Design of a Multisensory Device for Tomato Volatile Compound Detection Based on a Mixed Metal Oxide—Electrochemical Sensor Array and Optical Reader
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
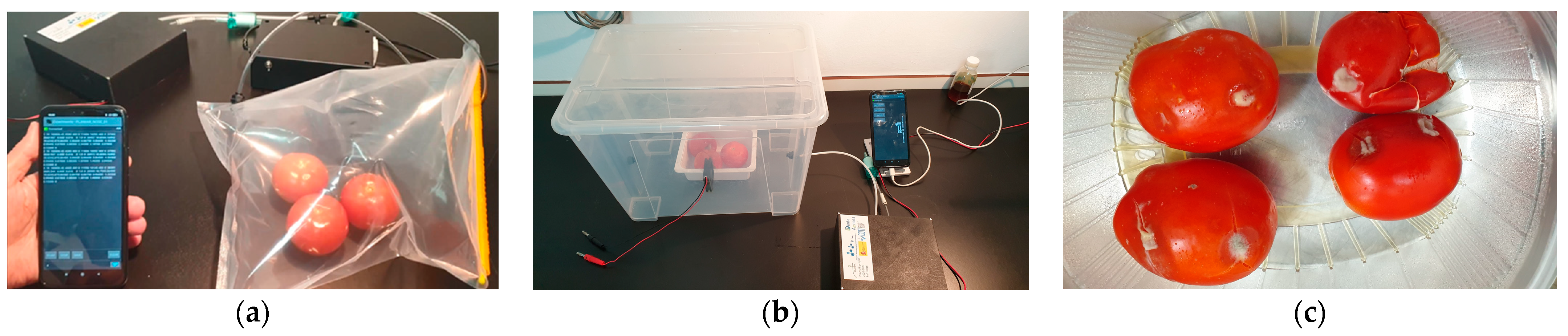
2.1.1. Samples of Bags and Air Containers
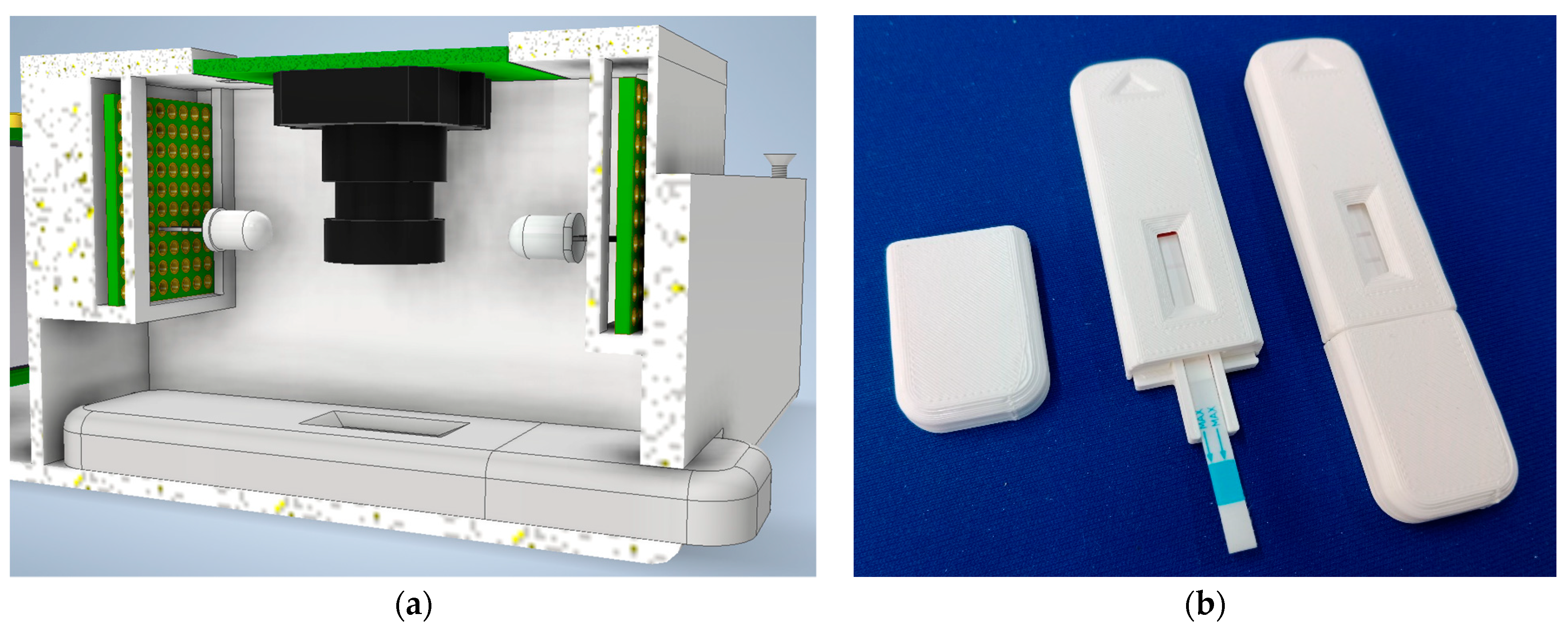
2.1.2. Lateral Flow Test Device (LFD)
2.2. Botrytis Cinerea Infection
2.3. Description of the Prototype
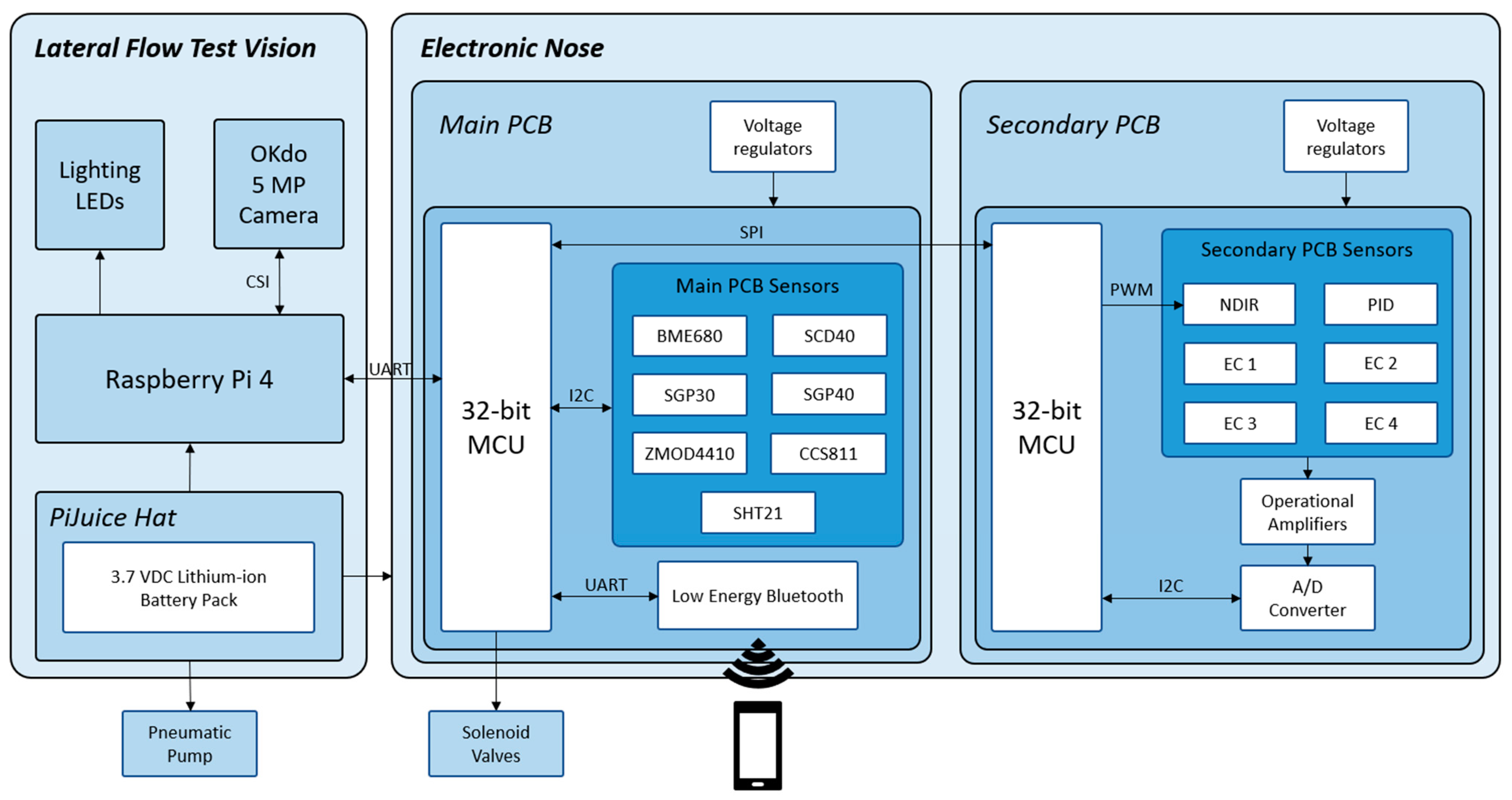
2.3.1. Electronic Nose Description
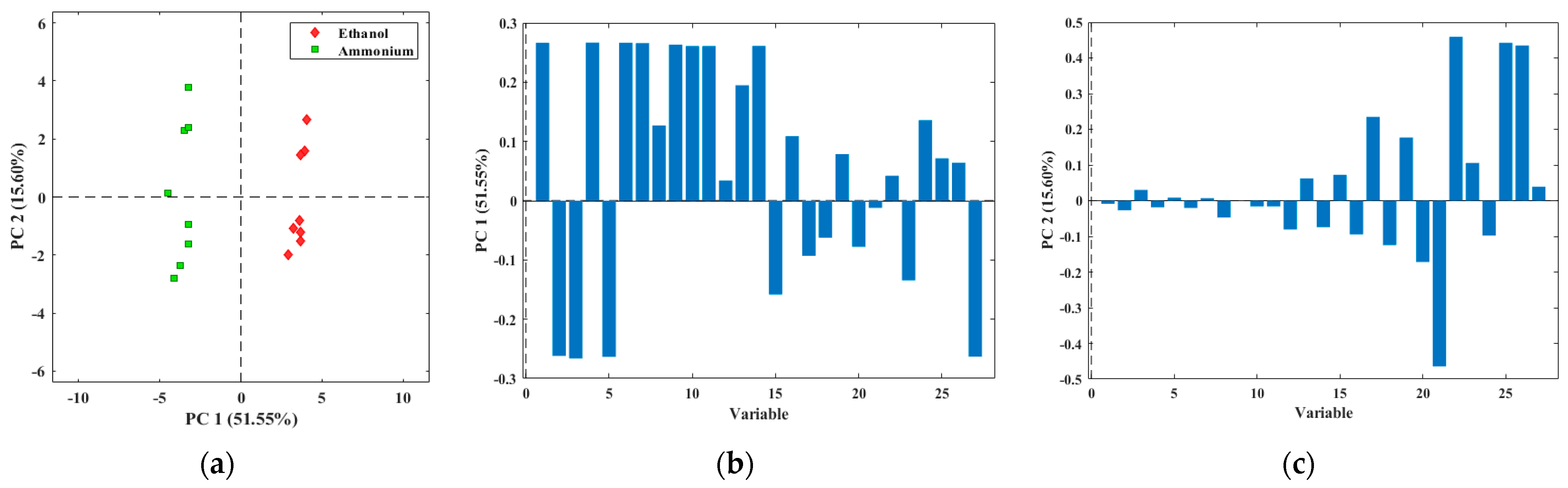
2.3.2. Multivariate Data Analysis
2.3.3. Vision Part Description
