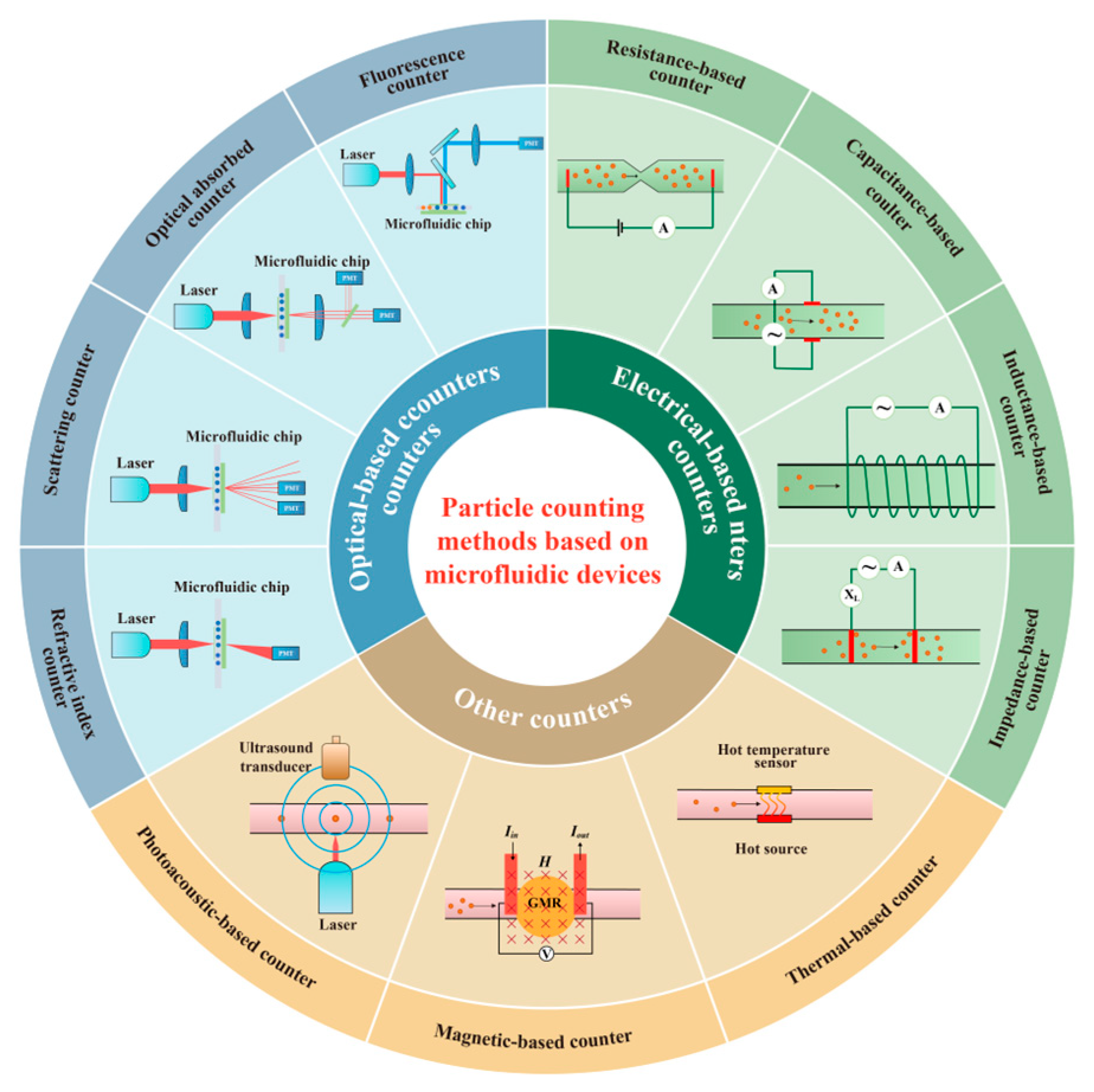
Particle Counting Methods Based on Microfluidic Devices
Abstract
1. Introduction
2. Optical-Based Counters
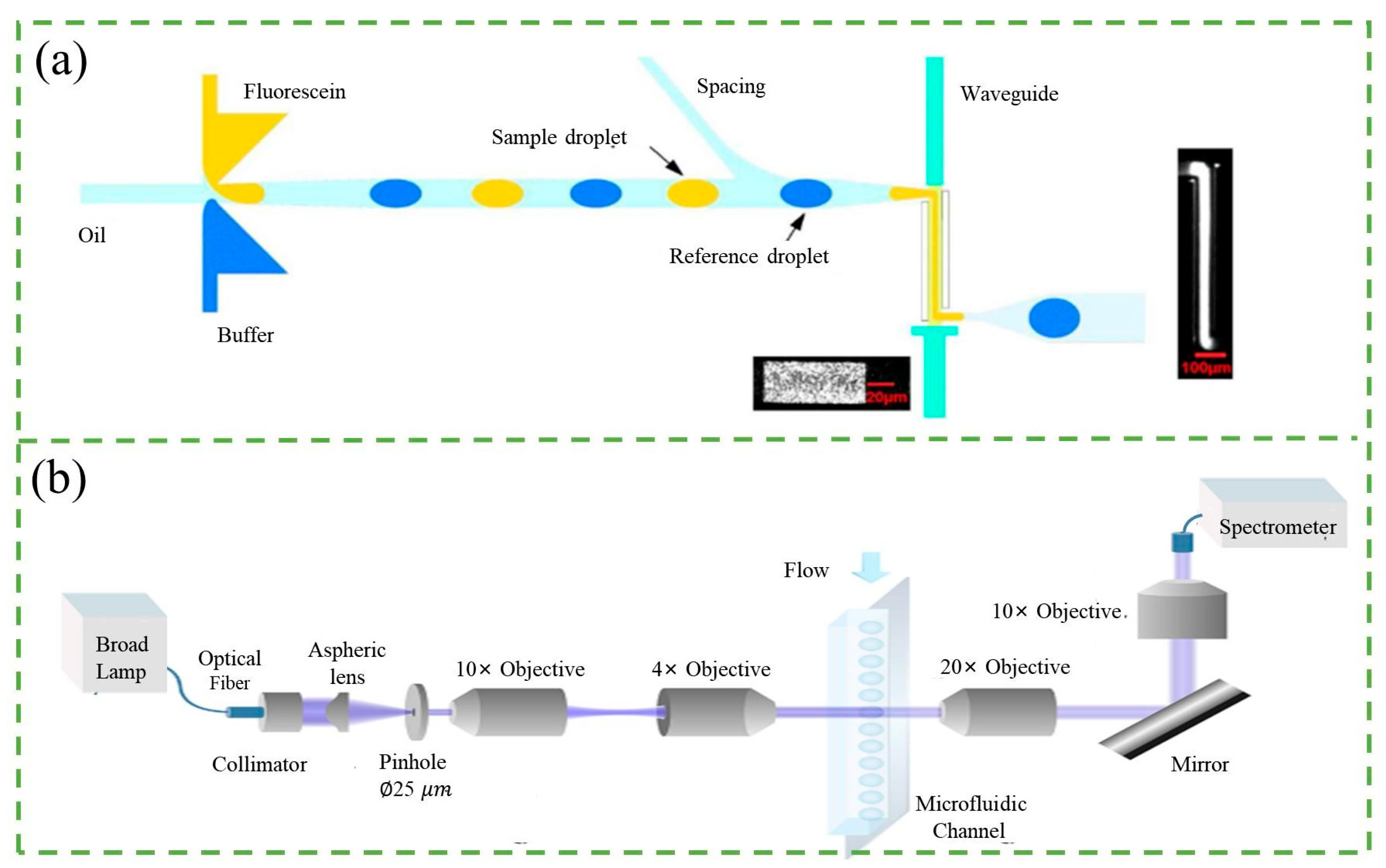
2.1. Fluorescence Counter
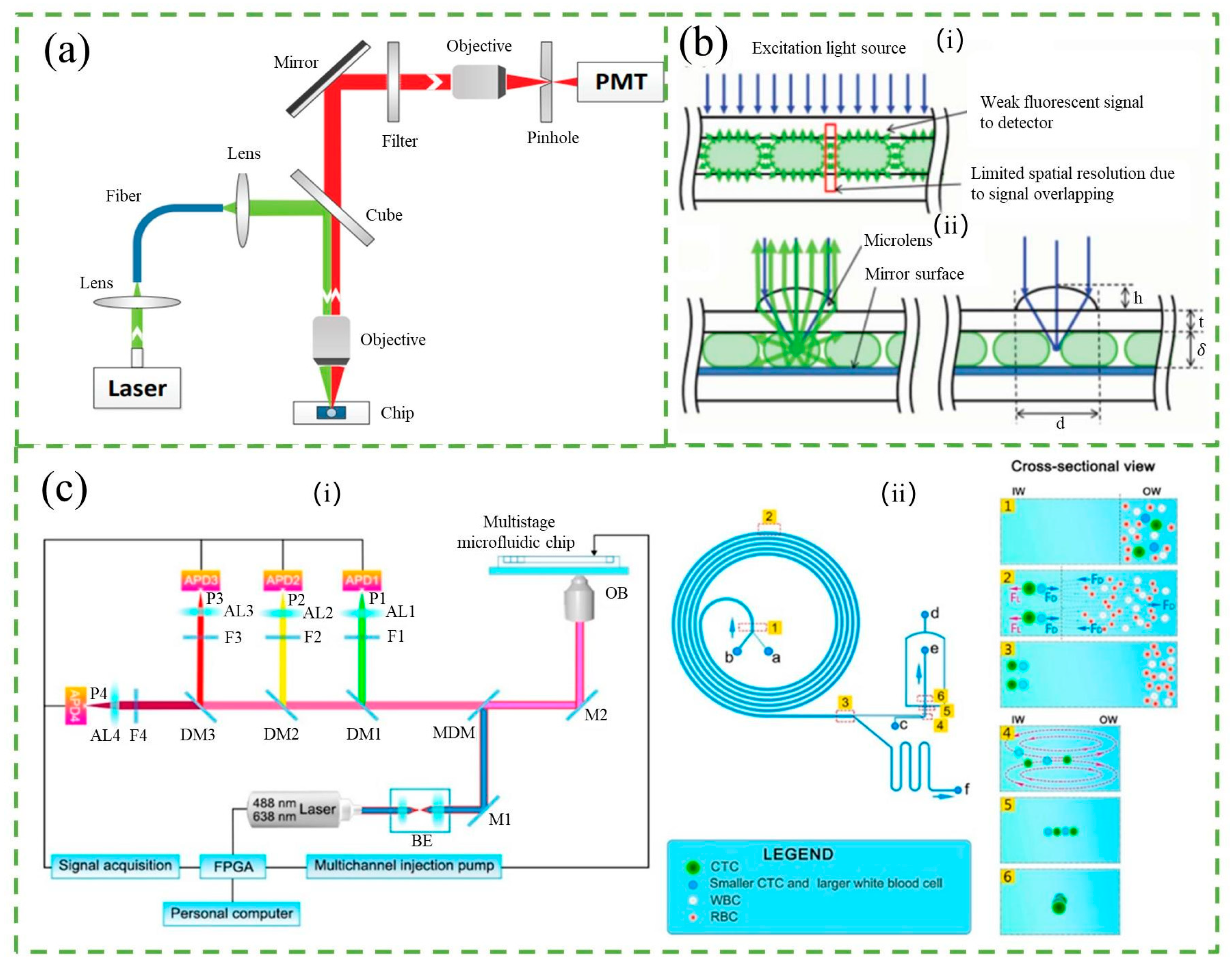
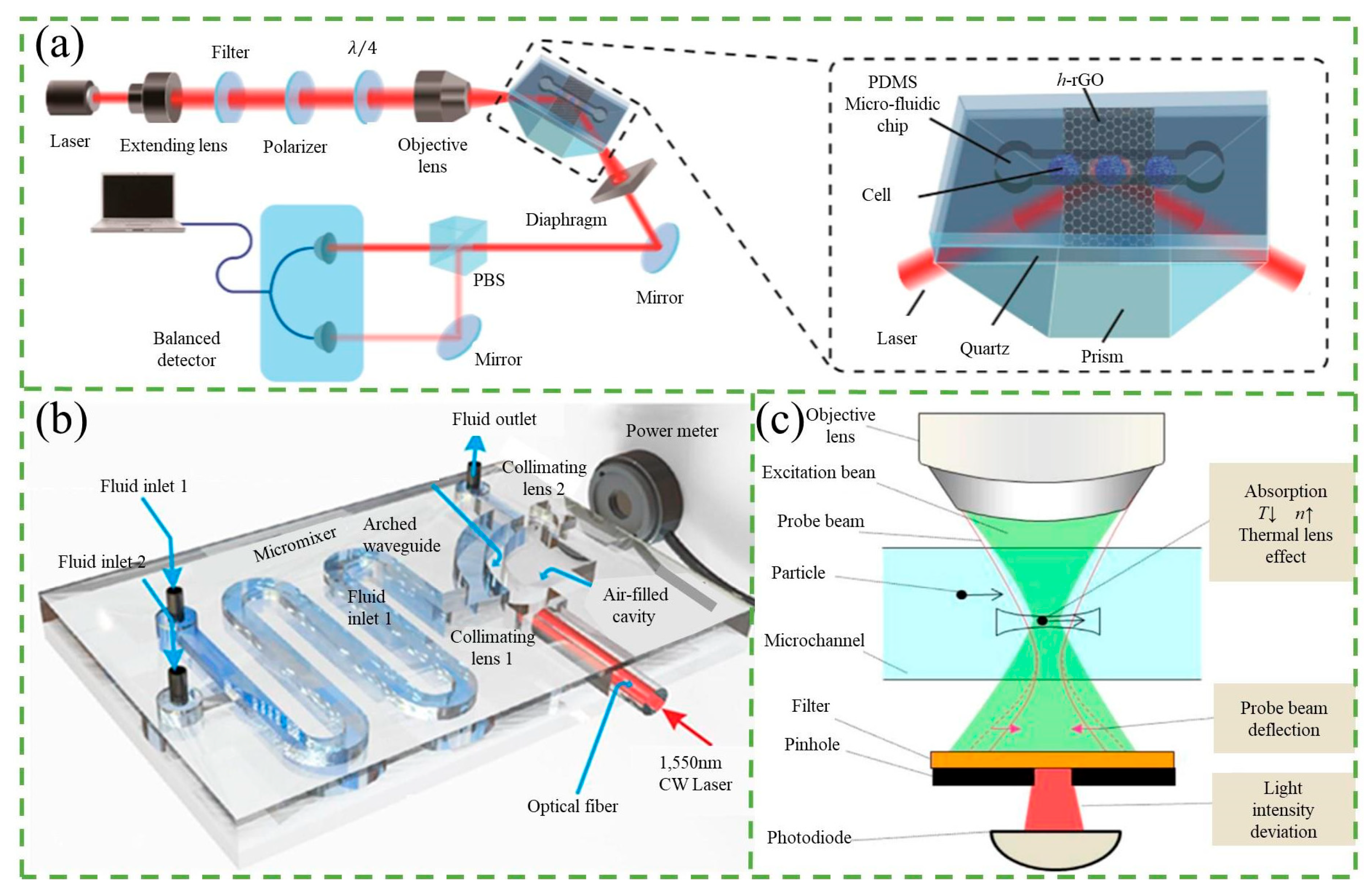
2.2. Optical Absorbed Counter
2.3. Scattering Counter
2.4. Refractive Index Counter
3. Electrical-Based Counters
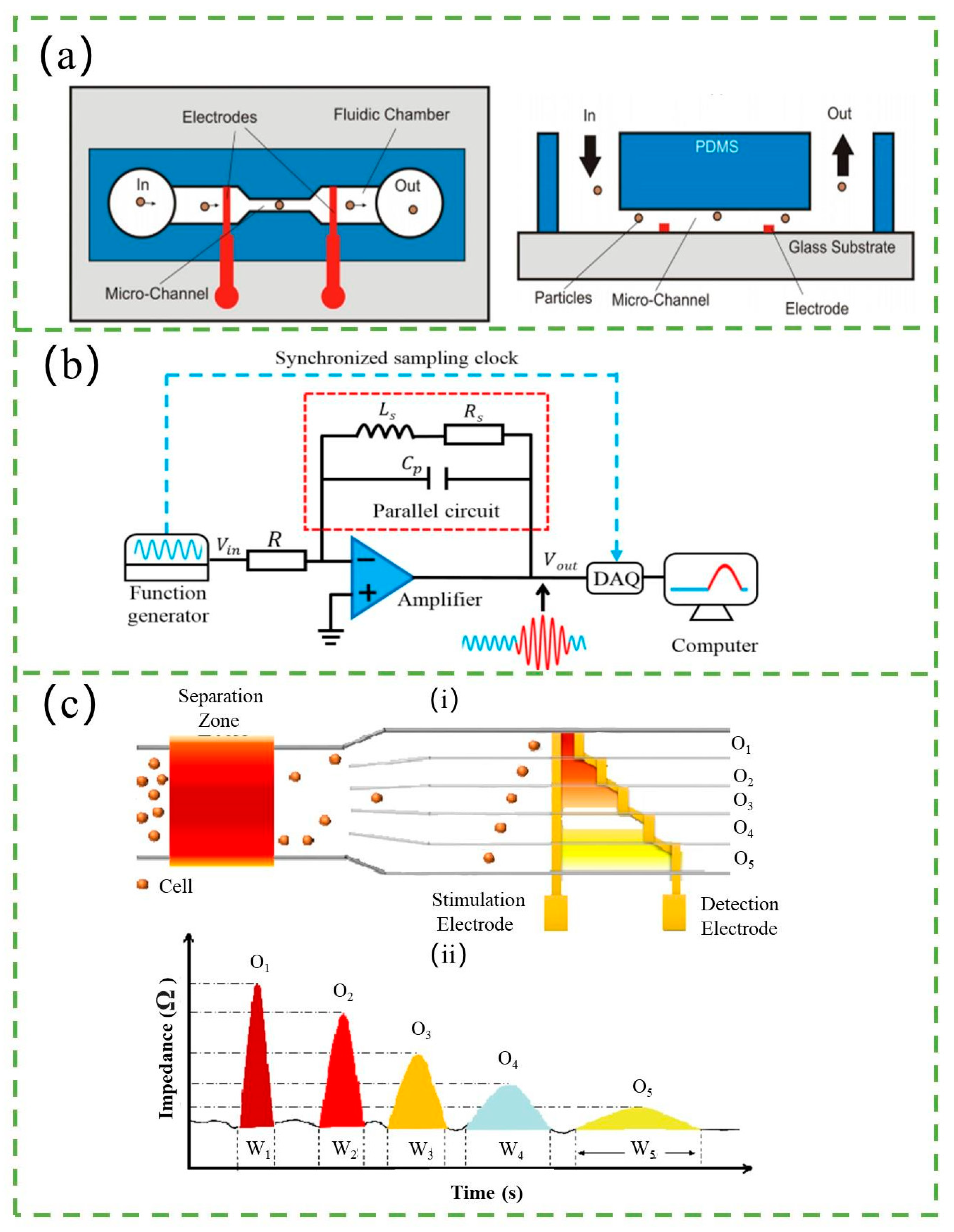
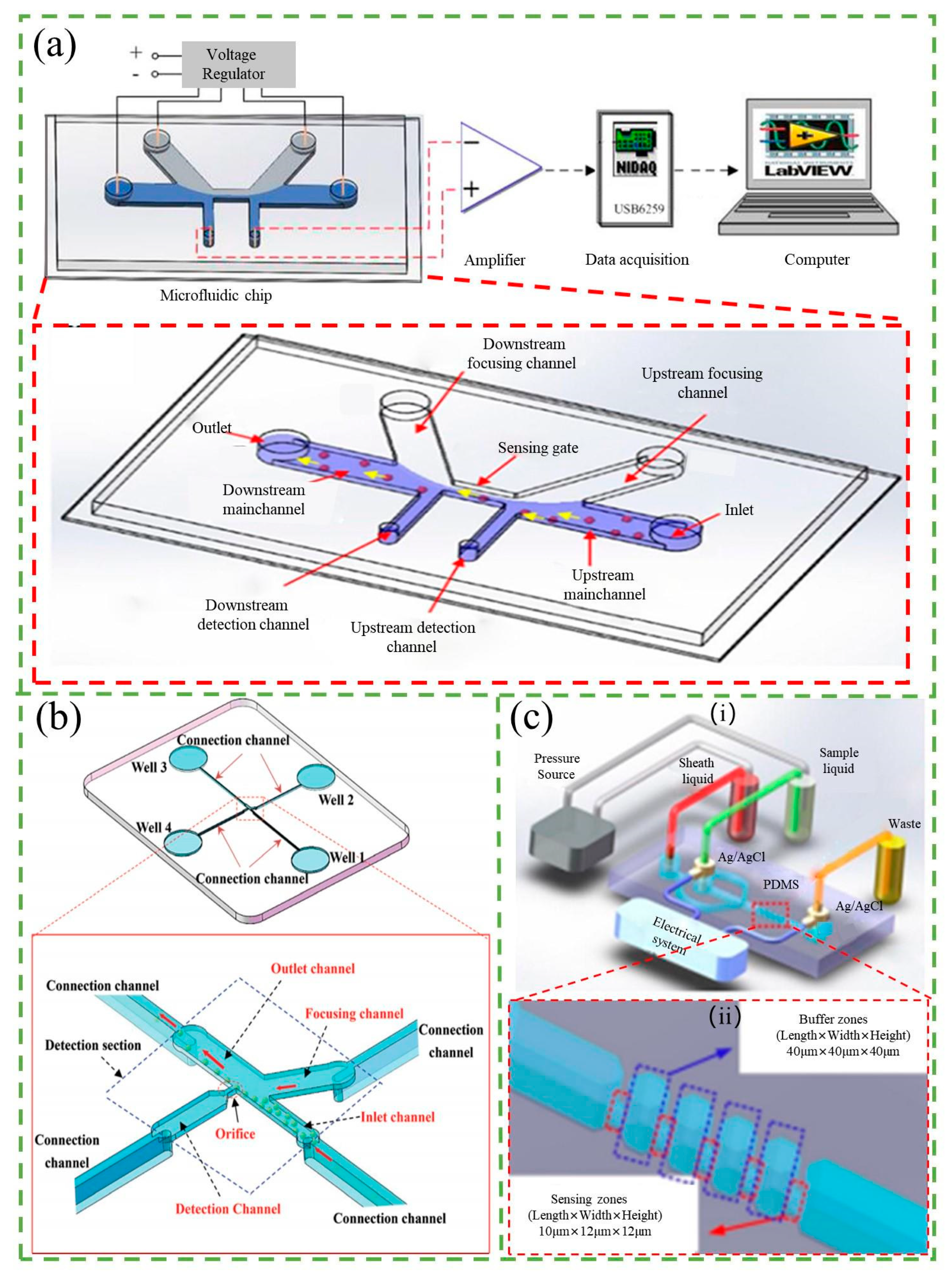
3.1. Resistance-Based Counter
3.2. Capacitance-Based Counter
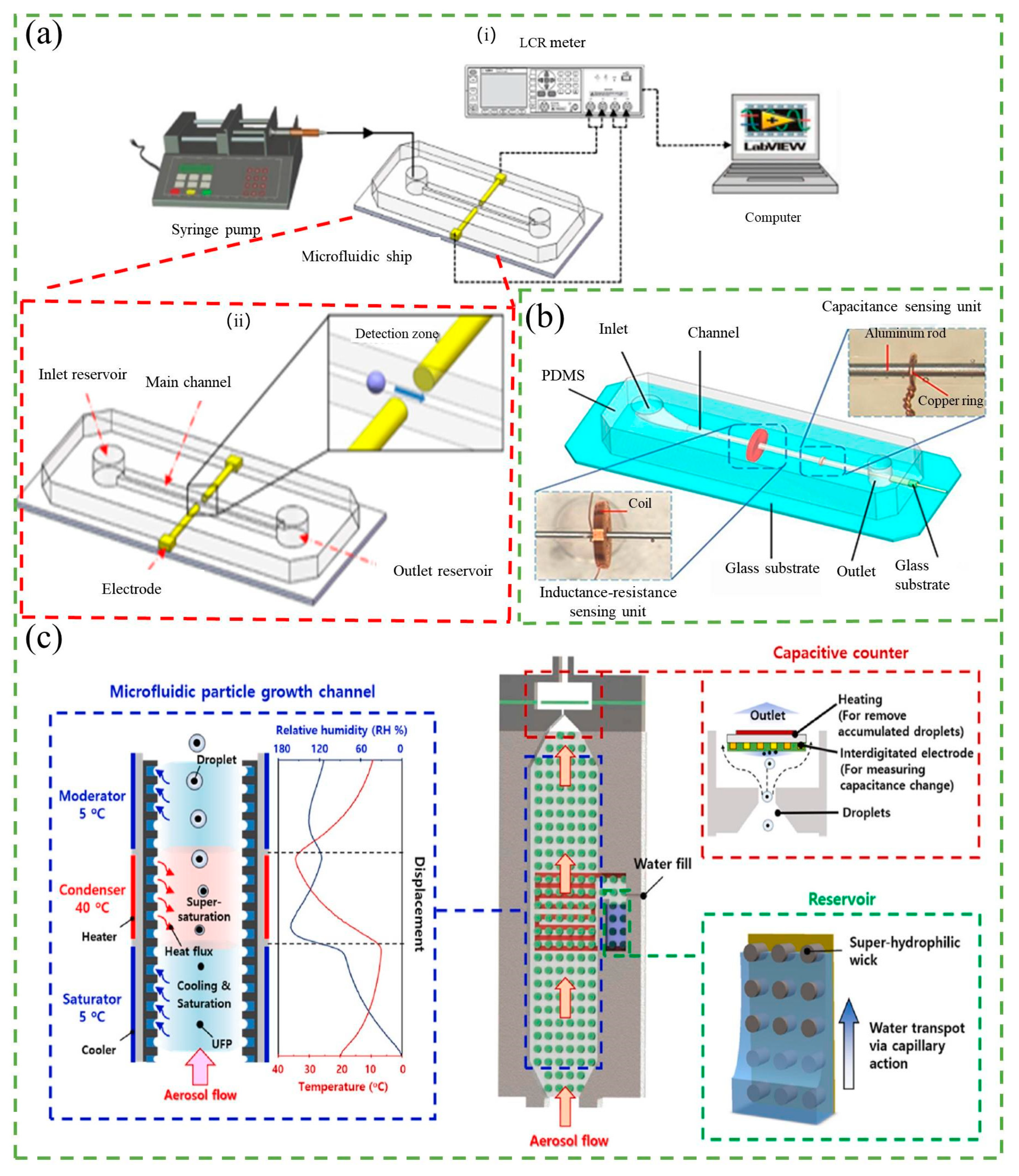
3.3. Inductance-Based Counter
3.4. Impedance-Based Counter
4. Other Counters
4.1. Photoacoustic-Based Counter
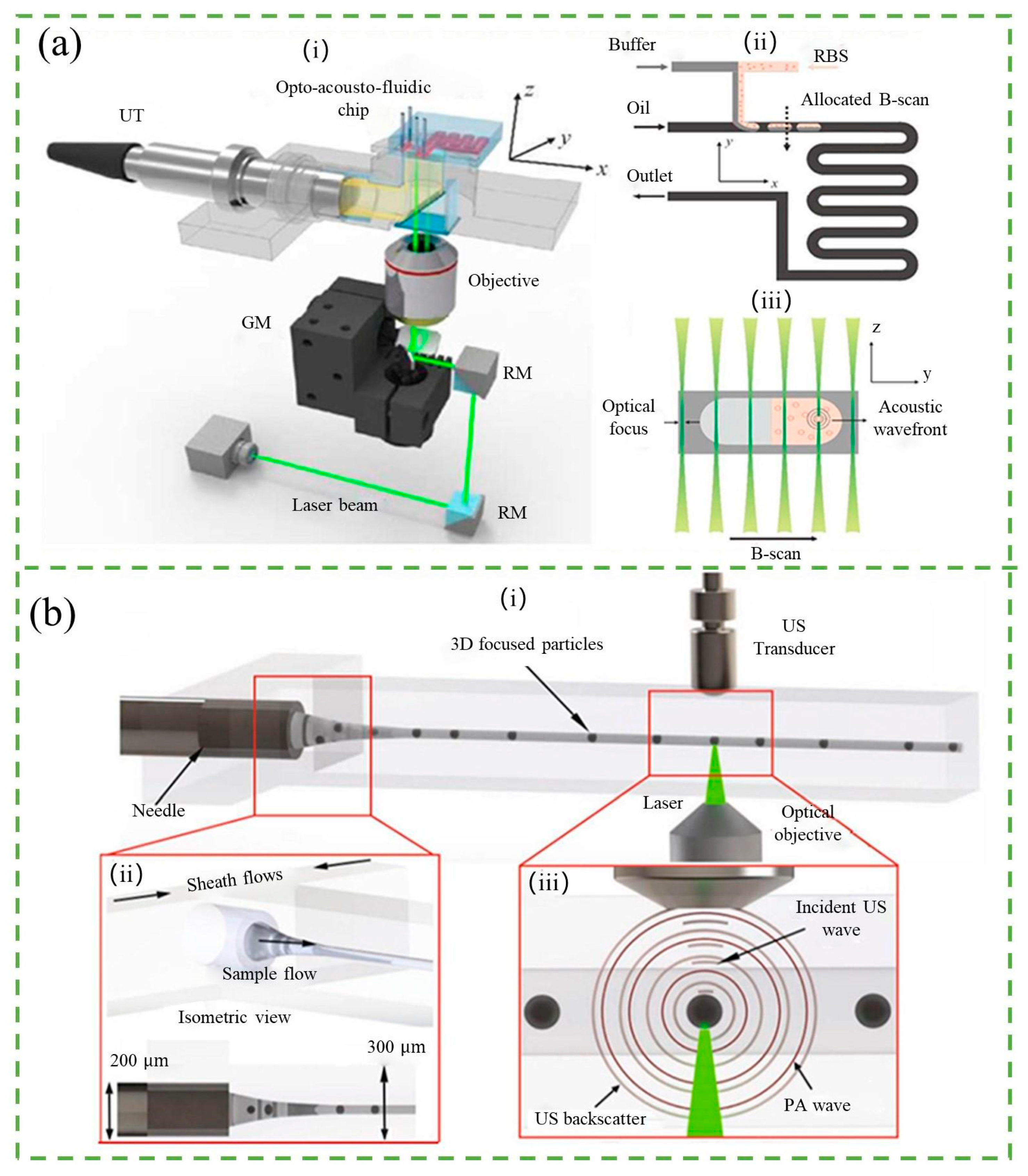
4.2. Magnetic-Based Counter
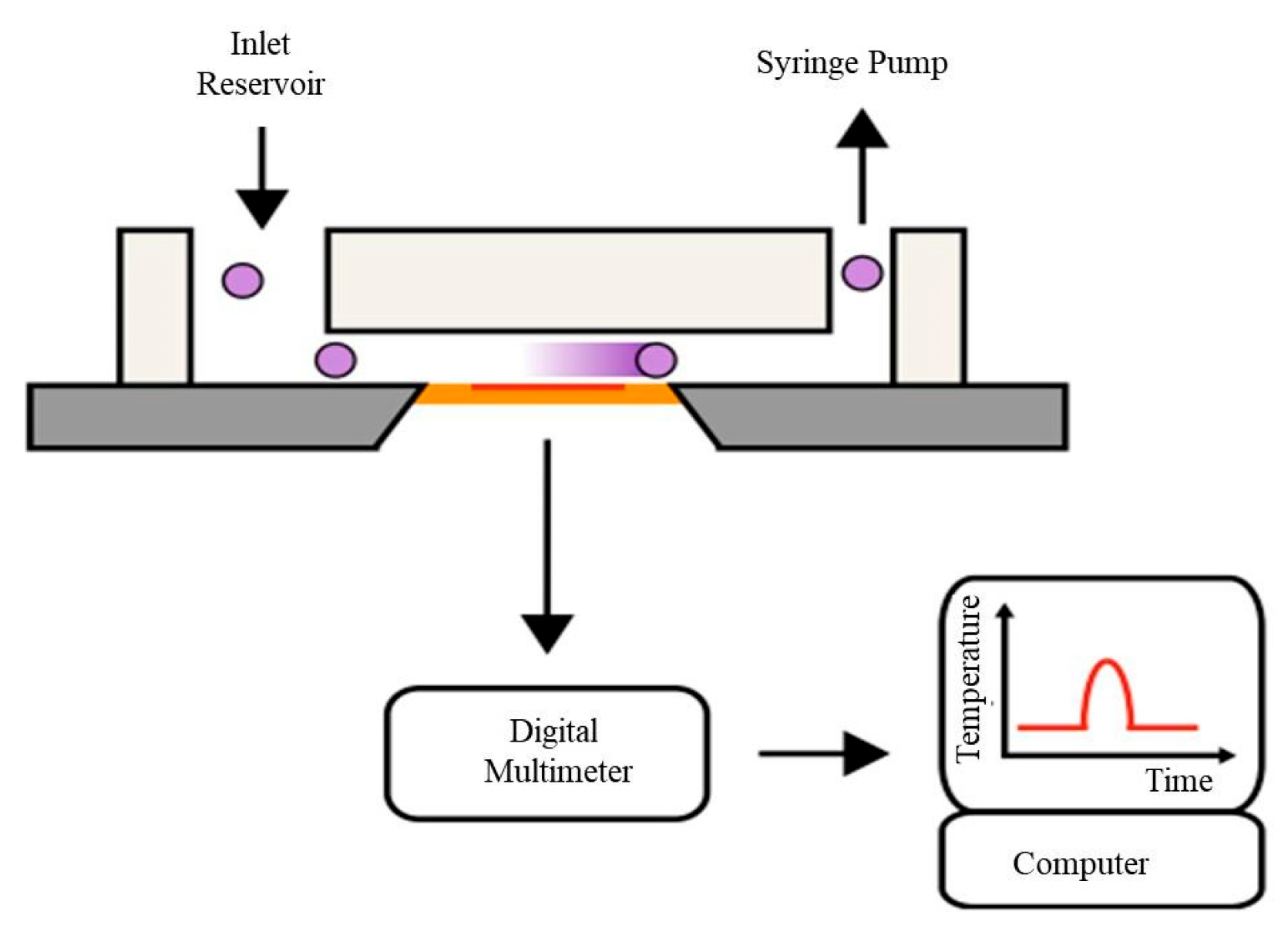
4.3. Thermal-Based Counter
5. Future Directions and Challenges
| Classification | Method | Limit of Detection | Integration Difficulty | Instrument Price | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Optical-Based Counters | Fluorescence Counter | (Escherichia coli O157:H7) | Medium | Moderately high | High sensitivity, multiple labeling | Complex equipment |
| Optical Absorbed Counter | (Rhodamine 6G concentration) | Low | Low | No labeling, easy to operate | Lower flux, low light transmission, poor effect | |
| Scattering Counter | (Particle size) | High | High | Label-free, differentiates between different particles | Complex equipment, difficult to integrate | |
| Refractive Index Counter | (Refractive index change) | Low | Medium | Label-free, can monitor cell status | Mainly used for analytical testing, not suitable for opaque particles | |
| Electrical-Based Counters | Resistance-Based Counter | (Particle size) | Low | Low | Simple, label-free, low cost | Only suitable for conductive solutions, limited flux |
| Capacitance-Based Counter | (Bubble diameter) | High | Medium | Can be used in non-conductive solutions | Complicated electrode production, low sensitivity | |
| Inductance-Based Counter | (Abrasive particle diameter) | Low | Low | Simple, low cost | Only detect metal abrasion particles, low throughput | |
| Impedance-Based Counter | (Bollstein microsphere diameter) | Low | Low | Simple, low cost | Electric field may damage biological particles | |
| Other Counters | Photoacoustic-Based Counter | No clear data | High | High | Label-free, real-time detection | Complex equipment, high cost |
| Magnetic-Based Counter | (Iron particle diameter) | Low | Low | Integrable, Low Cost | Only magnetic particles can be detected | |
| Thermal-Based Counter | (Particle diameter) | Medium | Medium | Label-free, in situ detection | Low sensitivity to small particles, susceptible to flow velocity and temperature |
5.1. Data Analysis Capabilities
5.2. Material
5.3. Equipment Manufacturing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dittrich, P.S.; Manz, A. Single-molecule fluorescence detection in microfluidic channels—The Holy Grail in muTAS? Anal. Bioanal. Chem. 2005, 382, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Amirifar, L.; Besanjideh, M.; Nasiri, R.; Shamloo, A.; Nasrollahi, F.; de Barros, N.R.; Davoodi, E.; Erdem, A.; Mahmoodi, M.; Hosseini, V.; et al. Droplet-based microfluidics in biomedical applications. Biofabrication 2022, 14, 022001. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Goel, S. Recent advancements in integrated microthermofluidic systems for biochemical and biomedical applications—A review. Sens. Actuators A Phys. 2022, 341, 113590. [Google Scholar] [CrossRef]
- Pan, Y.-L. Detection and characterization of biological and other organic-carbon aerosol particles in atmosphere using fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Du, L.; Zhe, J. An integrated ultrasonic–inductive pulse sensor for wear debris detection. Smart Mater. Struct. 2013, 22, 025003. [Google Scholar] [CrossRef]
- Hong, W.; Wang, S.; Tomovic, M.; Han, L.; Shi, J. Radial inductive debris detection sensor and performance analysis. Meas. Sci. Technol. 2013, 24, 125103. [Google Scholar] [CrossRef]
- Pagaduan, J.V.; Sahore, V.; Woolley, A.T. Applications of microfluidics and microchip electrophoresis for potential clinical biomarker analysis. Anal. Bioanal. Chem. 2015, 407, 6911–6922. [Google Scholar] [CrossRef]
- Tahirbegi, I.B.; Ehgartner, J.; Sulzer, P.; Zieger, S.; Kasjanow, A.; Paradiso, M.; Strobl, M.; Bouwes, D.; Mayr, T. Fast pesticide detection inside microfluidic device with integrated optical pH, oxygen sensors and algal fluorescence. Biosens. Bioelectron. 2017, 88, 188–195. [Google Scholar] [CrossRef]
