Bessel Beams in Ophthalmology: A Review
Abstract
:1. Introduction
2. Bessel Beams
3. Phakometry with Bessel Beams
4. Bessel Beam-Based Light Sheet Fluorescence Microscopy (LSFM) for Ocular Imaging
5. Retinal Imaging Using Bessel Beams
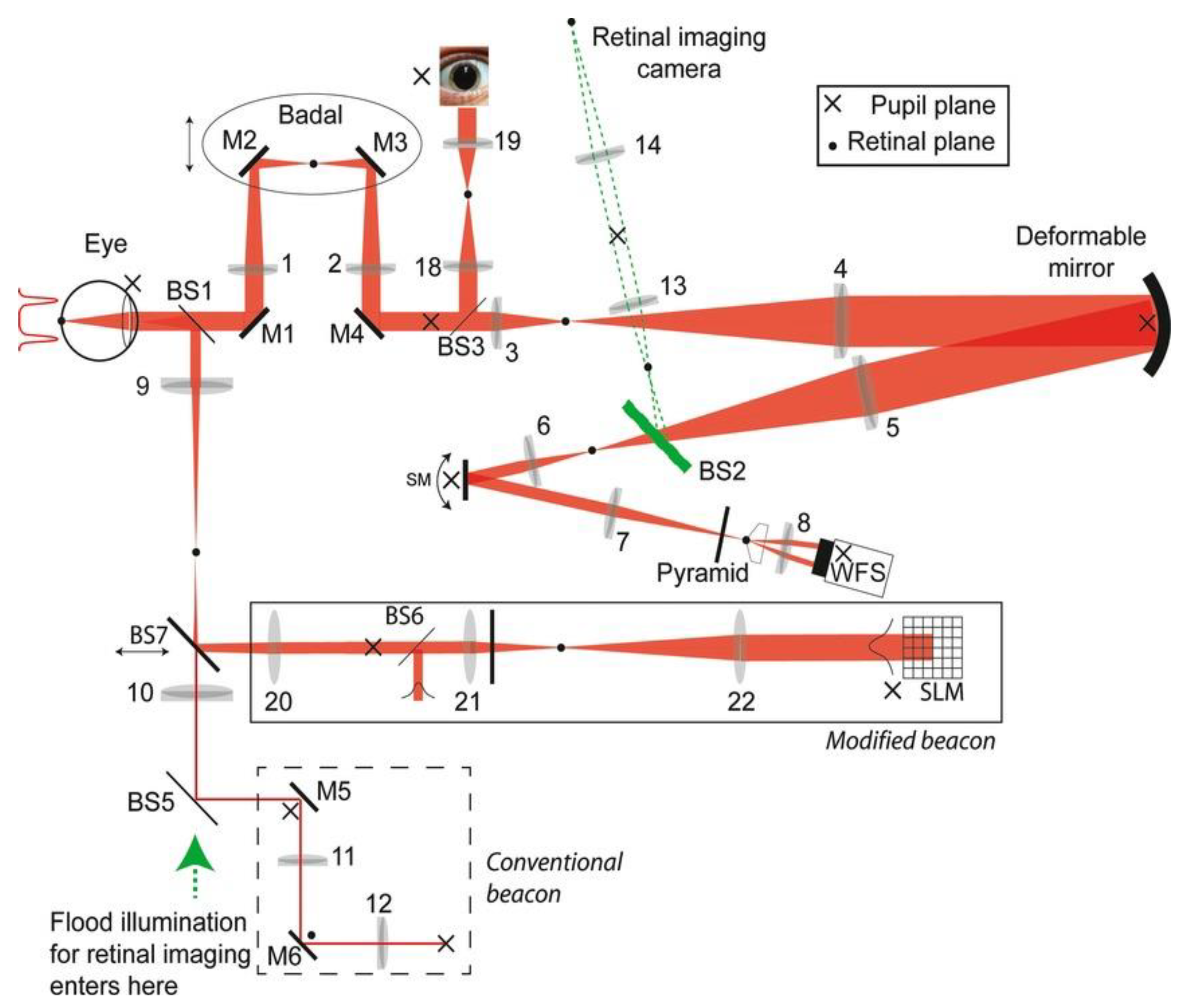
6. Eye Fixation Using Bessel Beams
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bille, J.F. High Resolution Imaging in Microscopy and Ophthalmology, 1st ed.; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Perinchery, S.M.; Shinde, A.; Fu, C.Y.; Jeesmond Hong, X.J.; Baskaran, M.; Aung, T.; Murukeshan, V.M. High resolution iridocorneal angle imaging system by axicon lens assisted gonioscopy. Sci. Rep. 2016, 6, 30844. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Hossain, P.; Malik, R.A. Clinical applications of corneal confocal microscopy. Clin. Ophthalmol. 2008, 2, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Suchand Sandeep, C.S.; Chan Lwin, N.; Liu, Y.-C.; Barathi, V.A.; Aung, T.; Baskaran, M.; Murukeshan, V.M. High-resolution, non-contact, cellular level imaging of the cornea of the eye in vivo. Opt. Laser Technol. 2022, 150, 107922. [Google Scholar] [CrossRef]
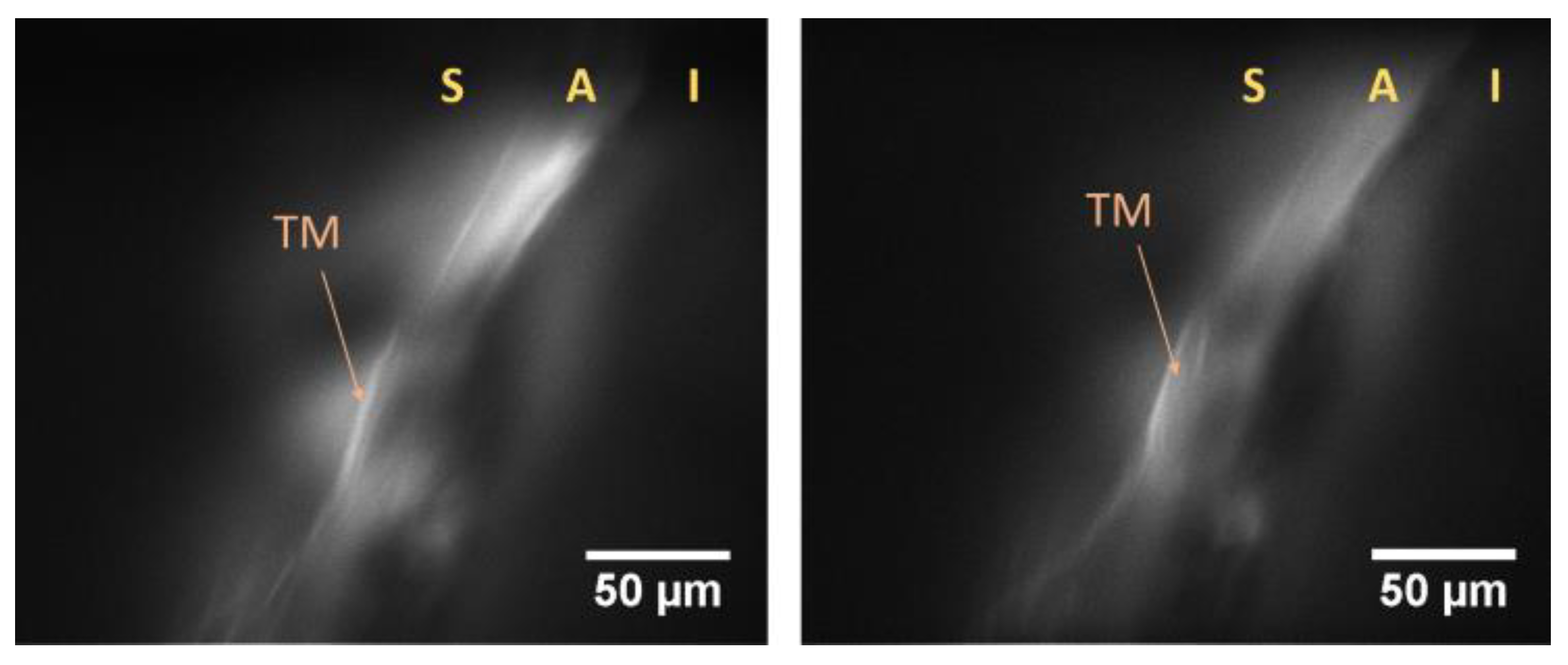
- Hong, X.J.J.; Shinoj, V.K.; Murukeshan, V.M.; Baskaran, M.; Aung, T. Imaging of trabecular meshwork using Bessel–Gauss light sheet with fluorescence. Laser Phys. Lett. 2017, 14, 035602. [Google Scholar] [CrossRef]
- Shinoj, V.K.; Hong, X.J.; Murukeshan, V.M.; Baskaran, M.; Tin, A. Progress in anterior chamber angle imaging for glaucoma risk prediction—A review on clinical equipment, practice and research. Med. Eng. Phys. 2016, 38, 1383–1391. [Google Scholar] [CrossRef]
- Horie, S.; Ohno-Matsui, K. Progress of Imaging in Diabetic Retinopathy—From the Past to the Present. Diagnostics 2022, 12, 1684. [Google Scholar] [CrossRef]
- Nanegrungsunk, O.; Patikulsila, D.; Sadda, S.R. Ophthalmic imaging in diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2022, 50, 1082–1096. [Google Scholar] [CrossRef]
- Nadler, Z.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Clinical application of ocular imaging. Optom. Vis. Sci. 2012, 89, E543–E553. [Google Scholar] [CrossRef]
- Lai, T.Y.Y. Ocular imaging at the cutting-edge. Eye 2021, 35, 1–3. [Google Scholar] [CrossRef]