2.3.4. Holder Cassette Design
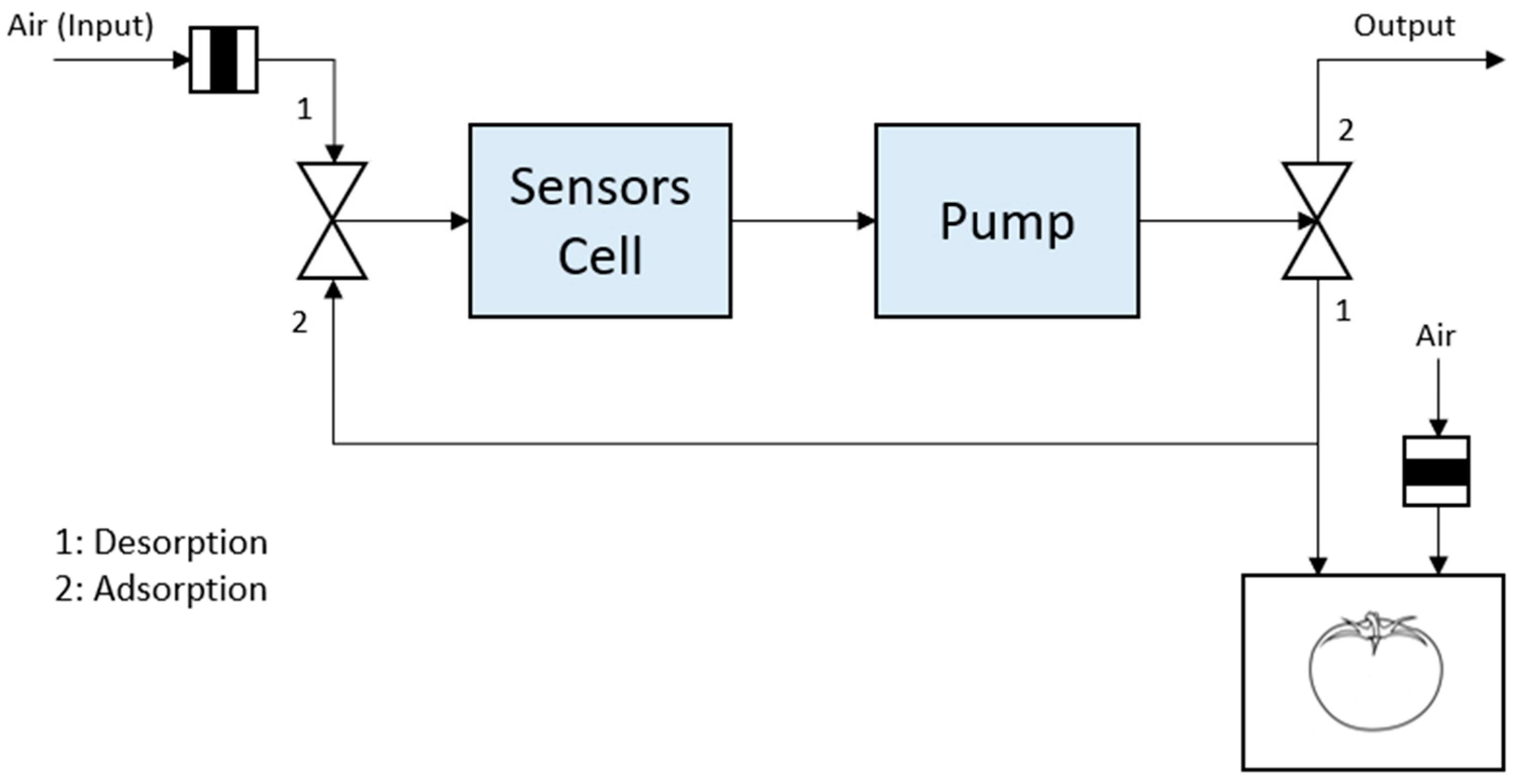
2.3.5. Pneumatics Description
2.3.6. Prototype External Design
2.3.7. Image Processing
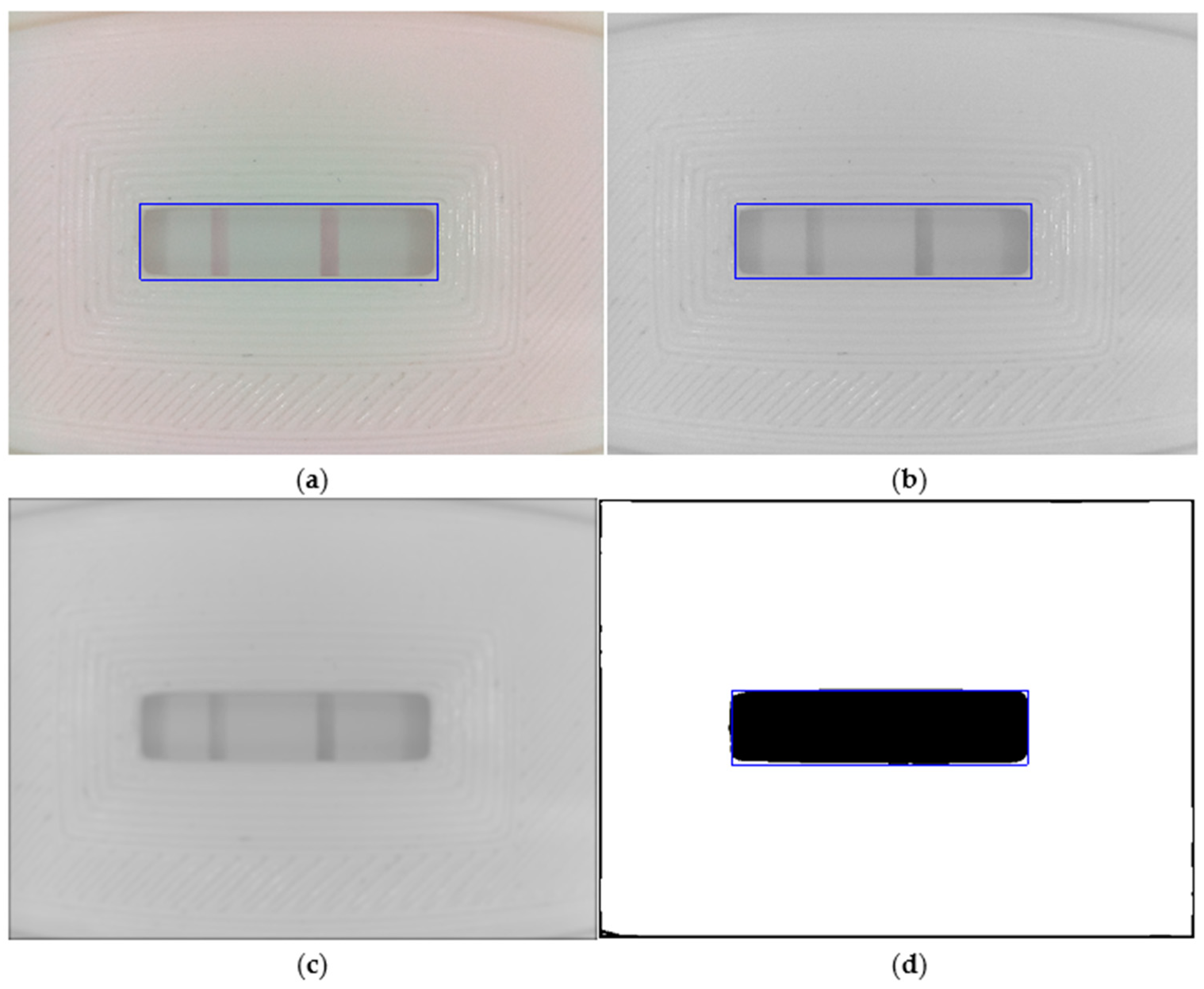
- Gaussian smoothing. The image is low-pass-filtered to reduce bright and dark spots that appear on the textured surface of the test cassette (Figure 5c). We use a 2D Gaussian filter with sigma = 10.
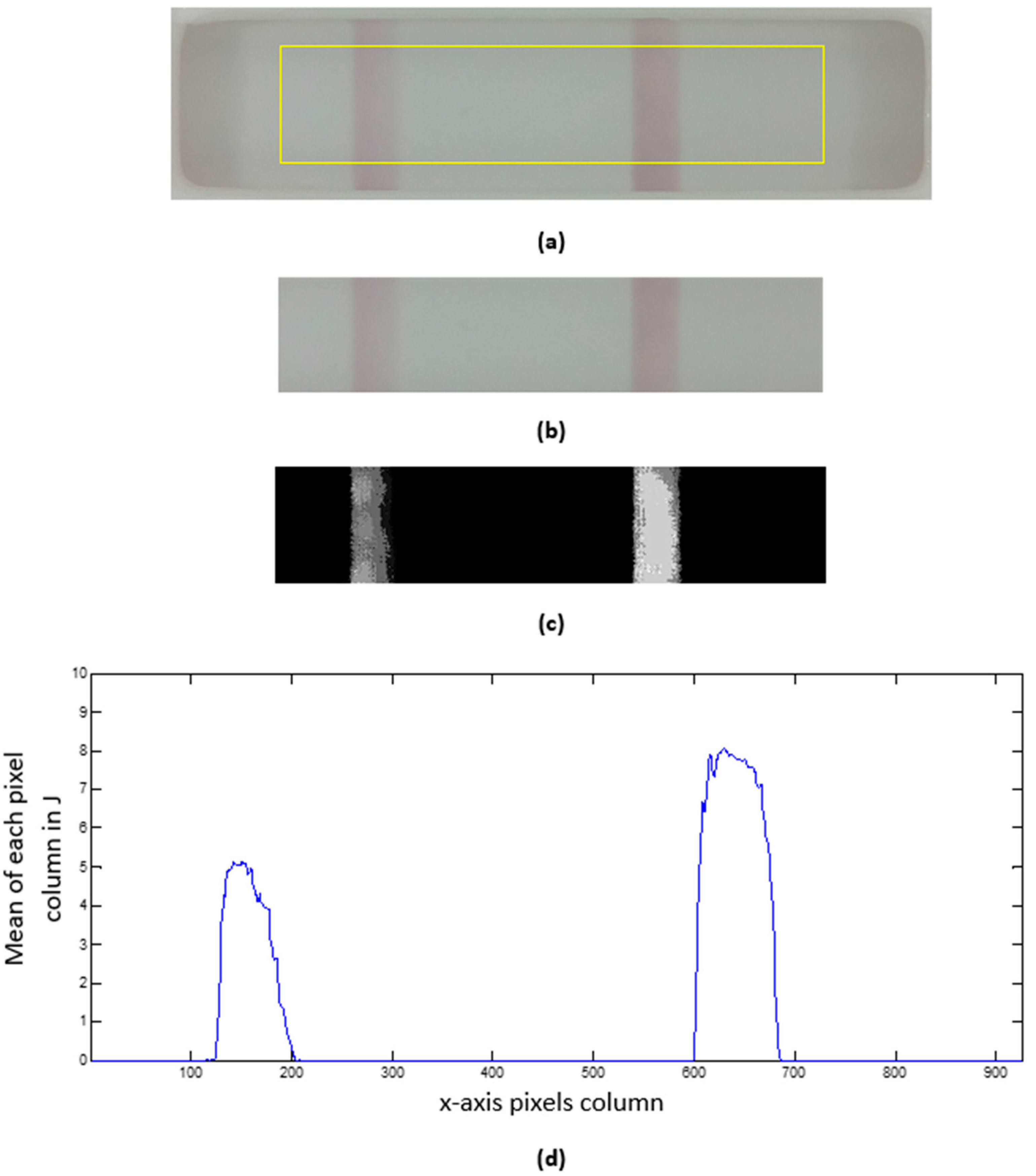
- Window location. As Figure 5d shows, the binarized image separates the test window area (in black) from the cassette. However, to safely locate the window sub-image, we first reconstruct