- Hu, J.; Cui, X.; Gong, Y.; Xu, X.; Gao, B.; Wen, T.; Lu, T.J.; Xu, F. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol. Adv. 2016, 34, 305–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Tseng, T.M.; Schlichtmann, U. Portable all-in-one automated microfluidic system (PAMICON) with 3D-printed chip using novel fluid control mechanism. Sci. Rep. 2021, 11, 19189. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.-Q.; Liu, Z.; Hou, C.-J.; Yang, J.; Luo, X.-G.; Fa, H.-B.; Dong, J.-L.; Zhang, Y.-C.; Zhang, G.-P.; Li, J.-J. Recent Advances on Optical Detection Methods and Techniques for Cell-based Microfluidic Systems. Chin. J. Anal. Chem. 2010, 38, 1357–1365. [Google Scholar] [CrossRef]
- Zhou, P.; He, H.; Ma, H.; Wang, S.; Hu, S. A Review of Optical Imaging Technologies for Microfluidics. Micromachines 2022, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Font, L.; Pellefigues, C.; Mayer, J.U.; Small, S.J.; Jaimes, M.C.; Price, K.M. Panel Design and Optimization for High-Dimensional Immunophenotyping Assays Using Spectral Flow Cytometry. Curr. Protoc. Cytom. 2020, 92, e70. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef]
- Li, T.; Díaz-Real, J.A.; Holm, T. Design of Electrochemical Microfluidic Detectors: A Review. Adv. Mater. Technol. 2021, 6, 2100569. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluidics 2021, 25, 99. [Google Scholar] [CrossRef]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal. Bioanal. Chem. 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Zhang, H.; Chon, C.H.; Pan, X.; Li, D. Methods for counting particles in microfluidic applications. Microfluid. Nanofluidics 2009, 7, 739–749. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhu, Y. “Development and application of analytical detection techniques for droplet-based microfluidics”—A review. Anal. Chim. Acta 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Colin, P.Y.; Kintses, B.; Gielen, F.; Miton, C.M.; Fischer, G.; Mohamed, M.F.; Hyvonen, M.; Morgavi, D.P.; Janssen, D.B.; Hollfelder, F. Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics. Nat. Commun. 2015, 6, 10008. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Chung, M.T.; Yao, Y.; Nidetz, R.; Lee, L.M.; Liu, A.P.; Feng, Y.; Kurabayashi, K.; Yang, G.Y. Efficient molecular evolution to generate enantioselective enzymes using a dual-channel microfluidic droplet screening platform. Nat. Commun. 2018, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, X.; Song, Y.; Pan, X.; Li, D. Detection of activity of single microalgae cells in a new microfluidic cell capturing chip. Meas. Sci. Technol. 2016, 27, 125701. [Google Scholar] [CrossRef]
- Parnamets, K.; Pardy, T.; Koel, A.; Rang, T.; Scheler, O.; le Moullec, Y.; Afrin, F. Optical Detection Methods for High-Throughput Fluorescent Droplet Microflow Cytometry. Micromachines 2021, 12, 345. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, T.; Morgan, H. Optical particle detection integrated in a dielectrophoretic lab-on-a-chip. J. Micromech. Microeng. 2002, 12, 7–12. [Google Scholar] [CrossRef]
- Lim, J.; Gruner, P.; Konrad, M.; Baret, J.C. Micro-optical lens array for fluorescence detection in droplet-based microfluidics. Lab Chip 2013, 13, 1472–1475. [Google Scholar] [CrossRef]
- Cao, X.; Du, Y.; Kuffner, A.; van Wyk, J.; Arosio, P.; Wang, J.; Fischer, P.; Stavrakis, S.; deMello, A. A Counter Propagating Lens-Mirror System for Ultrahigh Throughput Single Droplet Detection. Small 2020, 16, e1907534. [Google Scholar] [CrossRef]
- Gallina, M.E.; Kim, T.J.; Shelor, M.; Vasquez, J.; Mongersun, A.; Kim, M.; Tang, S.K.Y.; Abbyad, P.; Pratx, G. Toward a Droplet-Based Single-Cell Radiometric Assay. Anal. Chem. 2017, 89, 6472–6481. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.Y. Sensitive detection of microRNA with isothermal amplification and a single-quantum-dot-based nanosensor. Anal. Chem. 2012, 84, 224–231. [Google Scholar] [CrossRef]
- Yin, B.; Wang, Y.; Dong, M.; Wu, J.; Ran, B.; Xie, M.; Joo, S.W.; Chen, Y. One-step multiplexed detection of foodborne pathogens: Combining a quantum dot-mediated reverse assaying strategy and magnetic separation. Biosens. Bioelectron. 2016, 86, 996–1002. [Google Scholar] [CrossRef]
- Li, Q.; Cui, S.; Xu, Y.; Wang, Y.; Jin, F.; Si, H.; Li, L.; Tang, B. Consecutive Sorting and Phenotypic Counting of CTCs by an Optofluidic Flow Cytometer. Anal. Chem. 2019, 91, 14133–14140. [Google Scholar] [CrossRef]
- Yelleswarapu, V.R.; Jeong, H.H.; Yadavali, S.; Issadore, D. Ultra-high throughput detection (1 million droplets per second) of fluorescent droplets using a cell phone camera and time domain encoded optofluidics. Lab Chip 2017, 17, 1083–1094. [Google Scholar] [CrossRef]
- Kim, M.; Pan, M.; Gai, Y.; Pang, S.; Han, C.; Yang, C.; Tang, S.K. Optofluidic ultrahigh-throughput detection of fluorescent drops. Lab Chip 2015, 15, 1417–1423. [Google Scholar] [CrossRef]
- Chen, B.; Han, X.; Su, Z.; Liu, Q. Fluorescence detection system for microfluidic droplets. AIP Conf. Proc. 2018, 1967, 030016. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett. 2018, 18, 4226–4232. [Google Scholar] [CrossRef]