- Mohammadpour, M. Diagnostics in Ocular Imaging, 1st ed.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Shu, X.; Wang, J.; Hu, L. A review of functional slit lamp biomicroscopy. Eye Vis. 2019, 6, 15. [Google Scholar] [CrossRef]
- Lee, J.-S. (Ed.) Slit Lamp Exam. In Primary Eye Examination; Springer: Singapore, 2019; pp. 101–111. [Google Scholar]
- Ye, Y.; Jiang, H.; Zhang, H.; Karp, C.L.; Zhong, J.; Tao, A.; Shao, Y.; Wang, J. Resolution of slit-lamp microscopy photography using various cameras. Eye Contact Lens 2013, 39, 205–213. [Google Scholar] [CrossRef]
- Beckman, C.; Bond-Taylor, L.; Lindblom, B.; Sjostrand, J. Confocal fundus imaging with a scanning laser ophthalmoscope in eyes with cataract. Br. J. Ophthalmol. 1995, 79, 900–904. [Google Scholar] [CrossRef]
- Sakono, T.; Terasaki, H.; Sonoda, S.; Funatsu, R.; Shiihara, H.; Uchino, E.; Yamashita, T.; Sakamoto, T. Comparison of multicolor scanning laser ophthalmoscopy and optical coherence tomography angiography for detection of microaneurysms in diabetic retinopathy. Sci. Rep. 2021, 11, 17017. [Google Scholar] [CrossRef]
- Terasaki, H.; Sonoda, S.; Tomita, M.; Sakamoto, T. Recent Advances and Clinical Application of Color Scanning Laser Ophthalmoscope. J. Clin. Med. 2021, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quant. Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Staurenghi, G.; Gale, R.; Vision Academy Steering, C. The role of OCT-A in retinal disease management. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2019–2026. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98 (Suppl. 2), ii15–ii19. [Google Scholar] [CrossRef] [PubMed]
- Arabi, P.M.; Krishna, N.; Ashwini, V.; Prathibha, H.M. Identification of Age-Related Macular Degeneration Using OCT Images. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012096. [Google Scholar] [CrossRef]
- de Boer, J.F.; Leitgeb, R.; Wojtkowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomed. Opt. Express 2017, 8, 3248–3280. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Shearwood, C.; Keey, S.L.; Matham, M.V. Quantitative optical coherence microscopy for the in situ investigation of the biofilm. J. Biomed. Opt. 2016, 21, 127002. [Google Scholar] [CrossRef] [PubMed]
