Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure
Abstract
:1. Introduction
2. Materials and Methods
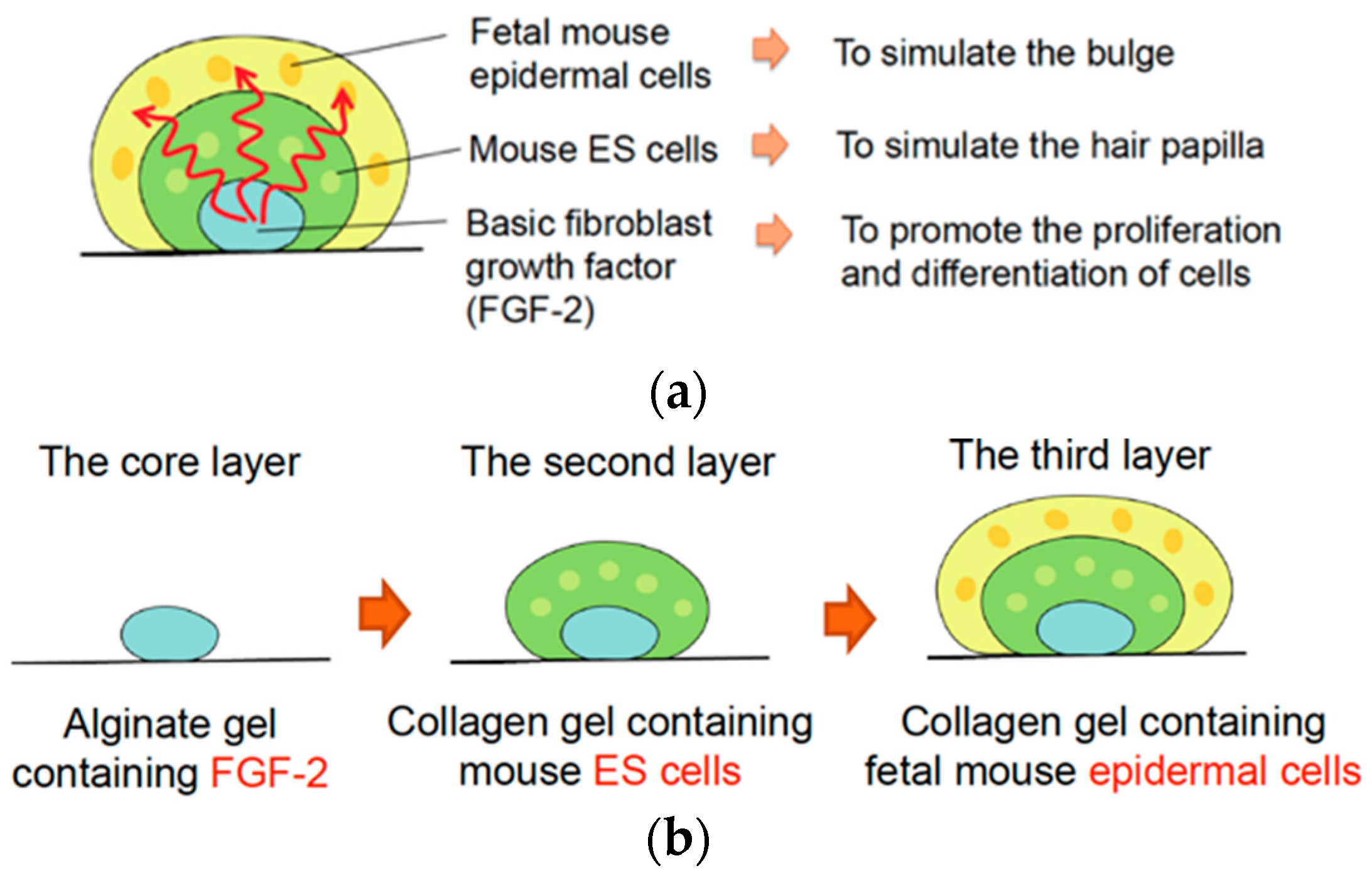
2.1. Experimental Design for In Vitro Reconstruction of Skin Appendages
2.2. Precise Microgel-Spotting Device for Fabricating Multilayered Gel Constructs Containing Cells
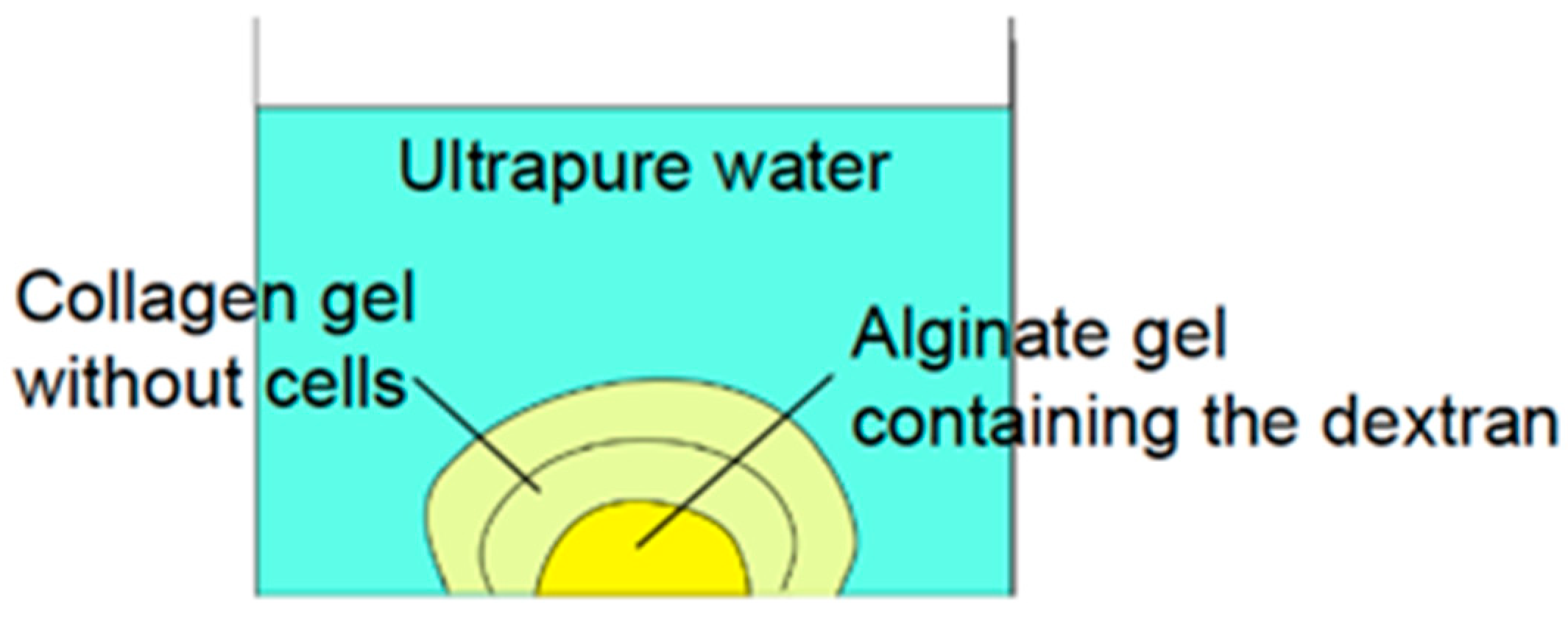
2.3. Experimental Analyses of Diffusion Process of Cytokines in Core Layer of Gel Construct
2.4. In Vitro Culture of Cell-Laden Three-Layered Gel Constructs
2.5. Histological Analysis
3. Results
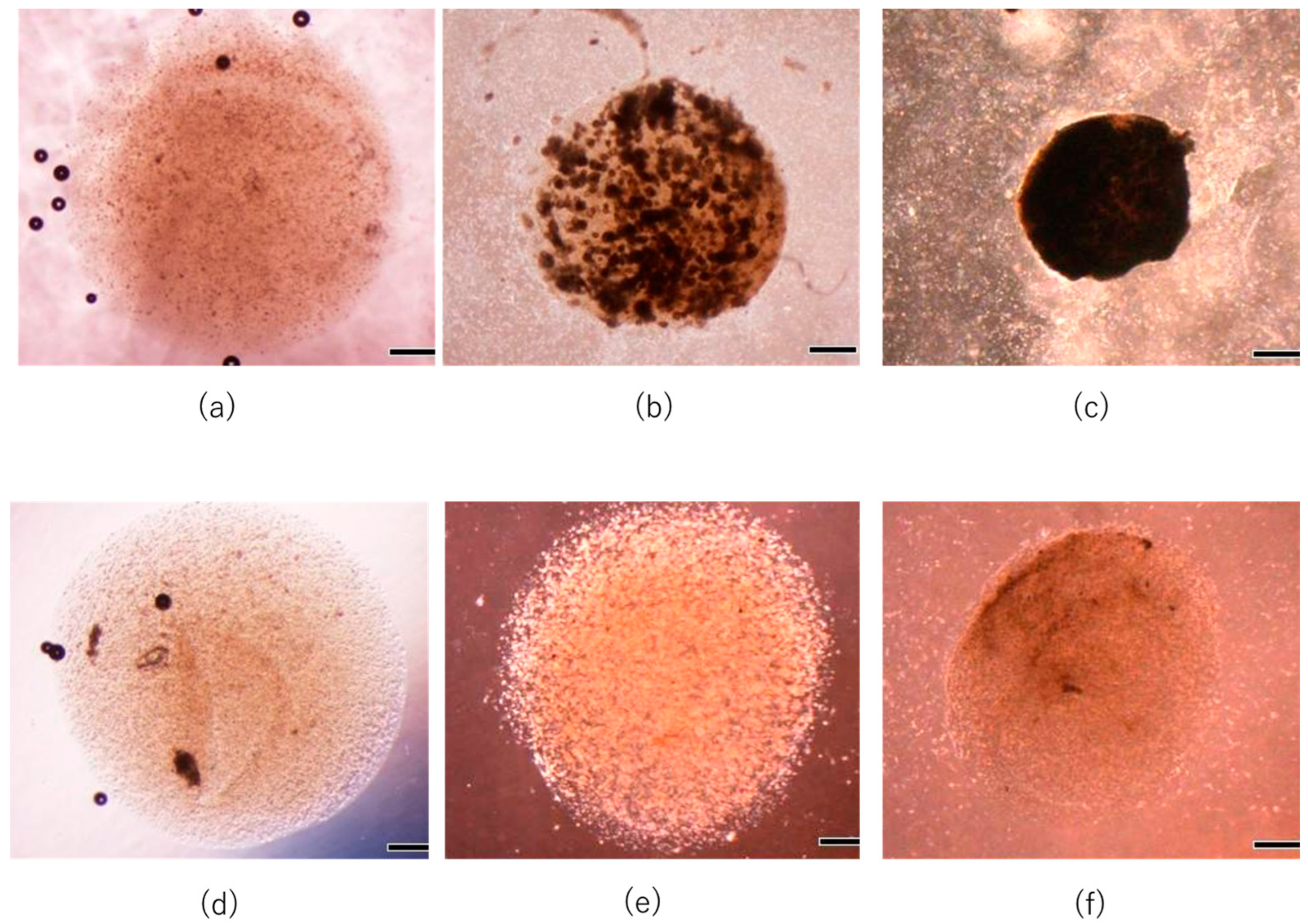
3.1. Diffusion Process of Cytokines in Core Layer of Gel Construct
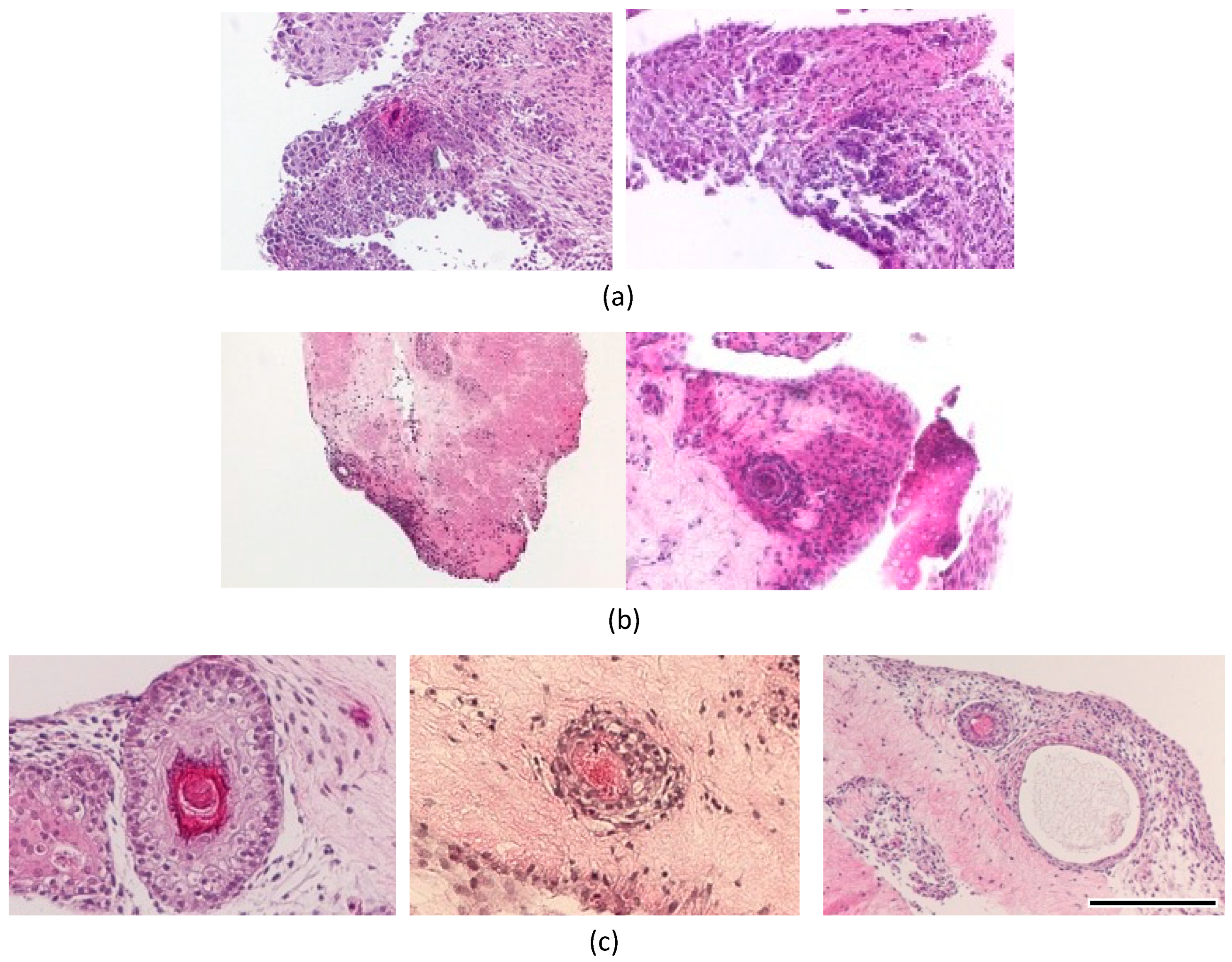
3.2. In Vitro Reconstruction of Hair Follicle-like Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jahoda, C.A.B.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Mahjour, S.B.; Ghaffarpasand, F.; Wang, H. Hair follicle regeneration in skin grafts: Current concepts and future perspectives. Tissue Eng. Part B Rev. 2012, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shah, J.D.; Wang, H. Nanofiber enabled layer-by-layer approach toward three-dimensional tissue formation. Tissue Eng. Part A 2009, 15, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Bíró, T.; Mescalchin, A.; Arenberger, P.; Paus, R. Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial-mesenchymal interactions. Br. J. Dermatol. 2004, 151, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Pirraco, R.P.; Reis, R.L.; Cerqueira, M.T.; Marques, A.P. Interfollicular epidermal stem-like cells for the recreation of the hair follicle epithelial compartment. Stem Cell Res. Ther. 2021, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhu, T.Y.; Lu, Y.G.; Liu, R.Q.; Mai, Y.; Cheng, B.; Lu, Z.F.; Zhong, B.Y.; Tang, S.Q. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch. Dermatol. Res. 2006, 298, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Limat, A.; Breitkreutz, D.; Hunziker, T.; Klein, C.E.; Noser, F.; Fusenig, N.E.; Braathen, L.R. Outer root sheath (ORS) cells organize into epidermoid cyst-like spheroids when cultured inside matrigel: A light-microscopic and immunohistological comparison between human ors cells and interfollicular keratinocytes. Cell Tissue Res. 1994, 275, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Turetsky, A.; Kemp, P.; Teumer, J. Hair morphogenesis in vitro: Formation of hair structures suitable for implantation. Regen. Med. 2008, 3, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Anakama, R.; Togashi, H.; Fukuda, J. Impacts of manipulating cell sorting on in vitro hair follicle regeneration. J. Biosci. Bioeng. 2022, 134, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Osada, A.; Kobayashi, K.; Masui, S.; Hamazaki, T.S.; Yasuda, K.; Okochi, H. Cloned cells from the murine dermal papilla have hair-inducing ability. J. Dermatol. Sci. 2009, 54, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Miyata, S. Three-dimensional cell drawing technique in hydrogel using micro injection system. Micromachines 2022, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Tomioka, M.; Watabe, A.; Aihara, M.; Shimba, S.; Inoue, H. The clock gene brain and muscle arnt-like protein-1 (BMAL1) is involved in hair growth. Arch. Dermatol. Res. 2013, 305, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Zemmyo, D.; Miyata, S. Evaluation of lipid accumulation using electrical impedance measurement under three-dimensional culture condition. Micromachines 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines 2021, 12, 1486. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Sanzen, N.; Sasaki, H.; Hayashi, T.; Umeda, M.; Yoshimura, M.; Yamamoto, T.; Shibata, T.; Abe, T.; Kiyonari, H.; et al. Tracing the origin of hair follicle stem cells. Nature 2021, 594, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Inamatsu, M.; Matsuzaki, T.; Iwanari, H.; Yoshizato, K. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J. Investig. Dermatol. 1998, 111, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Lappi, D.A.; Baird, A.; Majack, R.A.; Reidy, M.A. Role of basic fibroblast growth factor in vascular lesion formation. Circ. Res. 1991, 68, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Müller-Röver, S.; Van Der Veen, C.; Maurer, M.; Eichmüller, S.; Ling, G.; Hofmann, U.; Foitzik, K.; Mecklenburg, L.; Handjiski, B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Investig. Dermatol. 1999, 113, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.F. A Study of the post-natal development of the skin and hair of the mouse. Anat. Rec. 1941, 80, 61–81. [Google Scholar] [CrossRef]




| Experimental Group | FGF-2 | Gel Spot Structure (2nd and 3rd Layer) | Evaluation | |
|---|---|---|---|---|
| FGF(+)-mixed | (+) | Mixed | Cellular organization | |
| FGF(+)-layered | Layered | Cytokine concentration gradient | ||
| FGF(−)-layered | (−) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugeno, A.; Sumi, T.; Sato-Yazawa, H.; Yazawa, T.; Inoue, H.; Miyata, S. Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure. Micromachines 2023, 14, 1651. https://doi.org/10.3390/mi14091651
Sugeno A, Sumi T, Sato-Yazawa H, Yazawa T, Inoue H, Miyata S. Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure. Micromachines. 2023; 14(9):1651. https://doi.org/10.3390/mi14091651
Chicago/Turabian StyleSugeno, Aki, Takahiro Sumi, Hanako Sato-Yazawa, Takuya Yazawa, Hajime Inoue, and Shogo Miyata. 2023. "Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure" Micromachines 14, no. 9: 1651. https://doi.org/10.3390/mi14091651
APA StyleSugeno, A., Sumi, T., Sato-Yazawa, H., Yazawa, T., Inoue, H., & Miyata, S. (2023). Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure. Micromachines, 14(9), 1651. https://doi.org/10.3390/mi14091651