- Akgonullu, S.; Bakhshpour, M.; Piskin, A.K.; Denizli, A. Microfluidic Systems for Cancer Diagnosis and Applications. Micromachines 2021, 12, 1349. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, W.; Huang, J.; Dong, J.; Xiao, X.; Li, G. Size-resolved counting of circulating tumor cells on pinched flow-based microfluidic cytometry. Electrophoresis 2022, 44, 82–88. [Google Scholar] [CrossRef]
- Chiu, T.K.; Lei, K.F.; Hsieh, C.H.; Hsiao, H.B.; Wang, H.M.; Wu, M.H. Development of a microfluidic-based optical sensing device for label-free detection of circulating tumor cells (CTCs) through their lactic acid metabolism. Sensors 2015, 15, 6789–6806. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, P.; Basumatary, J.; Mondal, P.P. Light sheet based volume flow cytometry (VFC) for rapid volume reconstruction and parameter estimation on the go. Sci. Rep. 2022, 12, 78. [Google Scholar] [CrossRef]
- Probst, J.; Howes, P.; Arosio, P.; Stavrakis, S.; deMello, A. Broad-Band Spectrum, High-Sensitivity Absorbance Spectroscopy in Picoliter Volumes. Anal. Chem. 2021, 93, 7673–7681. [Google Scholar] [CrossRef]
- Yang, T.; Stavrakis, S.; deMello, A. A High-Sensitivity, Integrated Absorbance and Fluorescence Detection Scheme for Probing Picoliter-Volume Droplets in Segmented Flows. Anal. Chem. 2017, 89, 12880–12887. [Google Scholar] [CrossRef]
- Mao, Z.; Guo, F.; Xie, Y.; Zhao, Y.; Lapsley, M.I.; Wang, L.; Mai, J.D.; Costanzo, F.; Huang, T.J. Label-free measurements of reaction kinetics using a droplet-based optofluidic device. J. Lab. Autom. 2015, 20, 17–24. [Google Scholar] [CrossRef]
- Ro, K.W.; Lim, K.; Shim, B.C.; Hahn, J.H. Integrated Light Collimating System for Extended Optical-Path-Length Absorbance Detection in Microchip-Based Capillary Electrophoresis. Anal. Chem. 2005, 77, 5160–5166. [Google Scholar] [CrossRef]
- Gotz, S.; Karst, U. Recent developments in optical detection methods for microchip separations. Anal. Bioanal. Chem. 2007, 387, 183–192. [Google Scholar] [CrossRef]
- Onoshima, D.; Wang, J.; Aki, M.; Arinaga, K.; Kaji, N.; Tokeshi, M.; Fujita, S.; Yokoyama, N.; Baba, Y. A deep microfluidic absorbance detection cell replicated from a thickly stacked SU-8 dry film resist mold. Anal. Methods 2012, 4, 4368–4372. [Google Scholar] [CrossRef]
- Lamprecht, B.; Kraker, E.; Sagmeister, M.; Kostler, S.; Galler, N.; Ditlbacher, H.; Ungerbock, B.; Abel, T.; Mayr, T. Integrated waveguide sensor utilizing organic photodiodes. Phys. Status Solidi-Rapid Res. Lett. 2011, 5, 344–346. [Google Scholar] [CrossRef]
- Wu, D.; Niu, L.G.; Wu, S.Z.; Xu, J.; Midorikawa, K.; Sugioka, K. Ship-in-a-bottle femtosecond laser integration of optofluidic microlens arrays with center-pass units enabling coupling-free parallel cell counting with a 100% success rate. Lab Chip 2015, 15, 1515–1523. [Google Scholar] [CrossRef]
- Gielen, F.; Hours, R.; Emond, S.; Fischlechner, M.; Schell, U.; Hollfelder, F. Ultrahigh-throughput-directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proc. Natl. Acad. Sci. USA 2016, 113, E7383–E7389. [Google Scholar] [CrossRef]
- Banoth, E.; Jagannadh, V.K.; Gorthi, S.S. Single-Cell Optical Absorbance Characterization With High-Throughput Microfluidic Microscopy. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 111–116. [Google Scholar] [CrossRef]
- Masrie, M.; Majlis, B.Y.; Yunas, J.; Menon, P.S. Design Study of Integrated Optical Transducer for Bioparticles Detection. In Proceedings of the 10th IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 19–21 September 2012; pp. 205–209. [Google Scholar]
- Deal, K.S.; Easley, C.J. Self-Regulated, Droplet-Based Sample Chopper for Microfluidic Absorbance Detection. Anal. Chem. 2012, 84, 1510–1516. [Google Scholar] [CrossRef]
- Banoth, E.; Kasula, V.K.; Gorthi, S.S. Portable optofluidic absorption flow analyzer for quantitative malaria diagnosis from whole blood. Appl. Opt. 2016, 55, 8637–8643. [Google Scholar] [CrossRef]
- Banoth, E.; Kasula, V.K.; Jagannadh, V.K.; Gorthi, S.S. Optofluidic single-cell absorption flow analyzer for point-of-care diagnosis of malaria. J. Biophotonics 2016, 9, 610–618. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Hu, X.; Lo, Y. Microfluidic cytometers with integrated on-chip optical systems for red blood cell and platelet counting. Biomicrofluidics 2016, 10, 064119. [Google Scholar] [CrossRef]
- Kwon, H.B.; Yoo, S.J.; Kim, Y.J. Microfluidic condensation nanoparticle counter using water as the condensing liquid for assessing individual exposure to airborne nanoparticles. Lab Chip 2020, 20, 1092–1102. [Google Scholar] [CrossRef]
- Guo, T.; Wei, Y.; Xu, C.; Watts, B.R.; Zhang, Z.; Fang, Q.; Zhang, H.; Selvaganapathy, P.R.; Deen, M.J. Counting of Escherichia coli by a microflow cytometer based on a photonic-microfluidic integrated device. Electrophoresis 2015, 36, 298–304. [Google Scholar] [CrossRef]