- Aumann, S.; Donner, S.; Fischer, J.; Muller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Ratheesh, K.M.; Prabhathan, P.; Seah, L.K.; Murukeshan, V.M. Gold nanorods with higher aspect ratio as potential contrast agent in optical coherence tomography and for photothermal applications around 1300 nm imaging window. Biomed. Phys. Eng. Express 2016, 2, 055005. [Google Scholar] [CrossRef]
- Xu, C.; Ye, J.; Marks, D.L.; Boppart, S.A. Near-infrared dyes as contrast-enhancing agents for spectroscopic optical coherence tomography. Opt. Lett. 2004, 29, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, E.; Boccara, A.C.; Lebec, M.; Blanchot, L.; Saint-Jalmes, H. Full-field optical coherence microscopy. Opt. Lett. 1998, 23, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Boccara, A.C. Full-Field Optical Coherence Tomography. In Optical Coherence Tomography; Biological and Medical Physics, Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 565–591. [Google Scholar]
- Everett, M.; Magazzeni, S.; Schmoll, T.; Kempe, M. Optical coherence tomography: From technology to applications in ophthalmology. Transl. Biophotonics 2020, 3, e202000012. [Google Scholar] [CrossRef]
- Jalbert, I.; Stapleton, F.; Papas, E.; Sweeney, D.F.; Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 2003, 87, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Stachs, O.; Guthoff, R.F.; Aumann, S. In Vivo Confocal Scanning Laser Microscopy. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 263–284. [Google Scholar]
- Minsky, M. Microscopy Apparatus. U.S. Patent US3013467A, 19 December 1961. [Google Scholar]
- Minsky, M. Memoir on inventing the confocal scanning microscope. Scanning 1988, 10, 128–138. [Google Scholar] [CrossRef]
- Schweitzer, D.; Hammer, M.; Schweitzer, F.; Anders, R.; Doebbecke, T.; Schenke, S.; Gaillard, E.R.; Gaillard, E.R. In vivo measurement of time-resolved autofluorescence at the human fundus. J. Biomed. Opt. 2004, 9, 1214–1222. [Google Scholar] [CrossRef]
- Sauer, L.; Vitale, A.S.; Modersitzki, N.K.; Bernstein, P.S. Fluorescence lifetime imaging ophthalmoscopy: Autofluorescence imaging and beyond. Eye 2021, 35, 93–109. [Google Scholar] [CrossRef]
- Schweitzer, D.; Deutsch, L.; Klemm, M.; Jentsch, S.; Hammer, M.; Peters, S.; Haueisen, J.; Muller, U.A.; Dawczynski, J. Fluorescence lifetime imaging ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic retinopathy. J. Biomed. Opt. 2015, 20, 61106. [Google Scholar] [CrossRef]
