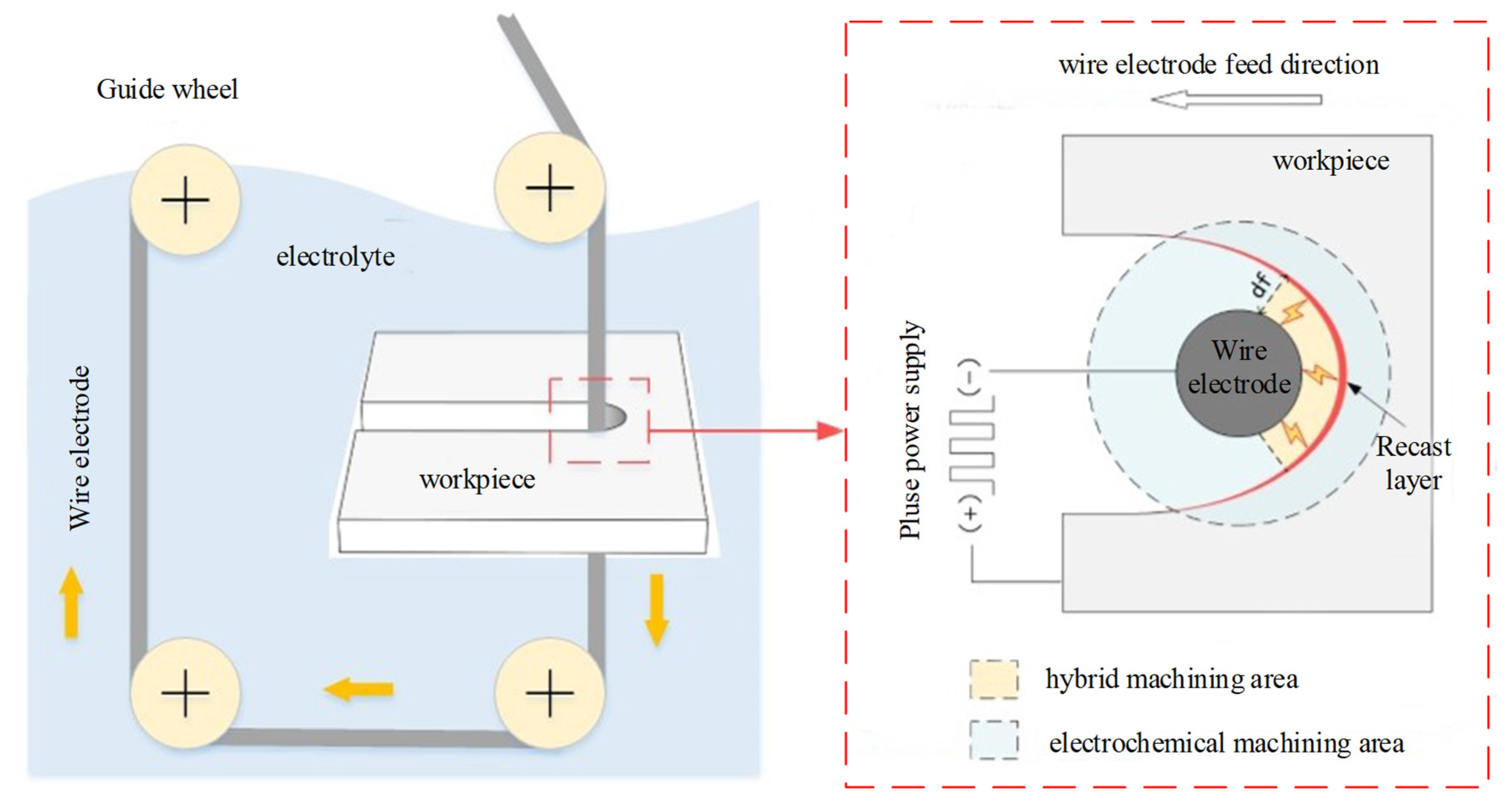
2. Machining Principle of WECDMM
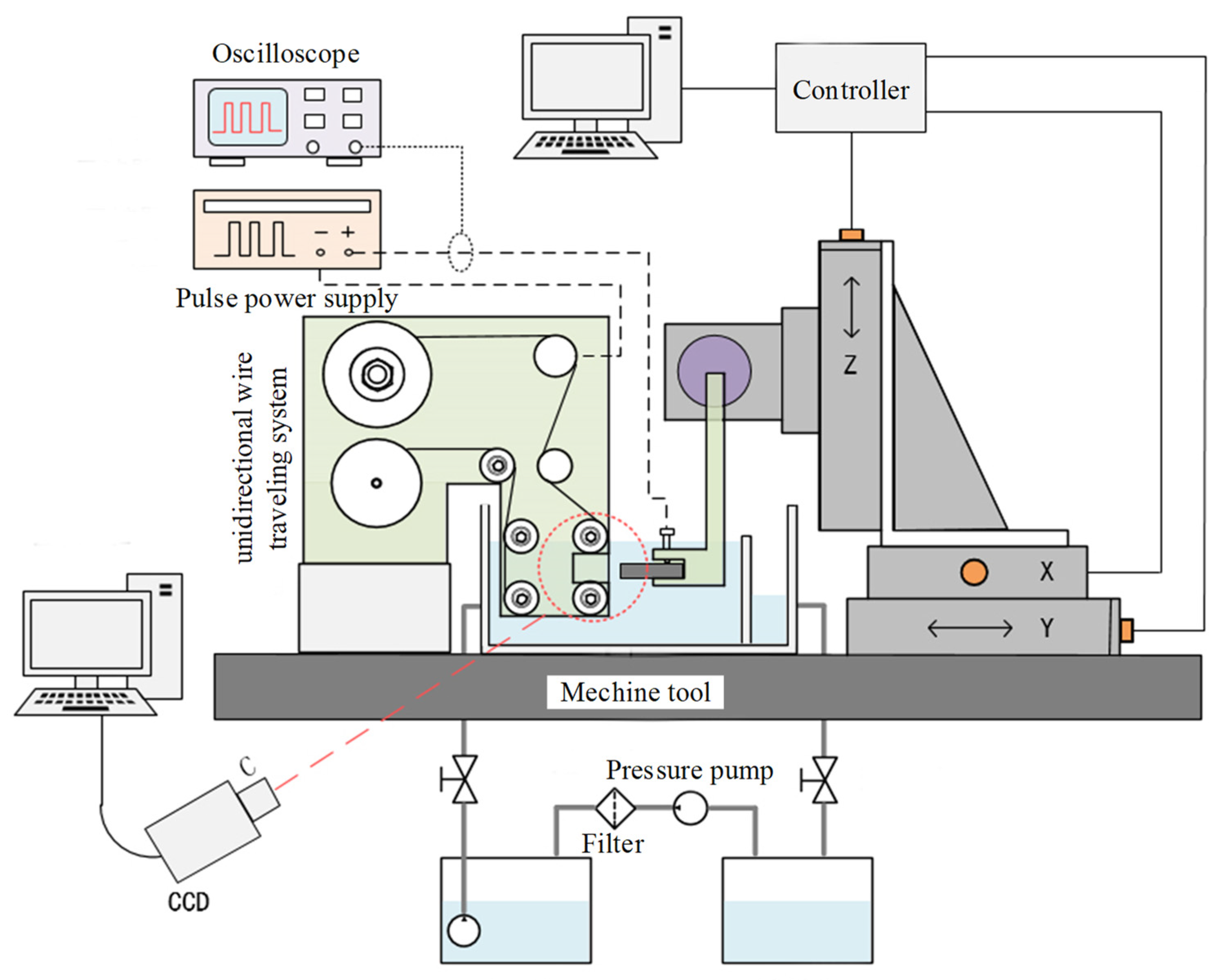
3. Experimental Apparatus
4. Results and Discussions
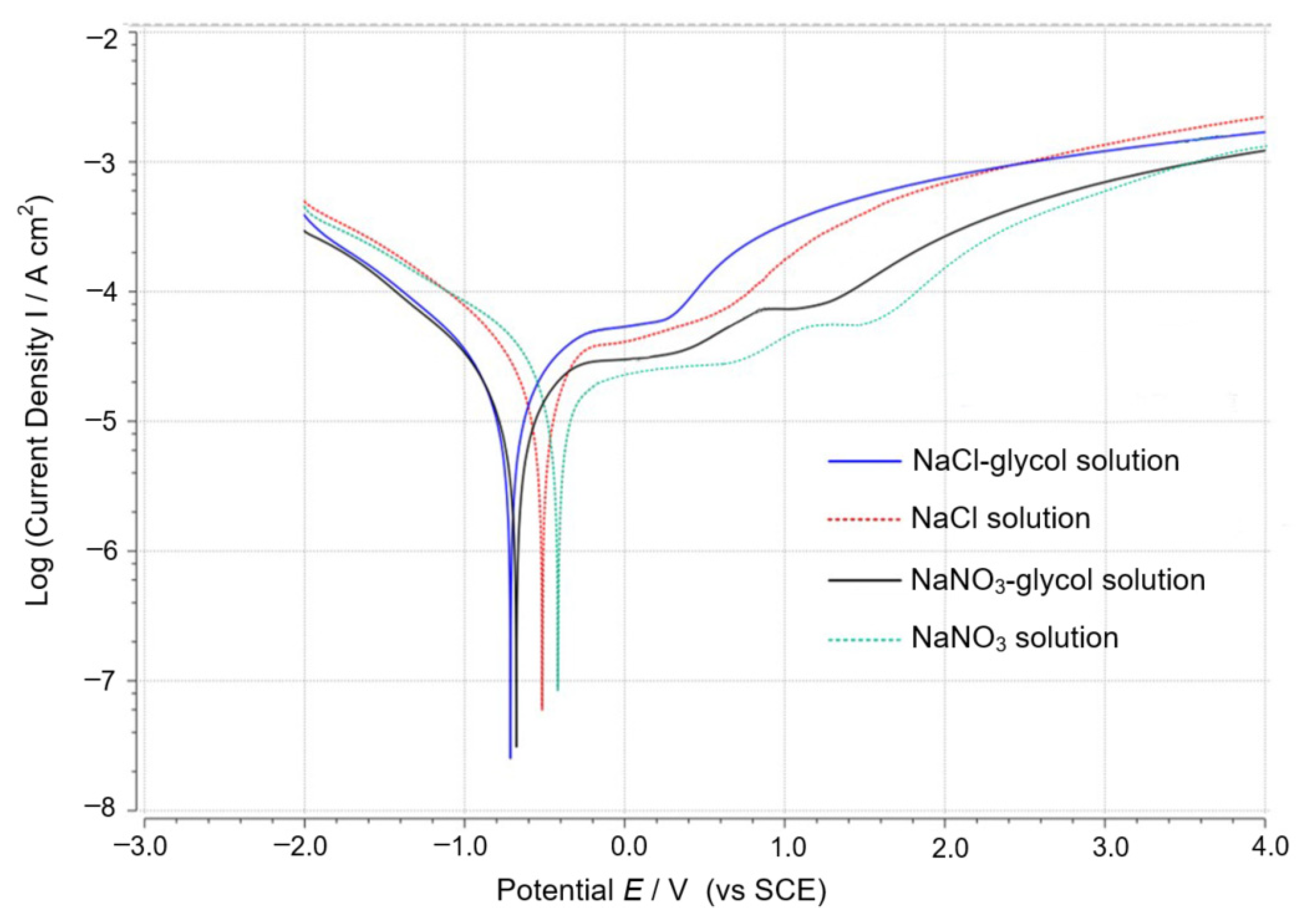
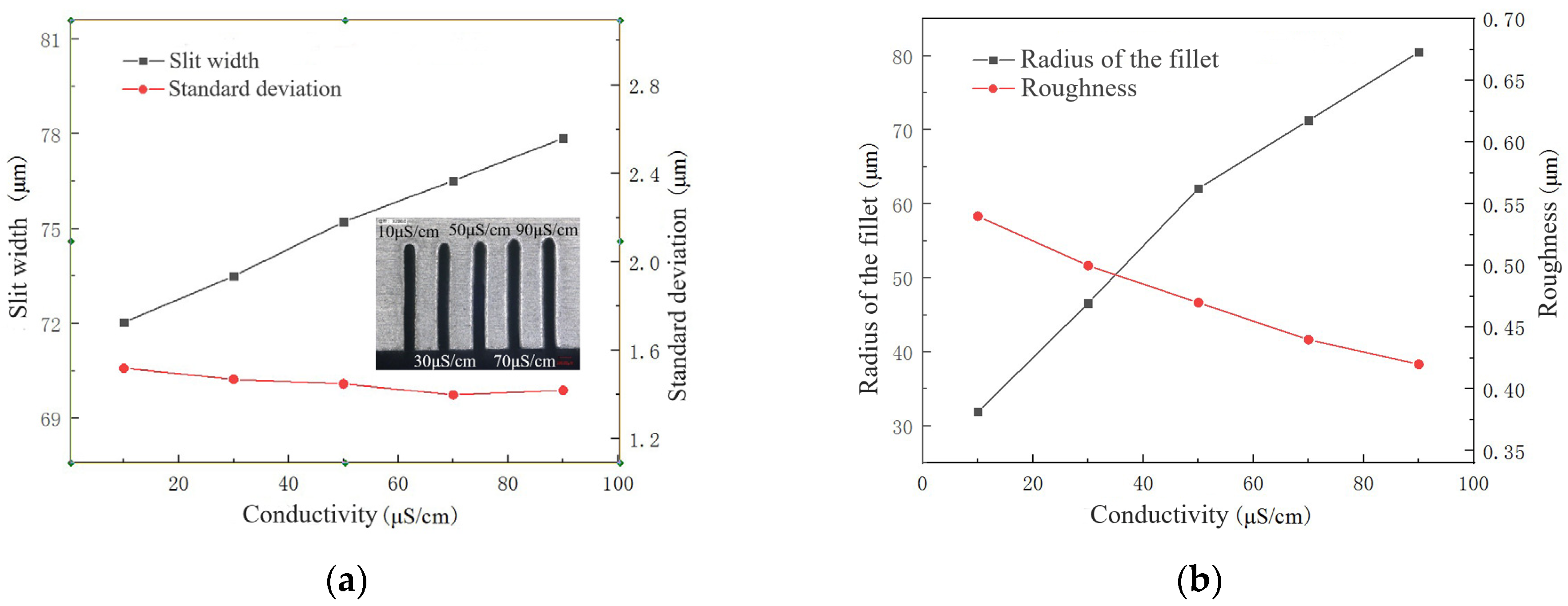
4.1. Selection of Electrolyte
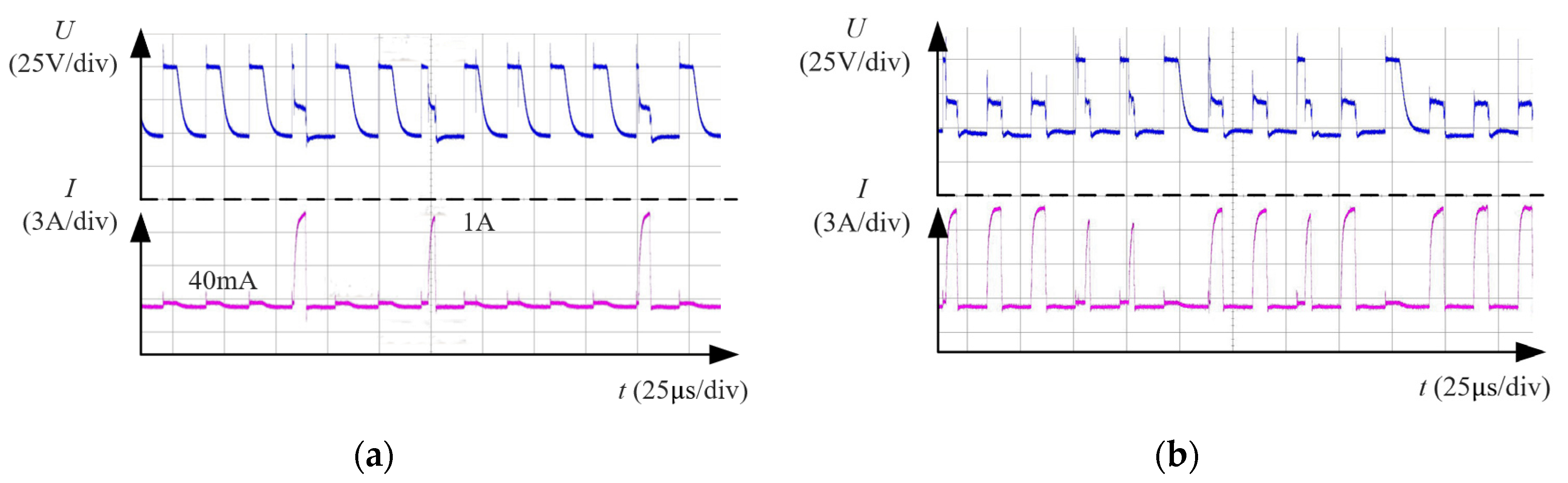
4.2. Phenomenon of WECDMM
4.3. Effect of Voltage, Pulse On-Time and Wire Travelling Speed
4.3.1. Effect of Pulse Voltage
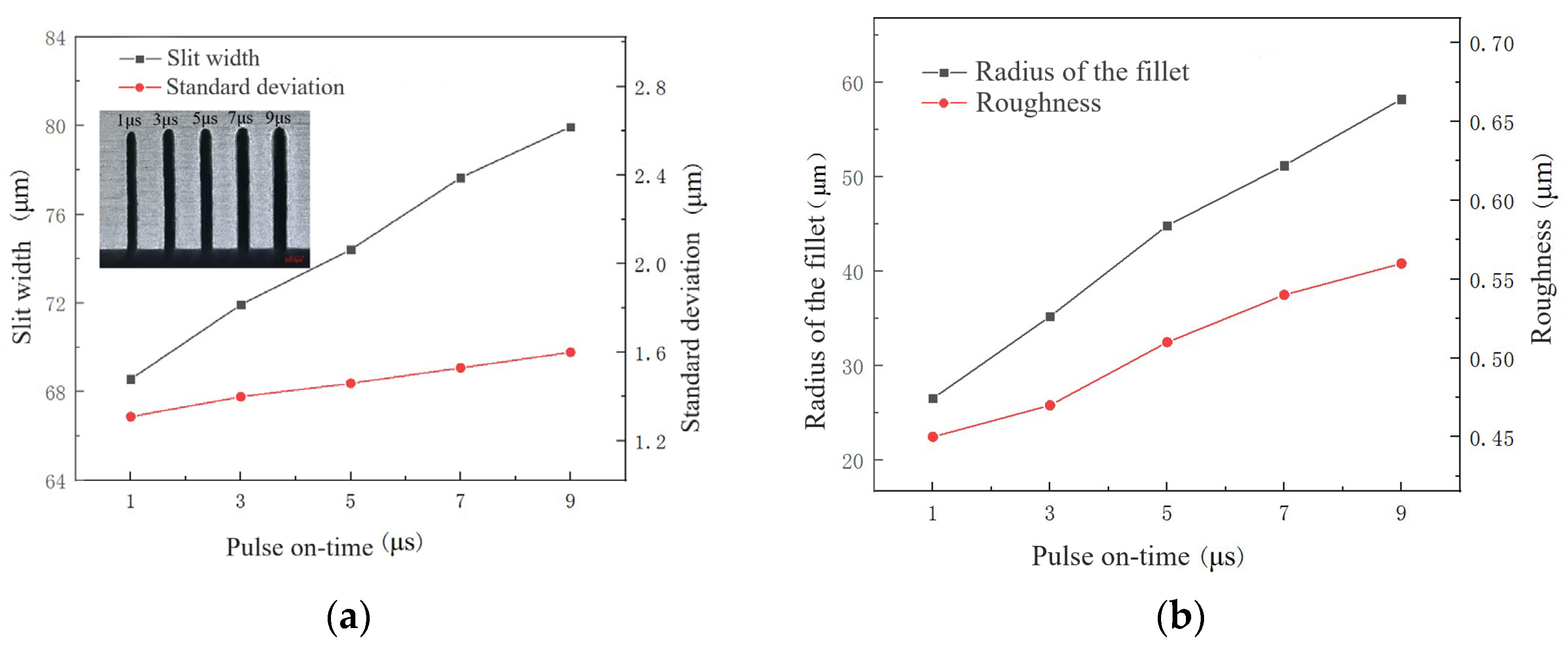
4.3.2. Effect of Pulse On-Time
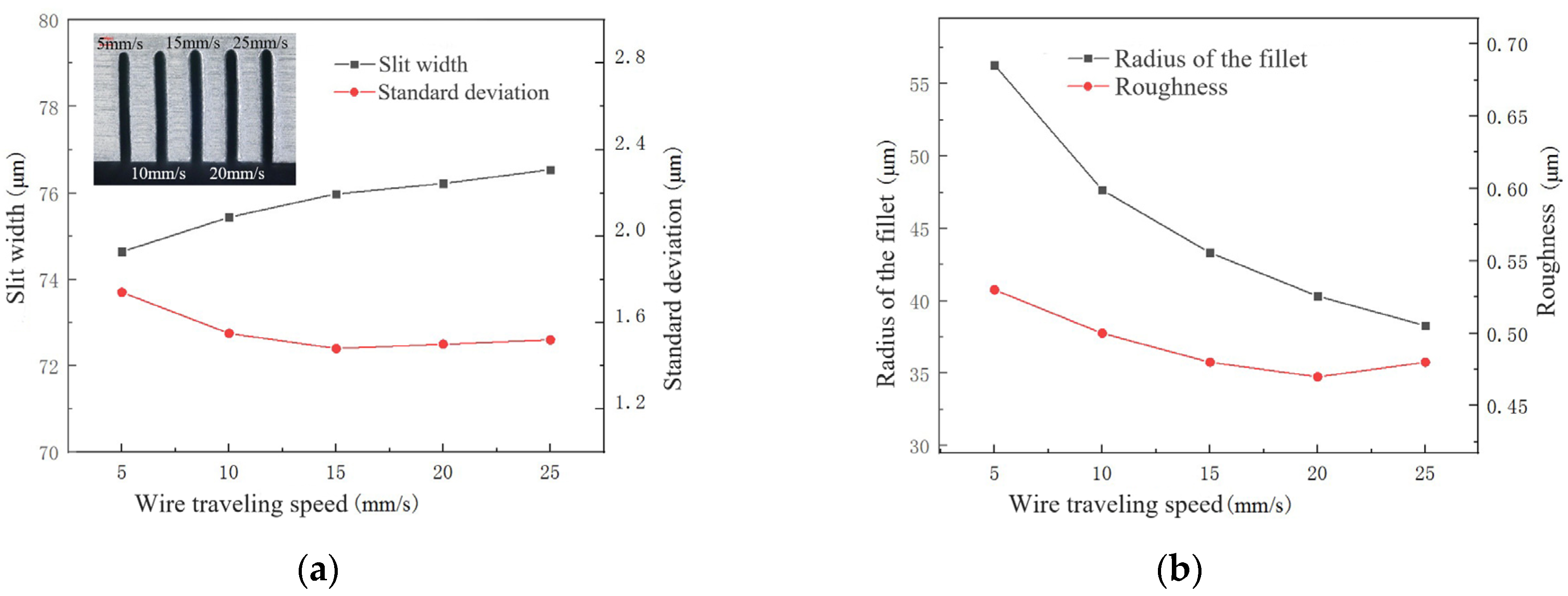
4.3.3. Effect of Wire Travelling Speed
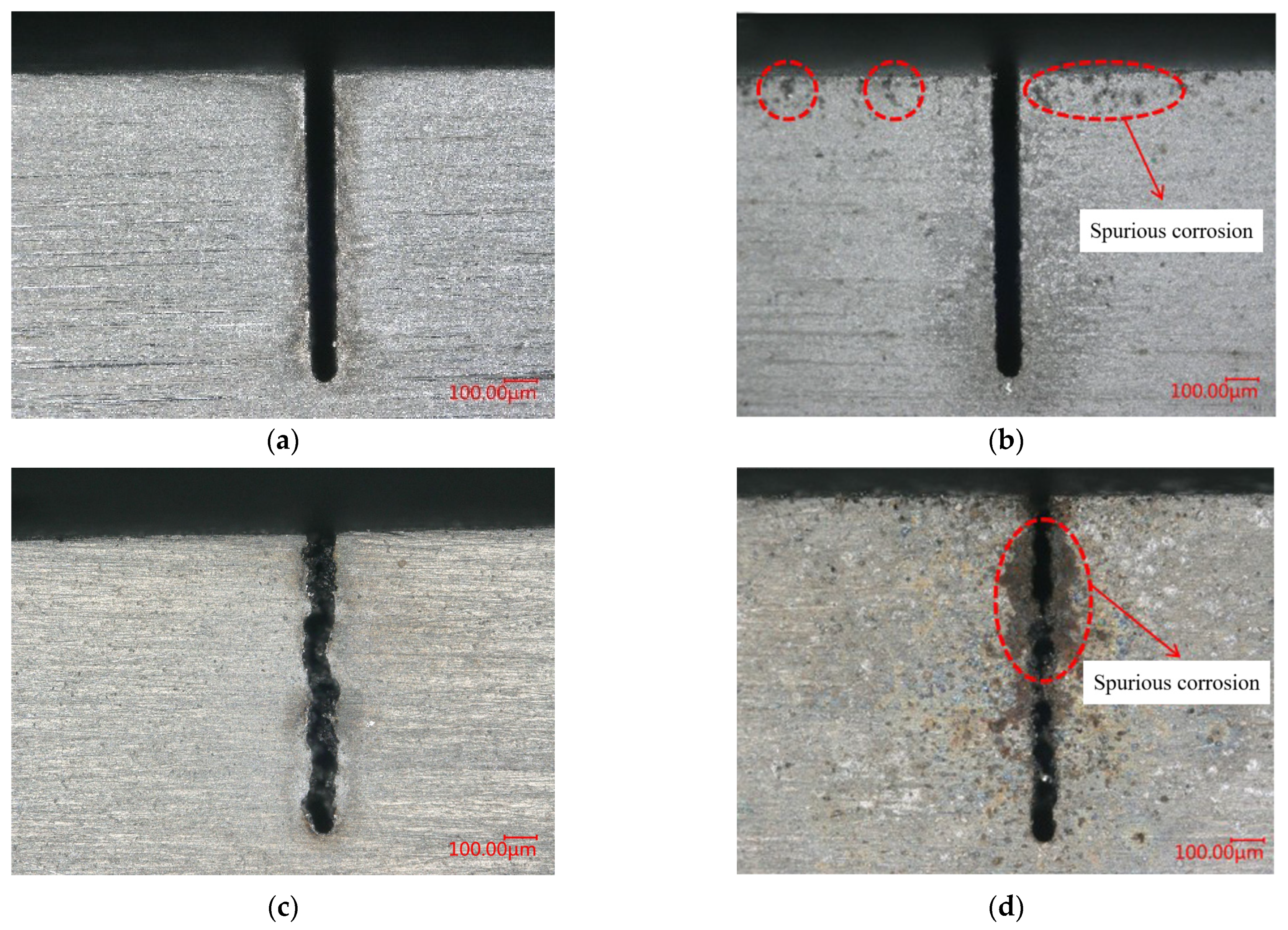
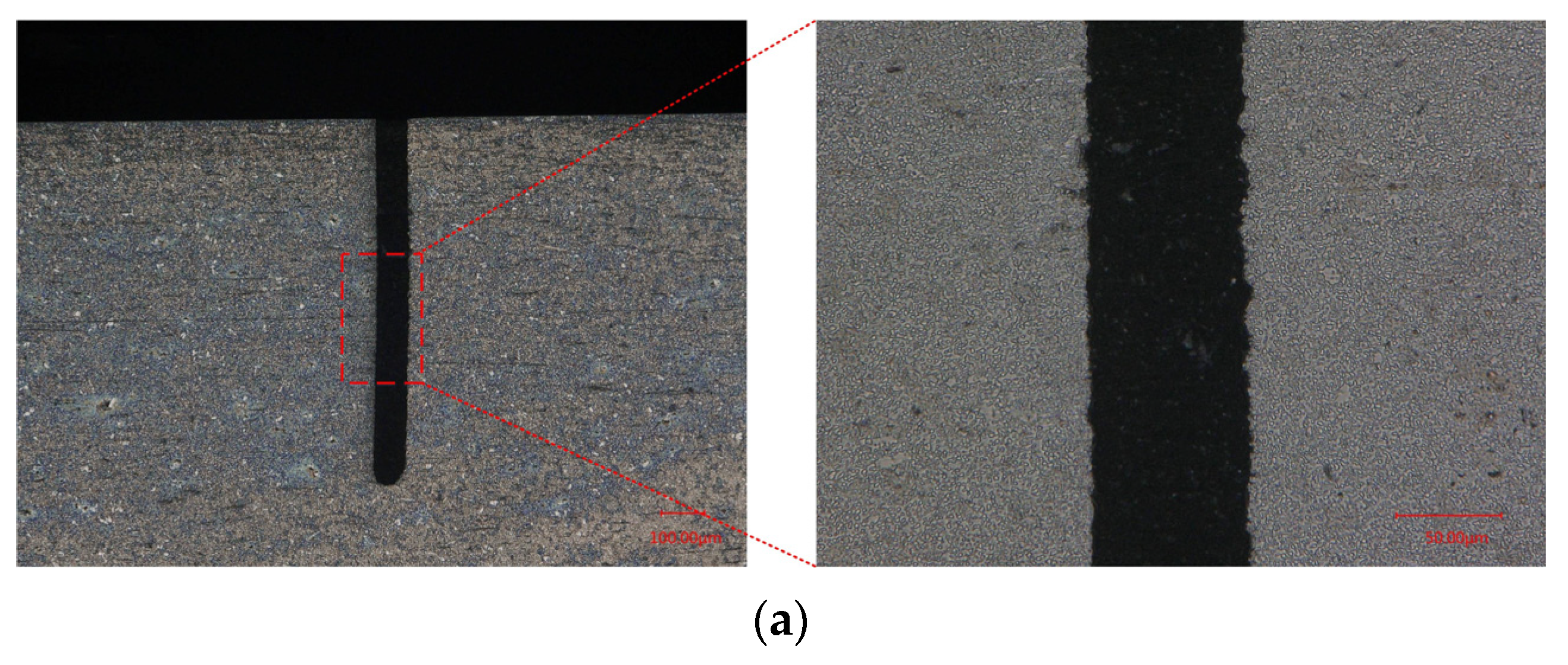
4.4. Recast Layer in WECDMM
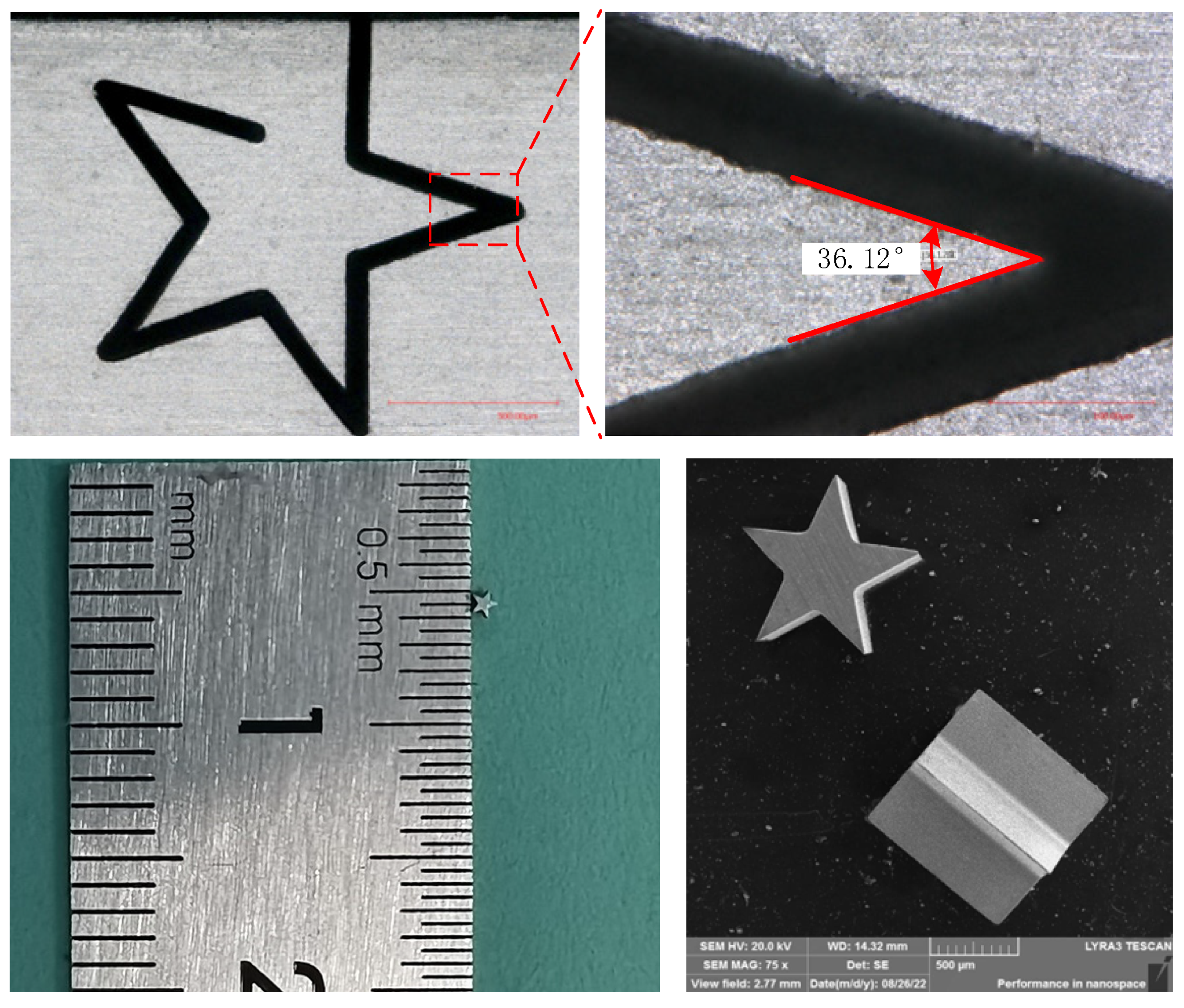
4.5. Manufacturing of Typical Structure
5. Conclusions
- The electrolyte must not only achieve an electrochemical reaction but also meet the requirements of discharge. The electrolyte was optimized through a comparison experiment, and NaNO3–glycol solution was determined as the best working solution.