- Dannhauser, D.; Romeo, G.; Causa, F.; Netti, P.A. Small angle light scattering characterization of single micrometric particles in microfluidic flows. In Optical Methods for Inspection, Characterization, and Imaging of Biomaterials; Ferraro, P., RitschMarte, M., Grilli, S., Stifter, D., Eds.; SPIE: Dresden, Germany, 2013; Volume 8792. [Google Scholar]
- Dannhauser, D.; Romeo, G.; Causa, F.; de Santo, I.; Netti, P.A. Multiplex single particle analysis in microfluidics. Analyst 2014, 139, 5239–5246. [Google Scholar] [CrossRef]
- Shivhare, P.K.; Prabhakar, A.; Sen, A.K. Optofluidics based lab-on-chip device for in situ measurement of mean droplet size and droplet size distribution of an emulsion. J. Micromech. Microeng. 2017, 27, 035003. [Google Scholar] [CrossRef]
- Hengoju, S.; Shvydkiv, O.; Tovar, M.; Roth, M.; Rosenbaum, M.A. Advantages of optical fibers for facile and enhanced detection in droplet microfluidics. Biosens. Bioelectron. 2022, 200, 113910. [Google Scholar] [CrossRef]
- Szymborski, T.R.; Czaplicka, M.; Nowicka, A.B.; Trzcinska-Danielewicz, J.; Girstun, A.; Kaminska, A. Dielectrophoresis-Based SERS Sensors for the Detection of Cancer Cells in Microfluidic Chips. Biosensors 2022, 12, 681. [Google Scholar] [CrossRef]
- Freitag, I.; Beleites, C.; Dochow, S.; Clement, J.H.; Krafft, C.; Popp, J. Recognition of tumor cells by immuno-SERS-markers in a microfluidic chip at continuous flow. Analyst 2016, 141, 5986–5989. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Wang, Y.-N.; Wang, Y.; Xu, Z.-R. Highly reproducible and fast detection of 6-thioguanine in human serum using a droplet-based microfluidic SERS system. Sens. Actuators B Chem. 2019, 283, 532–537. [Google Scholar] [CrossRef]
- Yue, S.; Ye, W.; Xu, Z. SERS monitoring of the Fenton degradation reaction based on microfluidic droplets and alginate microparticles. Analyst 2019, 144, 5882–5889. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, E.L.; Veneman, P.A.; Simmonds, A.; Zacher, B.; Huebner, D.; Saavedra, S.S.; Armstrong, N.R. A Planar, Chip-Based, Dual-Beam Refractometer Using an Integrated Organic Light-Emitting Diode (OLED) Light Source and Organic Photovoltaic (OPV) Detectors. Anal. Chem. 2010, 82, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Meng, G.X.; Zhang, Q.; Pan, L.T.; Wang, P.; Liu, Z.B.; Jiang, W.S.; Chen, Y.; Tian, J.G. Ultrasensitive flow sensing of a single cell using graphene-based optical sensors. Nano Lett. 2014, 14, 3563–3569. [Google Scholar] [CrossRef]
- Yan, R.; Cui, E.; Zhao, S.; Zhou, F.; Wang, D.; Lei, C. Real-time and high-sensitivity refractive index sensing with an arched optofluidic waveguide. Opt. Express 2022, 30, 16031–16043. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, S.; Kataoka, Y.; Kazama, Y.; Fujii, Y.; Hibara, A. Efficient thermal lens nanoparticle detection in a flow-focusing microfluidic device. Sens. Actuators B Chem. 2016, 228, 581–586. [Google Scholar] [CrossRef]
- Mawatari, K.; Kitamori, T.; Sawada, T. Individual Detection of Single-Nanometer-Sized Particles in Liquid by Photothermal Microscope. Anal. Chem. 1998, 70, 5037–5041. [Google Scholar] [CrossRef]
- Seta, N.; Mawatari, K.; Kitamori, T. Individual Nanoparticle Detection in Liquids by Thermal Lens Microscopy and Improvement of Detection Efficiency Using a 1-mu m Microfluidic Channel. Anal. Sci. 2009, 25, 275–278. [Google Scholar] [CrossRef]
- Mahdieh, M.H.; Asl, M.S. Thermal lensing in liquid ethanol induced by high-power diode laser and its effect in beam quality factor M2. Indian J. Phys. 2022, 97, 235–242. [Google Scholar] [CrossRef]
- Shimizu, H.; Mawatari, K.; Kitamori, T. Femtoliter-scale separation and sensitive detection of nonfluorescent samples in an extended-nano fluidic device. Analyst 2014, 139, 2154–2157. [Google Scholar] [CrossRef]
- Guo, T.; Liu, F.; Liang, X.; Qiu, X.H.; Huang, Y.Y.; Xie, C.; Xu, P.; Mao, W.; Guan, B.O.; Albert, J. Highly sensitive detection of urinary protein variations using tilted fiber grating sensors with plasmonic nanocoatings. Biosens. Bioelectron. 2016, 78, 221–228. [Google Scholar] [CrossRef]
- Jahns, S.; Gutekunst, S.B.; Selhuber-Unkel, C.; Nazirizadeh, Y.; Gerken, M. Human blood microfluidic test chip for imaging, label-free biosensor. Microsyst. Technol.-Micro-Nanosyst.-Inf. Storage Process. Syst. 2016, 22, 1513–1518. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, Q.; Li, C.-W.; Yang, J.; Zhao, J.; Yang, M. Optical and electrochemical detection techniques for cell-based microfluidic systems. Anal. Bioanal. Chem. 2006, 384, 1259–1268. [Google Scholar] [CrossRef]
- Coulter, W.I. Means for Counting Partciles Suspended in a Fluid. U.S. Patent No. 2,656,508, 20 October 1953. [Google Scholar]
- Guo, J.; Lei, W.; Ma, X.; Xue, P.; Chen, Y.; Kang, Y. Design of a fluidic circuit-based microcytometer for circulating tumor cell detection and enumeration. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 35–41. [Google Scholar] [CrossRef]