- Schmidt, J.; Peters, S.; Sauer, L.; Schweitzer, D.; Klemm, M.; Augsten, R.; Muller, N.; Hammer, M. Fundus autofluorescence lifetimes are increased in non-proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Vitale, A.; Modersitzki, N.K.; Sauer, L. Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) in Patients with Diabetes and Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1852. [Google Scholar]
- Liu, W.; Zhang, H.F. Photoacoustic imaging of the eye: A mini review. Photoacoustics 2016, 4, 112–123. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Lim, H.T.; Maekawa, T.; Vadakke Matham, M.; Sakthi Kumar, D. Gold nanocages entering into the realm of high-contrast photoacoustic ocular imaging. Nanoscale 2018, 10, 13959–13968. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, W.; Nguyen, V.P.; Huang, Z.; Wang, X.; Paulus, Y.M. Integrated photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy for multimodal chorioretinal imaging. Proc. SPIE 2018, 10494, 104945U. [Google Scholar] [CrossRef]
- Sreejith, S.; Joseph, J.; Nguyen, K.T.; Murukeshan, V.M.; Lye, S.W.; Zhao, Y. Graphene Oxide Wrapping of Gold-Silica Core-Shell Nanohybrids for Photoacoustic Signal Generation and Bimodal Imaging. ChemNanoMat 2015, 1, 39–45. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Paulus, Y.M. Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions. J. Imaging 2018, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Murukeshan, V.M.; Woh, L.S. Integrated photoacoustic, ultrasound and fluorescence platform for diagnostic medical imaging-proof of concept study with a tissue mimicking phantom. Biomed. Opt. Express 2014, 5, 2135–2144. [Google Scholar] [CrossRef]
- Zheng, S.; Jiejie, D.; Yue, Y.; Qi, M.; Huifeng, S. A Deep Learning Method for Motion Artifact Correction in Intravascular Photoacoustic Image Sequence. IEEE Trans. Med. Imaging 2023, 42, 66–78. [Google Scholar] [CrossRef]
- Gulenko, O.; Yang, H.; Kim, K.; Youm, J.Y.; Kim, M.; Kim, Y.; Jung, W.; Yang, J.M. Deep-Learning-Based Algorithm for the Removal of Electromagnetic Interference Noise in Photoacoustic Endoscopic Image Processing. Sensors 2022, 22, 3961. [Google Scholar] [CrossRef]
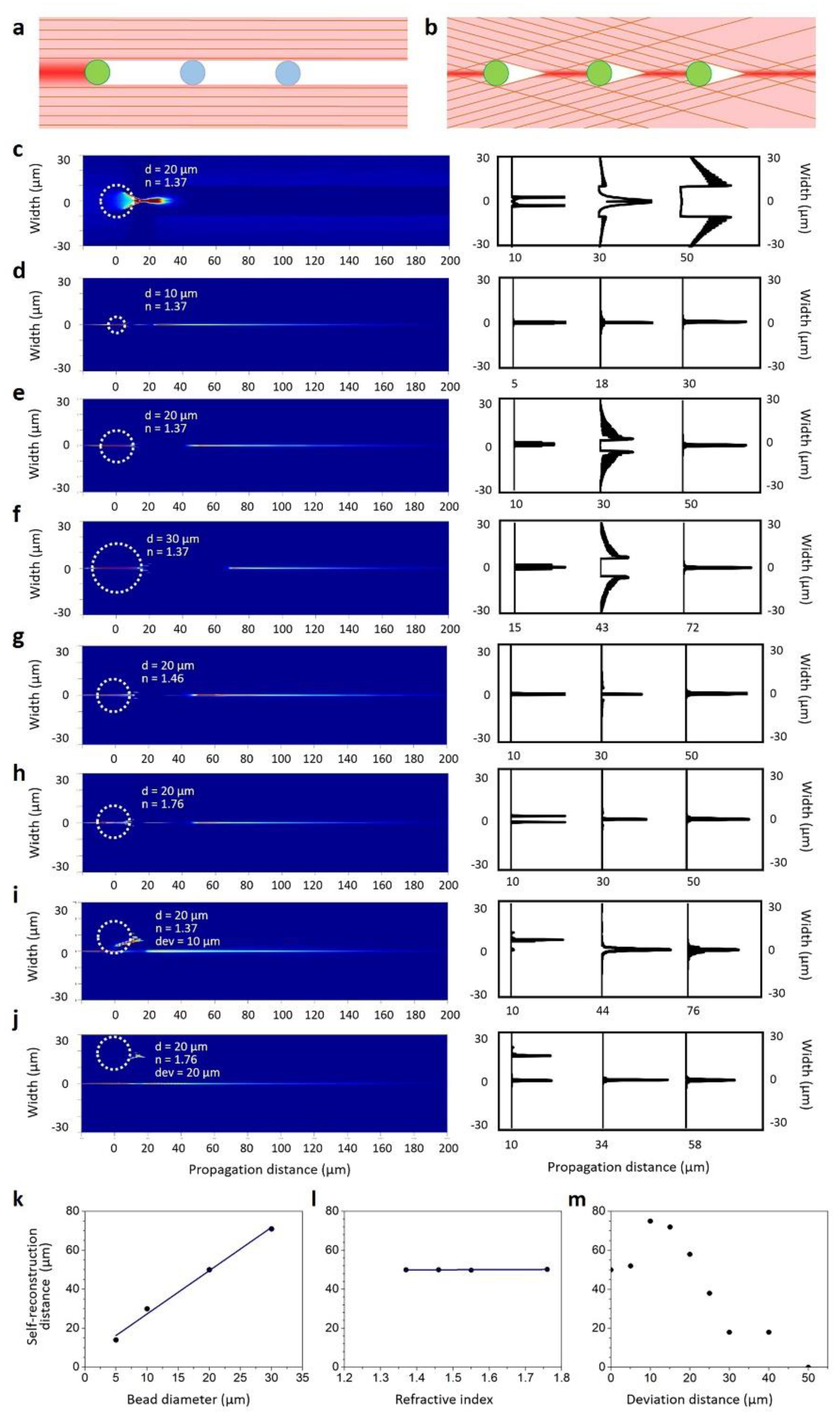