- Through the observation of machining phenomena and waveform analysis, the discharge characteristics in low conductivity dielectric were investigated. The type of discharge is related to the arrangement of bubbles in the machining gap before discharge. Discharge breakdown of the low conductivity NaNO3–glycol solution in an environment of few bubbles. The medium of discharge breakdown is a bubble in an environment of numerous bubbles.
- The influences of key process parameters including conductivity of the electrolyte, pulse voltage, pulse-on time and wire feed rate were analyzed on the slit width, standard deviation, radius of fillet at the entrance of the slit and roughness.
- According to the influence on the machining results, the parametric combination is as follows: the NaNO3–glycol solution with a conductivity of 30 μS/cm, a voltage of 45 V, pulse on-time of 5 μs, pulse off-time of 12 μs, wire traveling speed of 10 mm/s and feed rate of 2 μm/s. Typical microstructures were machined, which verified the machining ability of WECDMM.
Author Contributions
Funding
Conflicts of Interest
References
- Kienle, F.; Achenbach, D. More Powerful Femtosecond Lasers. Laser Tech. J. 2014, 11, 45–47. [Google Scholar] [CrossRef]
- Miller, D.S. Micromachining with abrasive waterjets. J. Mater. Process. Technol. 2004, 149, 37–42. [Google Scholar] [CrossRef]
- Koch, O.; Ehrfeld, W.; Michel, F. Recent progress in micro-electro discharge machining technology. In Proceedings of the 13th International Symposium for Electro machining ISEM XIII, Bilbao, Spain, 9–11 May 2001; Volume 4, pp. 71–79. [Google Scholar]
- Sharma, V.; Patel, D.S.; Jain, V.K.; Ramkumar, J. Wire electrochemical micromachining: An overview. Int. J. Mach. Tools Manuf. 2020, 155, 103579. [Google Scholar] [CrossRef]