- Liu, Z.J.; Li, J.; Yang, J.D.; Song, Y.X.; Pan, X.X.; Li, D.Q. Improving particle detection sensitivity of a microfluidic resistive pulse sensor by a novel electrokinetic flow focusing method. Microfluid. Nanofluidics 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, T.; Liu, Q.; Liu, Z.; Li, D. Nanoparticle and microorganism detection with a side-micron-orifice-based resistive pulse sensor. Analyst 2020, 145, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.; Choi, G.; Liang, S.; Liu, M.; Guan, W. Microfluidic multiple cross-correlated Coulter counter for improved particle size analysis. Sens. Actuators B Chem. 2019, 296, 162215. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, H.; Chon, C.H.; Chen, S.; Pan, X.; Li, D. Counting bacteria on a microfluidic chip. Anal. Chim. Acta 2010, 681, 82–86. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Detection and sizing of nanoparticles and DNA on PDMS nanofluidic chips based on differential resistive pulse sensing. Nanoscale 2017, 9, 5964–5974. [Google Scholar] [CrossRef]
- Ashley, B.K.; Hassan, U. Frequency-Time Domain (FTD) Impedance Data Analysis to Improve Accuracy of Microparticle Enumeration in a Microfluidic Electronic Counter. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Ashley, B.K.; Hassan, U. Time-domain signal averaging to improve microparticles detection and enumeration accuracy in a microfluidic impedance cytometer. Biotechnol. Bioeng. 2021, 118, 4428–4440. [Google Scholar] [CrossRef]
- Pollard, M.; Hunsicker, E.; Platt, M. A tunable three-dimensional printed microfluidic resistive pulse sensor for the characterization of algae and microplastics. ACS Sens. 2020, 5, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Riordon, J.; M-Catafard, N.; Godin, M. Using the fringing electric field in microfluidic volume sensors to enhance sensitivity and accuracy. Appl. Phys. Lett. 2012, 101, 154105. [Google Scholar] [CrossRef]
- Bernabini, C.; Holmes, D.; Morgan, H. Micro-impedance cytometry for detection and analysis of micron-sized particles and bacteria. Lab Chip 2011, 11, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Riordon, J.; Nash, M.; Calderini, M.; Godin, M. Using active microfluidic flow focusing to sort particles and cells based on high-resolution volume measurements. Microelectron. Eng. 2014, 118, 35–40. [Google Scholar] [CrossRef]
- Jagtiani, A.V.; Carletta, J.; Zhe, J. A microfluidic multichannel resistive pulse sensor using frequency division multiplexing for high throughput counting of micro particles. J. Micromech. Microeng. 2011, 21, 065004. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Pan, X.; Li, D. High-throughput and sensitive particle counting by a novel microfluidic differential resistive pulse sensor with multidetecting channels and a common reference channel. Electrophoresis 2015, 36, 495–501. [Google Scholar] [CrossRef]
- Olmos, C.M.; Rosero, G.; Fernandez-Cabada, T.; Booth, R.; Der, M.; Cabaleiro, J.M.; Debut, A.; Cumbal, L.; Perez, M.S.; Lerner, B. Hybrid microchannel-solid state micropore device for fast and optical cell detection. RSC Adv. 2020, 10, 5361–5370. [Google Scholar] [CrossRef]
- Song, Y.; Han, X.; Li, D.; Liu, Q.; Li, D. Simultaneous and continuous particle separation and counting via localized DC-dielectrophoresis in a microfluidic chip. RSC Adv. 2021, 11, 3827–3833. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2020, 122, 115701. [Google Scholar] [CrossRef]
- Do, Q.L.; Bui, T.T.; Tran, T.T.H.; Kikuchi, K.; Aoyagi, M.; Duc, T.C. Differential Capacitively Coupled Contactless Conductivity Detection ((DCD)-D-4) Sensor for Detection of Object in Microfluidic Channel. In Proceedings of the 2015 IEEE Sensors, Busan, Republic of Korea, 1–4 November 2015; pp. 1546–1549. [Google Scholar]
- Shi, H.; Yu, S.; Xie, Y.; Li, W.; Zhang, H.; Zheng, Y.; Zhang, S.; Li, G.; Sun, Y.; Chen, H. Comprehensive detection method for multi-contaminants in hydraulic oil based on inductance-resistance-capacitance analysis. Tribol. Int. 2022, 173, 107609. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Yoo, S.-J.; Kim, Y.-J. An integrated electrical condensation particle counter for compact and low-cost ultrafine particle measurement system. J. Aerosol Sci. 2022, 163, 105996. [Google Scholar] [CrossRef]
- Murali, S.V.; Xia, X.; Jagtiani, A.V.; Carletta, J.; Zhe, J. A Microfluidic Device for Wear Detection in Lubricants. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Boston, MA, USA, 31 October–6 November 2008; pp. 859–863. [Google Scholar] [CrossRef]
- Murali, S.; Jagtiani, A.V.; Xia, X.; Carletta, J.; Zhe, J. A microfluidic Coulter counting device for metal wear detection in lubrication oil. Rev. Sci. Instrum. 2009, 80, 016105. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Silva, C.A.; Dong, T. Integratable capacitive bubble counter for lab-on-chip devices. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisbon, Portugal, 11–12 June 2014. [Google Scholar]