- Fahrbach, F.O.; Simon, P.; Rohrbach, A. Microscopy with self-reconstructing beams. Nature Photon. 2010, 4, 780–785. [Google Scholar] [CrossRef]
- Nagar, H.; Dekel, E.; Kasimov, D.; Roichman, Y. Non-diffracting beams for label-free imaging through turbid media. Opt. Lett. 2018, 43, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-X.; He, H.; Tang, H.; Wong, K.K.Y. Non-Diffracting Light Wave: Fundamentals and Biomedical Applications. Front. Phys. 2021, 9, 698343. [Google Scholar] [CrossRef]
- Nylk, J.; McCluskey, K.; Aggarwal, S.; Tello, J.A.; Dholakia, K. Enhancement of image quality and imaging depth with Airy light-sheet microscopy in cleared and non-cleared neural tissue. Biomed. Opt. Express 2016, 7, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Kozawa, Y.; Sato, S. Resolution enhancement of confocal microscopy by subtraction method with vector beams. Opt. Lett. 2014, 39, 3118–3121. [Google Scholar] [CrossRef]
- Tsou, C.H.; Wu, T.W.; Tung, J.C.; Liang, H.C.; Tuan, P.H.; Chen, Y.F. Generation of pseudonondiffracting optical beams with superlattice structures. Opt. Express 2013, 21, 23441–23449. [Google Scholar] [CrossRef]
- Murukeshan, V.M.; Jesmond, H.X.J.; Shinoj, V.K.; Baskaran, M.; Tin, A. Non-contact high resolution Bessel beam probe for diagnostic imaging of cornea and trabecular meshwork region in eye. Proc. SPIE 2015, 9537, 953728. [Google Scholar] [CrossRef]
- Rohrbach, A. Artifacts resulting from imaging in scattering media: A theoretical prediction. Opt. Lett. 2009, 34, 3041–3043. [Google Scholar] [CrossRef]
- Bouchal, Z.; Wagner, J.; Chlup, M. Self-reconstruction of a distorted nondiffracting beam. Opt. Commun. 1998, 151, 207–211. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L.; Karpeev, S.V.; Butt, M.A. Bessel Beam: Significance and Applications—A Progressive Review. Micromachines 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
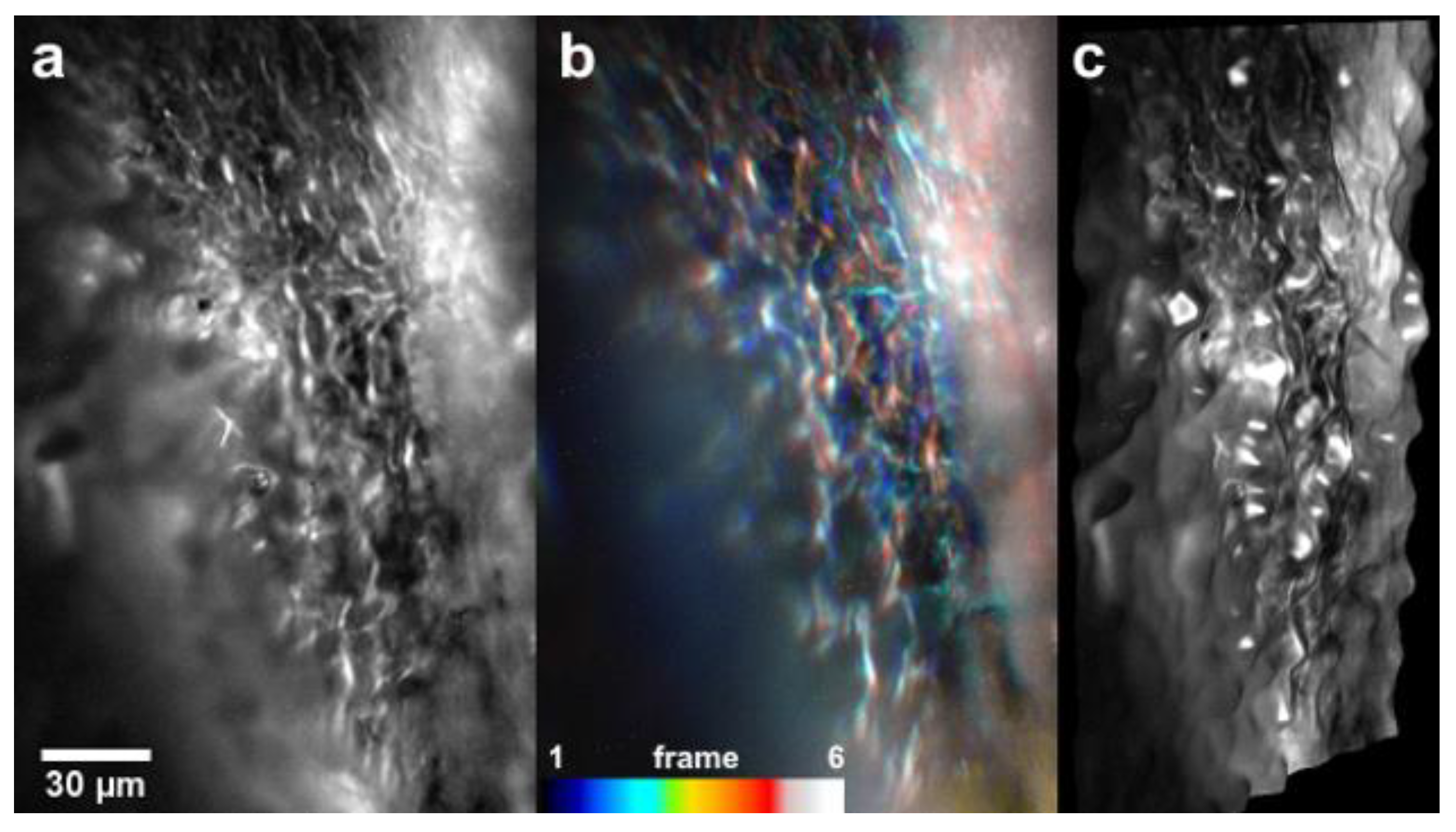
- Suchand Sandeep, C.S.; Sarangapani, S.; Hong, X.J.J.; Aung, T.; Baskaran, M.; Murukeshan, V.M. Optical sectioning and high resolution visualization of trabecular meshwork using Bessel beam assisted light sheet fluorescence microscopy. J. Biophotonics 2019, 12, e201900048. [Google Scholar] [CrossRef]