- Oza, A.D.; Kumar, A.; Badheka, V.; Arora, A. Traveling Wire Electrochemical Discharge Machining (TW-ECDM) of Quartz Using Zinc Coated Brass Wire: Investigations on Material Removal Rate and Kerf Width Characteristics. Silicon 2019, 11, 2873–2884. [Google Scholar] [CrossRef]
- Rattan, N.; Mulik, R.S. Improvement in material removal rate (MRR) using magnetic field in TW-ECSM process. Mater. Manuf. Process. 2016, 32, 101–107. [Google Scholar] [CrossRef]
- Rattan, N.; Mulik, R.S. Experimental Set Up to Improve Machining Performance of Silicon Dioxide (Quartz) in Magnetic Field Assisted TW-ECSM Process. Silicon 2018, 10, 2783–2791. [Google Scholar] [CrossRef]
- Rattan, N.; Mulik, R.S. Experimental Investigations and Multi-response Optimization of Silicon Dioxide (Quartz) Machining in Magnetic Field Assisted TW-ECSM Process. Silicon 2017, 9, 663–673. [Google Scholar] [CrossRef]
- Kuo, K.Y.; Wu, K.L.; Yang, C.K.; Yan, B.H. Wire electrochemical discharge machining (WECDM) of quartz glass with titrated electrolyte flow. Int. J. Mach. Tools Manuf. 2013, 72, 50–57. [Google Scholar] [CrossRef]