- Song, Y.; Li, M.; Yang, J.; Wang, J.; Pan, X.; Sun, Y.; Li, D. Capacitive detection of living microalgae in a microfluidic chip. Sens. Actuators B Chem. 2014, 194, 164–172. [Google Scholar] [CrossRef]
- Du, L.; Zhe, J.A.; Carletta, J.; Veillette, R.; Choy, F. Real-time monitoring of wear debris in lubrication oil using a microfluidic inductive Coulter counting device. Microfluid. Nanofluidics 2010, 9, 1241–1245. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Ma, L.; Zeng, L. A multi-function sensor for online detection of contaminants in hydraulic oil. Tribol. Int. 2019, 138, 196–203. [Google Scholar] [CrossRef]
- Wang, C.; Bai, C.; Yang, Z.; Zhang, H.; Li, W.; Wang, X.; Zheng, Y.; Ilerioluwa, L.; Sun, Y. Research on High Sensitivity Oil Debris Detection Sensor Using High Magnetic Permeability Material and Coil Mutual Inductance. Sensors 2022, 22, 1833. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Z.; Yu, K.; Fan, Z.; Yuan, Z.; Sui, Z.; Yin, Y.; Pan, X. A Novel Multichannel Inductive Wear Debris Sensor Based on Time Division Multiplexing. IEEE Sens. J. 2021, 21, 11131–11139. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Wang, W.; Zeng, L.; Sun, G.; Chen, H. An Integrated Inductive-Capacitive Microfluidic Sensor for Detection of Wear Debris in Hydraulic Oil. IEEE Sens. J. 2019, 19, 11583–11590. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, H.; Zeng, L.; Zhao, X.; Ma, L. Inductive Magnetic Nanoparticle Sensor based on Microfluidic Chip Oil Detection Technology. Micromachines 2020, 11, 183. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Wang, S.; Yin, Y.; Liu, D.; Wu, S.; Liu, Z.; Pan, X. Improving Sensitivity of a Micro Inductive Sensor for Wear Debris Detection with Magnetic Powder Surrounded. Micromachines 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Zhang, H.; Wang, C.; Li, W. A Highly Sensitive Wear Debris Sensor Based on Differential Detection. IEEE Sens. J. 2023, 23, 16746–16754. [Google Scholar] [CrossRef]
- Du, L.; Zhu, X.; Han, Y.; Zhe, J. High Throughput Wear Debris Detection in Lubricants Using a Resonance Frequency Division Multiplexed Sensor. Tribol. Lett. 2013, 51, 453–460. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Z.; Yuan, H.; Yu, K.; Gao, Y.; Liu, L.; Pan, X. Multichannel Inductive Sensor Based on Phase Division Multiplexing for Wear Debris Detection. Micromachines 2019, 10, 246. [Google Scholar] [CrossRef]
- Jagtiani, A.V.; Carletta, J.; Zhe, J. An impedimetric approach for accurate particle sizing using a microfluidic Coulter counter. J. Micromech. Microeng. 2011, 21, 045036. [Google Scholar] [CrossRef]
- Winkler, T.E.; Ben-Yoav, H.; Ghodssi, R. Hydrodynamic focusing for microfluidic impedance cytometry: A system integration study. Microfluid. Nanofluidics 2016, 20, 1–14. [Google Scholar] [CrossRef]
- Haandbæk, N.; With, O.; Burgel, S.C.; Heer, F.; Hierlemann, A. Resonance-enhanced microfluidic impedance cytometer for detection of single bacteria. Lab Chip 2014, 14, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Raihan, M.K.; Zhu, D.; Yu, K.; Wang, F.; Pan, X. The Optimization of Parallel Resonance Circuit for Wear Debris Detection by Adjusting Capacitance. Energies 2022, 15, 7318. [Google Scholar] [CrossRef]
- Demori, M.; Ferrari, V.; Farise, S.; Poesio, P.; Pedrazzani, R.; Steimberg, N.; Boniotti, J.; Mazzoleni, G. Microfluidic Sensor for Noncontact Detection of Cell Flow in a Microchannel. In Proceedings of the 26th European Conference on Solid-State Transducers, Eurosensor 2012, Kraków, Poland, 9–12 September 2012; Walczak, R., Dziuban, J., Eds.; Procedia Engineering. Volume 47, pp. 1247–1250. [Google Scholar]
- Emaminejad, S.; Javanmard, M.; Dutton, R.W.; Davis, R.W. Microfluidic diagnostic tool for the developing world: Contactless impedance flow cytometry. Lab Chip 2012, 12, 4499–4507. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Guo, J. Lab-on-PCB-based micro-cytometer for circulating tumor cells detection and enumeration. Microfluid. Nanofluidics 2017, 21. [Google Scholar] [CrossRef]
- Guler, M.T.; Bilican, I.; Agan, S.; Elbuken, C. A simple approach for the fabrication of 3D microelectrodes for impedimetric sensing. J. Micromech. Microeng. 2015, 25, 095019. [Google Scholar] [CrossRef]
- Tang, W.; Tang, D.; Ni, Z.; Xiang, N.; Yi, H. Microfluidic Impedance Cytometer with Inertial Focusing and Liquid Electrodes for High-Throughput Cell Counting and Discrimination. Anal. Chem. 2017, 89, 3154–3161. [Google Scholar] [CrossRef]
- Sobahi, N.; Han, A. High-throughput and label-free multi-outlet cell counting using a single pair of impedance electrodes. Biosens. Bioelectron. 2020, 166, 112458. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Wang, Y.; Rosano, J.M.; Prabhakarpandian, B.; Garson, C.; Pant, K.; Lai, E. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells. Lab Chip 2013, 13, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Yakdi, N.E.; Bricault, D.; Huet, F.; Ngo, K. Detection and sizing of single droplets flowing in a microfluidic device by impedance measurement. Procedia Eng. 2016, 168, 1466–1470. [Google Scholar] [CrossRef]