- Diouf, M.; Lin, Z.; Harling, M.; Toussaint, K.C., Jr. Demonstration of speckle resistance using space-time light sheets. Sci. Rep. 2022, 12, 14064. [Google Scholar] [CrossRef]
- Durnin, J. Exact solutions for nondiffracting beams I The scalar theory. J. Opt. Soc. Am. A 1987, 4, 651. [Google Scholar] [CrossRef]
- Bouchal, Z. Dependence of Bessel Beam Characteristics on Angular Spectrum Phase Variations. J. Mod. Opt. 1993, 40, 1325–1329. [Google Scholar] [CrossRef]
- McGloin, D.; Dholakia, K. Bessel beams: Diffraction in a new light. Contemp. Phys. 2005, 46, 15–28. [Google Scholar] [CrossRef]
- Li, S.; Jiao, J.; Boonruangkan, J.; Toh, H.T.; An, J.; Su, P.-C.; Suchand Sandeep, C.S.; Kim, Y.-J. Self-reconstructing Bessel beam created by two-photon-polymerized micro-axicon for light-sheet fluorescence microscopy. Results Phys. 2021, 24, 104111. [Google Scholar] [CrossRef]
- Gori, F.; Guattari, G.; Padovani, C. Bessel-Gauss beams. Opt. Commun. 1987, 64, 491–495. [Google Scholar] [CrossRef]
- Paterson, C.; Smith, R. Higher-order Bessel waves produced by axicon-type computer-generated holograms. Opt. Commun. 1996, 124, 121–130. [Google Scholar] [CrossRef]
- Anand, V.; Katkus, T.; Juodkazis, S. Randomly Multiplexed Diffractive Lens and Axicon for Spatial and Spectral Imaging. Micromachines 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Cheng, Z.; Lv, Q.; Wang, X. Tunable Axicons Generated by Spatial Light Modulator with High-Level Phase Computer-Generated Holograms. Appl. Sci. 2020, 10, 5127. [Google Scholar] [CrossRef]
- Fahrbach, F.O.; Rohrbach, A. A line scanned light-sheet microscope with phase shaped self-reconstructing beams. Opt. Express 2010, 18, 24229–24244. [Google Scholar] [CrossRef] [PubMed]
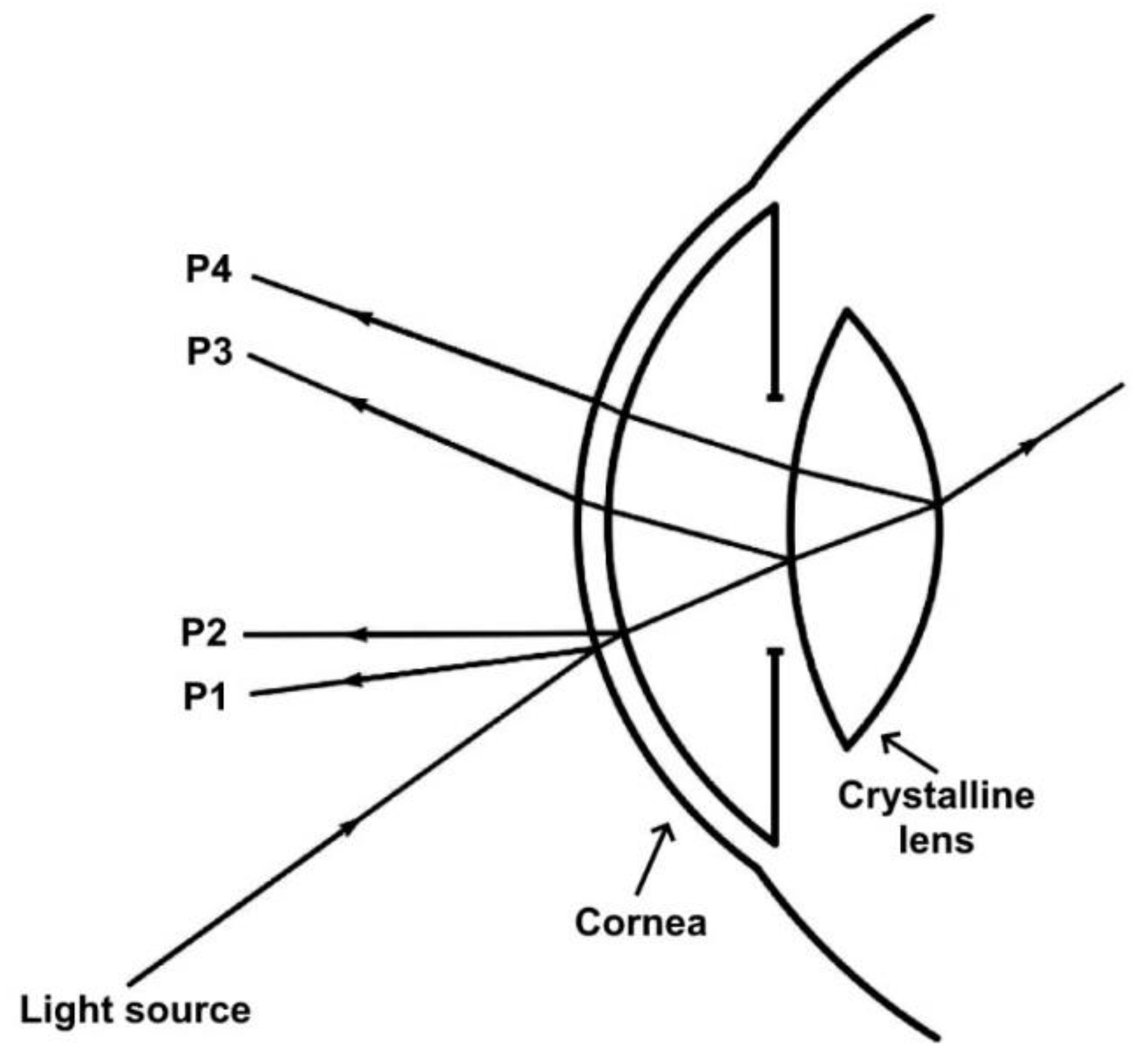
- Calderon-Uribe, U.; Hernandez-Gomez, G.; Gomez-Vieyra, A. Measurement of Longitudinal Chromatic Aberration in the Last Crystalline Lens Surface Using Hartmann Test and Purkinje Images. Sensors 2022, 22, 2653. [Google Scholar] [CrossRef]
- Suheimat, M.; Bhattarai, D.; Maher, H.K.; Chandra, M.; Chelepy, W.; Halloran, S.K.; Lambert, A.J.; Atchison, D.A. Improvements to Phakometry Using Bessel Beams. Optom. Vis. Sci. 2017, 94, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.J.J.; Suchand Sandeep, C.S.; Shinoj, V.K.; Aung, T.; Barathi, V.A.; Baskaran, M.; Murukeshan, V.M. Noninvasive and Noncontact Sequential Imaging of the Iridocorneal Angle and the Cornea of the Eye. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Llobet, A.; Gasull, X.; Gual, A. Understanding trabecular meshwork physiology: A key to the control of intraocular pressure? News Physiol. Sci. 2003, 18, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Suchand Sandeep, C.S.; Barathi, V.A.; Aung, T.; Baskaran, M.; Matham, M.V. Light Sheet Fluorescence Microscopy of the Trabecular Meshwork in Rodent Eyes. In Proceedings of the Imaging and Applied Optics Congress, Washington, DC, USA, 22 June 2020; p. JW5A.3. [Google Scholar]
- Kim, J.; Kim, Y.; Ahn, J.; Woo, S.J.; Park, K.H.; Oh, W.Y. High Three-Dimensional Resolution and Long Imaging Range Retinal Optical Frequency Domain Imaging using Dual-Axicon Lens Illumination. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1501. [Google Scholar]
- Bhattarai, D. Application of Bessel Beams in the Human Eye. Ph.D. Thesis, Queensland University of Technology, Brisbane City, Australia, 2019. [Google Scholar]
- Westheimer, G. Focused and defocused retinal images with Bessel and axicon pupil functions. J. Opt. Soc. Am. A 2020, 37, 108–114. [Google Scholar] [CrossRef]
- Lambert, A.J.; Daly, E.M.; Dainty, C.J. Improved fixation quality provided by a Bessel beacon in an adaptive optics system. Ophthalmic Physiol. Opt. 2013, 33, 403–411. [Google Scholar] [CrossRef]
- Ahluwalia, B.P.S.; Yuan, X.C.; Tao, S.H. Generation of self-imaged optical bottle beams. Opt. Commun. 2004, 238, 177–184. [Google Scholar] [CrossRef]
- Ding, Z.; Ren, H.; Zhao, Y.; Nelson, J.S.; Chen, Z. High-resolution optical coherence tomography over a large depth range with an axicon lens. Opt. Lett. 2002, 27, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Sun, L.; Ding, W. Multifocal spectral-domain optical coherence tomography based on Bessel beam for extended imaging depth. J. Biomed. Opt. 2017, 22, 106016. [Google Scholar] [CrossRef]
- Lee, K.S.; Rolland, J.P. Bessel beam spectral-domain high-resolution optical coherence tomography with micro-optic axicon providing extended focusing range. Opt. Lett. 2008, 33, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Classen, A.; Fernandez, A.; Gruner-Nielsen, L.; Gibbs, H.C.; Esmaeili, S.; Hemmer, P.; Baltuska, A.; Sokolov, A.V.; Leitgeb, R.A.; et al. Extended focal depth Fourier domain optical coherence microscopy with a Bessel-beam—LP(02) mode—From a higher order mode fiber. Biomed. Opt. Express 2021, 12, 7327–7337. [Google Scholar] [CrossRef]