- Kuo, K.Y.; Wu, K.L.; Yang, C.K.; Yan, B.H. Effect of adding SiC powder on surface quality of quartz glass microslit machined by WECDM. Int. J. Adv. Manuf. Technol. 2014, 78, 73–83. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Wang, M.; Zhang, J. Experimental investigation of micro wire electrochemical discharge machining by using a rotating helical tool. J. Manuf. Process. 2017, 29, 265–271. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, S.; Guo, C.; Wei, Z. Experimental Study of Micro Electrochemical Discharge Machining of Ultra-Clear Glass with a Rotating Helical Tool. Processes 2019, 7, 195. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Xin, G. Experimental Study on Ultrasonic Vibration-Assisted WECDM of Glass Microstructures with a High Aspect Ratio. Micromachines 2021, 12, 125. [Google Scholar] [CrossRef]
- Kumar, M.; Vaishya, R.O.; Oza, A.D.; Suri, N.M. Experimental Investigation of Wire-Electrochemical Discharge Machining (WECDM) Performance Characteristics for Quartz Material. Silicon 2019, 12, 2211–2220. [Google Scholar] [CrossRef]
- Yang, C.H.; Tsui, H.P. Study on ultrasonic-assisted WECDM of quartz wafer with continuous electrolyte flow. Int. J. Adv. Manuf. Technol. 2021, 118, 1061–1076. [Google Scholar] [CrossRef]
- Liu, J.W.; Yue, T.M.; Guo, Z.N. Wire Electrochemical Discharge Machining of Al2O3 Particle Reinforced Aluminum Alloy 6061. Mater. Manuf. Process. 2009, 24, 446–453. [Google Scholar] [CrossRef]
- El-Hofy, H.; Mcgeough, J.A. Evaluation of an Apparatus for Electrochemical Arc Wire-Machining. J. Eng. Ind. 1988, 110, 119–123. [Google Scholar] [CrossRef]
- Shamim, F.A.; Dvivedi, A.; Kumar, P. On near-dry wire ECDM of Al6063/SiC/10p MMC. Mater. Manuf. Process. 2020, 36, 122–134. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, S.J.; Zhao, W.; Tang, L.; Li, Z.P. Experiment investigation of using wire electrochemical machining in deionized water to reduce the wire electrical discharge machining surface roughness. Int. J. Adv. Manuf. Technol. 