- Wang, H.; Sobahi, N.; Han, A. Impedance spectroscopy-based cell/particle position detection in microfluidic systems. Lab Chip 2017, 17, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Jin, T.; Yan, R.; Qi, W.; Huang, T.; Ding, H.; Tan, S.H.; Nguyen, N.T.; Xi, L. Opto-acousto-fluidic microscopy for three-dimensional label-free detection of droplets and cells in microchannels. Lab Chip 2018, 18, 1292–1297. [Google Scholar] [CrossRef]
- Liu, F.; Jin, T.; Yan, R.; Li, T.; Hu, B.; Yao, L.; Huang, T.; Song, C.; Xi, L. An opto-acousto-fluidic microscopic system with a high spatiotemporal resolution for microfluidic applications. Opt. Express 2019, 27, 1425–1432. [Google Scholar] [CrossRef]
- NIGnyawali, V.; Strohm, E.M.; Wang, J.Z.; Tsai, S.S.H.; Kolios, M.C. Simultaneous acoustic and photoacoustic microfluidic flow cytometry for label-free analysis. Sci. Rep. 2019, 9, 1585. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Duarte, C.M.; Cardoso, F.A.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Lab-on-Chip Cytometry Based on Magnetoresistive Sensors for Bacteria Detection in Milk. Sensors 2014, 14, 15496–15524. [Google Scholar] [CrossRef]
- Huang, H.T.; Garu, P.; Li, C.H.; Chang, W.C.; Chen, B.W.; Sung, S.Y.; Lee, C.M.; Chen, J.Y.; Hsieh, T.F.; Sheu, W.J.; et al. Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. Spin 2019, 9, 1940002. [Google Scholar] [CrossRef]
- Loureiro, J.; Fermon, C.; Pannetier-Lecoeur, M.; Arrias, G.; Ferreira, R.; Cardoso, S.; Freitas, P.P. Magnetoresistive Detection of Magnetic Beads Flowing at High Speed in Microfluidic Channels. IEEE Trans. Magn. 2009, 45, 4873–4876. [Google Scholar] [CrossRef]
- Loureiro, J.; Andrade, P.Z.; Cardoso, S.; da Silva, C.L.; Cabral, J.M.; Freitas, P.P. Magnetoresistive chip cytometer. Lab Chip 2011, 11, 2255–2261. [Google Scholar] [CrossRef]
- Duarte, C.M.; Fernandes, A.C.; Cardoso, F.A.; Bexiga, R.; Cardoso, S.F.; Freitas, P.J.P. Magnetic Counter for Group B Streptococci Detection in Milk. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Kokkinis, G.; Cardoso, S.; Keplinger, F.; Giouroudi, I. Microfluidic platform with integrated GMR sensors for quantification of cancer cells. Sens. Actuators B Chem. 2017, 241, 438–445. [Google Scholar] [CrossRef]
- Gooneratne, C.P.; Kodzius, R.; Li, F.; Foulds, I.G.; Kosel, J. On-Chip Magnetic Bead Manipulation and Detection Using a Magnetoresistive Sensor-Based Micro-Chip: Design Considerations and Experimental Characterization. Sensors 2016, 16, 1369. [Google Scholar] [CrossRef]
- Lee, C.P.; Lai, M.F.; Huang, H.T.; Lin, C.W.; Wei, Z.H. Wheatstone bridge giant-magnetoresistance based cell counter. Biosens. Bioelectron. 2014, 57, 48–53. [Google Scholar] [CrossRef]
- Ren, C.; Bayin, Q.; Feng, S.; Fu, Y.; Ma, X.; Guo, J. Biomarkers detection with magnetoresistance-based sensors. Biosens. Bioelectron. 2020, 165, 112340. [Google Scholar] [CrossRef]
- Murzin, D.; Mapps, D.J.; Levada, K.; Belyaev, V.; Omelyanchik, A.; Panina, L.; Rodionova, V. Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569. [Google Scholar] [CrossRef]
- Davaji, B. Thermal Microfluidic Devices: Design, Fabrication and Applications. Ph.D. Thesis, Marquette University, Milwaukee, WI, USA, 2016. [Google Scholar]
- Yi, N.; Park, B.K.; Kim, D.; Park, J. Micro-droplet detection and characterization using thermal responses. Lab Chip 2011, 11, 2378–2384. [Google Scholar] [CrossRef]
- Vutha, A.K.; Davaji, B.; Lee, C.H.; Walker, G.M. A microfluidic device for thermal particle detection. Microfluid. Nanofluidics 2014, 17, 871–878. [Google Scholar] [CrossRef]
- Inomata, N.; Toda, M.; Ono, T. Microfabricated Temperature-Sensing Devices Using a Microfluidic Chip for Biological Applications. Int. J. Autom. Technol. 2018, 12, 15–23. [Google Scholar] [CrossRef]
- Inomata, N.; Pan, L.; Wang, Z.; Kimura, M.; Ono, T. Vanadium oxide thermal microsensor integrated in a microfluidic chip for detecting cholesterol and glucose concentrations. Microsyst. Technol. 2016, 23, 2873–2879. [Google Scholar] [CrossRef]
- Inomata, N.; van Toan, N.; Ono, T. Liquid Thermocouple Using Thermoelectric Ionic Liquids. IEEE Sens. Lett. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Nagdalian, A.A.; Povetkin, S.N.; Gvozdenko, A.A.; Verevkina, M.N.; Rzhepakovsky, I.V.; Lopteva, M.S.; Maglakelidze, D.G.; Kataeva, T.S.; Blinova, A.A.; et al. Surface-Oxidized Polymer-Stabilized Silver Nanoparticles as a Covering Component of Suture Materials. Micromachines 2022, 13, 1105. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical Systems (MEMS) for Biomedical Applications. Micromachines 2022, 13, 164. [Google Scholar] [CrossRef]
- Yunjia, L. Microelectromechanical systems (MEMS). Mater. Integr. Intell. Syst.-Technol. Appl. Technol. Appl. 2018, 81–106. [Google Scholar] [CrossRef]