- Mirg, S.; Turner, K.L.; Chen, H.; Drew, P.J.; Kothapalli, S.R. Photoacoustic imaging for microcirculation. Microcirculation 2022, 29, e12776. [Google Scholar] [CrossRef] [PubMed]
- Hosseinaee, Z.; Nima, A.; Pellegrino, N.; Khalili, L.; Mukhangaliyeva, L.; Haji Reza, P. Functional and structural ophthalmic imaging using noncontact multimodal photoacoustic remote sensing microscopy and optical coherence tomography. Sci. Rep. 2021, 11, 11466. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, T.; Wen, R.; Li, Y.; Puliafito, C.A.; Zhang, H.F.; Jiao, S. Optical coherence photoacoustic microscopy for in vivo multimodal retinal imaging. Opt. Lett. 2015, 40, 1370–1373. [Google Scholar] [CrossRef]
- Park, B.; Lee, H.; Jeon, S.; Ahn, J.; Kim, H.H.; Kim, C. Reflection-mode switchable subwavelength Bessel-beam and Gaussian-beam photoacoustic microscopy in vivo. J. Biophotonics 2019, 12, e201800215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yang, X.; Luo, Q. Reflection-mode Bessel-beam photoacoustic microscopy for in vivo imaging of cerebral capillaries. Opt. Express 2016, 24, 20167–20176. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Noordam, C.; Wang, L.V. Bessel-beam Grueneisen relaxation photoacoustic microscopy with extended depth of field. J. Biomed. Opt. 2015, 20, 116002. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchand Sandeep, C.S.; Khairyanto, A.; Aung, T.; Vadakke Matham, M. Bessel Beams in Ophthalmology: A Review. Micromachines 2023, 14, 1672. https://doi.org/10.3390/mi14091672
Suchand Sandeep CS, Khairyanto A, Aung T, Vadakke Matham M. Bessel Beams in Ophthalmology: A Review. Micromachines. 2023; 14(9):1672. https://doi.org/10.3390/mi14091672
Chicago/Turabian StyleSuchand Sandeep, C. S., Ahmad Khairyanto, Tin Aung, and Murukeshan Vadakke Matham. 2023. "Bessel Beams in Ophthalmology: A Review" Micromachines 14, no. 9: 1672. https://doi.org/10.3390/mi14091672
APA StyleSuchand Sandeep, C. S., Khairyanto, A., Aung, T., & Vadakke Matham, M. (2023). Bessel Beams in Ophthalmology: A Review. Micromachines, 14(9), 1672. https://doi.org/10.3390/mi14091672