2019, 102, 343–353. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.D.; Pan, H.W.; Deng, C.; Qiu, M.B. Effect of no-load rate on recast layer cutting by ultra fine wire-EDM. Chin. J. Aeronaut. 2021, 34, 124–131. [Google Scholar] [CrossRef]
- Kong, W.J.; Zeng, Y.B.; Liu, Z.Y.; Hu, X.Y.; Kong, H.H. Helical wire electrochemical discharge machining on large-thickness Inconel 718 alloy in low-conductivity salt-glycol solution. Chin. J. Aeronaut. 2023, 36, 522–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.Y.; Zhu, D.; Xing, J. Tube electrode high-speed electrochemical discharge drilling using low-conductivity salt solution. Int. J. Mach. Tools Manuf. 2015, 92, 10–18. [Google Scholar] [CrossRef]





| Item | WECDMM |
|---|---|
| Work material | 9Cr18Mo |
| Workpiece thickness (mm) | 1 mm |
| electrode (mm) | Φ50 μm tungsten wire |
| Pulse voltage (V) | 35, 40, 45, 50 V, 55 V |
| Pulse on-time (μs) | 1, 3, 5, 7, 9 |
| Pulse off-time (μs) | 12 |
| Pulse frequency (kHz) | 50 |
| Wire traveling speed (mm/s) | 5, 10, 15, 20, 25 |
| Feed rate (μm/s) | 2 |
| Conductivity of electrolyte (μS/cm) | 10, 30, 50, 70, 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Liu, Z.; Zhang, R.; Zeng, Y. Investigation on Wire Electrochemical Discharge Micro-Machining. Micromachines 2023, 14, 1505. https://doi.org/10.3390/mi14081505
Kong W, Liu Z, Zhang R, Zeng Y. Investigation on Wire Electrochemical Discharge Micro-Machining. Micromachines. 2023; 14(8):1505. https://doi.org/10.3390/mi14081505
Chicago/Turabian StyleKong, Weijing, Ziyu Liu, Rudong Zhang, and Yongbin Zeng. 2023. "Investigation on Wire Electrochemical Discharge Micro-Machining" Micromachines 14, no. 8: 1505. https://doi.org/10.3390/mi14081505
APA StyleKong, W., Liu, Z., Zhang, R., & Zeng, Y. (2023). Investigation on Wire Electrochemical Discharge Micro-Machining. Micromachines, 14(8), 1505. https://doi.org/10.3390/mi14081505