- Yi-Qiang, F.; Hong-Liang, W.; Ke-Xin, G.; Jing-Ji, L.; Dong-Ping, C.; ZHANG, Y.-J. Applications of modular microfluidics technology. Chin. J. Anal. Chem. 2018, 46, 1863–1871. [Google Scholar] [CrossRef]
- Shende, P.; Kasture, P. Dendrimeric biosensor for detection of E. coli O157: H7 in diet. Biointerface Res. Appl. Chem. 2020, 10, 5128–5131. Available online: https://biointerfaceresearch.com/wp-content/uploads/2020/01/20695837102128131.pdf (accessed on 28 July 2023).
- Leïchlé, T.; Nicu, L.; Alava, T. MEMS Biosensors and COVID-19: Missed Opportunity. ACS Sensors 2020, 5, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Kaisti, M.; Panula, T.; Leppänen, J.; Punkkinen, R.; Tadi, M.J.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P.; et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. Npj Digit. Med. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Doan, M.; Vorobjev, I.; Rees, P.; Filby, A.; Wolkenhauer, O.; Goldfeld, A.E.; Lieberman, J.; Barteneva, N.; Carpenter, A.E.; Hennig, H. Diagnostic Potential of Imaging Flow Cytometry. Trends Biotechnol. 2018, 36, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xie, D.; Wang, S.; Li, X.; Wu, G. Advances in early detection methods for solid tumors. Front. Genet. 2023, 14, 1091223. [Google Scholar] [CrossRef]
- Lippeveld, M.; Knill, C.; Ladlow, E.; Fuller, A.; Michaelis, L.J.; Saeys, Y.; Filby, A.; Peralta, D. Classification of Human White Blood Cells Using Machine Learning for Stain-Free Imaging Flow Cytometry. Cytom. Part A 2019, 97, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takahashi, L. Toward the Golden Age of Materials Informatics: Perspective and Opportunities. J. Phys. Chem. Lett. 2023, 14, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Frickenstein, A.N.; Wilhelm, S. High-throughput single-cell analysis of nanoparticle-cell interactions. TrAC Trends Anal. Chem. 2023, 166, 117172. [Google Scholar] [CrossRef]
- Zaman, Q.; Zia, K.M.; Zuber, M.; Mabkhot, Y.N.; Almalki, F.; Ben Hadda, T. A comprehensive review on synthesis, characterization, and applications of polydimethylsiloxane and copolymers. Int. J. Plast. Technol. 2019, 23, 261–282. [Google Scholar] [CrossRef]
- Peydro, M.; Parres, F.; Crespo, J.; Navarro, R. Recovery of recycled acrylonitrile–butadiene–styrene, through mixing with styrene–ethylene/butylene–styrene. J. Mater. Process. Technol. 2013, 213, 1268–1283. [Google Scholar] [CrossRef]
- Liu, B.; Shangguan, Y.; Song, Y.; Zheng, Q. Influences of compatibilizers on rheology and mechanical properties of propylene random copolymer/styrene-ethylene-butylene-styrene block copolymer/organic-montmorillonite nanocomposites. J. Appl. Polym. Sci. 2012, 129, 973–982. [Google Scholar] [CrossRef]
- Lu, X.; Qu, J. Morphology and properties of PBT/SEBS and PBT/SEBS-G-MAH blends. AIP Conf. Proc. 2016, 1713, 050004. [Google Scholar] [CrossRef]
- Hopcroft, M.A.; Nix, W.D.; Kenny, T.W. What is the Young’s Modulus of Silicon? J. Microelectromech. Syst. 2010, 19, 229–238. Available online: http://mems.stanford.edu/~hopcroft/Publications/Hopcroft_Youngs_Modulus_Silicon.pdf (accessed on 28 July 2023). [CrossRef]
- Yao, W.; Peddi, R. Customizable silicone materials for MEMS and semiconductor packages. In Proceedings of the 2016 IEEE 37th International Electronics Manufacturing Technology (IEMT) & 18th Electronics Materials and Packaging (EMAP) Conference, Georgetown, Malaysia, 20–22 September 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Zheng, B.; Guo, Q.; Duan, X.; Wulamu, A.; Zhang, D. Wearable Devices for Gait Analysis in Intelligent Healthcare. Front. Comput. Sci. 2021, 3, 1676. [Google Scholar] [CrossRef]
- Schmidt-Speicher, L.M.; Länge, K. Microfluidic integration for electrochemical biosensor applications. Curr. Opin. Electrochem. 2021, 29. [Google Scholar] [CrossRef]
- Xing, G.; Ai, J.; Wang, N.; Pu, Q. Recent progress of smartphone-assisted microfluidic sensors for point of care testing. TrAC Trends Anal. Chem. 2022, 157. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, Z.; Jiang, Y.; Su, X.; Wang, Z.; Wang, Y.; Sun, Z.; Zhao, Z.; Zhang, C.; Hong, Y.; Liu, Z. Particle Counting Methods Based on Microfluidic Devices. Micromachines 2023, 14, 1722. https://doi.org/10.3390/mi14091722
Dang Z, Jiang Y, Su X, Wang Z, Wang Y, Sun Z, Zhao Z, Zhang C, Hong Y, Liu Z. Particle Counting Methods Based on Microfluidic Devices. Micromachines. 2023; 14(9):1722. https://doi.org/10.3390/mi14091722
Chicago/Turabian StyleDang, Zenglin, Yuning Jiang, Xin Su, Zhihao Wang, Yucheng Wang, Zhe Sun, Zheng Zhao, Chi Zhang, Yuming Hong, and Zhijian Liu. 2023. "Particle Counting Methods Based on Microfluidic Devices" Micromachines 14, no. 9: 1722. https://doi.org/10.3390/mi14091722
APA StyleDang, Z., Jiang, Y., Su, X., Wang, Z., Wang, Y., Sun, Z., Zhao, Z., Zhang, C., Hong, Y., & Liu, Z. (2023). Particle Counting Methods Based on Microfluidic Devices. Micromachines, 14(9), 1722. https://doi.org/10.3390/mi14091722