the black border area, which is a product of the Gaussian filtering stage. Next, we compute the horizontal and vertical sum arrays from the reconstructed binary image. Finally, we select the window sub-image as those rows (columns) with horizontal (vertical) sum array values less than the mean value of that array. The resulting test window sub-image (1294 × 330 px) is shown in Figure 5a–d as a blue rectangle overlaid on each image.
- Cropping. The resulting image from Part 1 is a safely cropped sub-image where the test window is entirely preserved, but small portions of the cassette are regularly included at its border. While this sub-image may be useful for data logging, a further cropped image is more beneficial for data analysis when cropping is used to reject outliers. As Figure 6a shows, we discard the top and bottom fifths (1/5) of the image height and the left and right sevenths (1/7) of the image width to remove the cassette’s edge pixels and the lateral shadows it projects onto the test window. Thus, for the depicted example of size 1294 × 330 px, the centrally cropped sub-image (yellow rectangle in Figure 6a) has dimensions of 926 × 199 px.
- Background subtraction. The selected chromatography lateral flow test technique allows two visible bars to appear in the previous test window cropped image (Figure 6b). These bars are called the control and test lines, and our properly white-balanced camera perceives them as slightly red pixels on a mostly gray background. If the control line is not visible, the test is null; otherwise, the test is positive only if the test line appears in the image. To further facilitate analysis, we transform the cropped RGB image into an 8-bit grayscale image (J) by subtracting the green (G) channel from the red (R) channel. Figure 6c shows a contrast-stretched version of J for the represented example, with discernible test (left) and control (right) bars, showcasing the benefits of the method.
- Test profile. As a means of data reduction, we finally obtain a test profile array P that is computed as the mean of each pixel column in J (see Figure 6d). In this way, P is a more compact representation of the test image, allowing a straightforward analysis of the strength of the perceived test bars that can be characterized by, e.g., the local maximum of each peak in P.
2.3.8. Communication Protocol
2.4. Experimental Procedure
2.4.1. Laboratory Test
2.4.2. Tomato Analysis with the Electronic Nose
3. Results
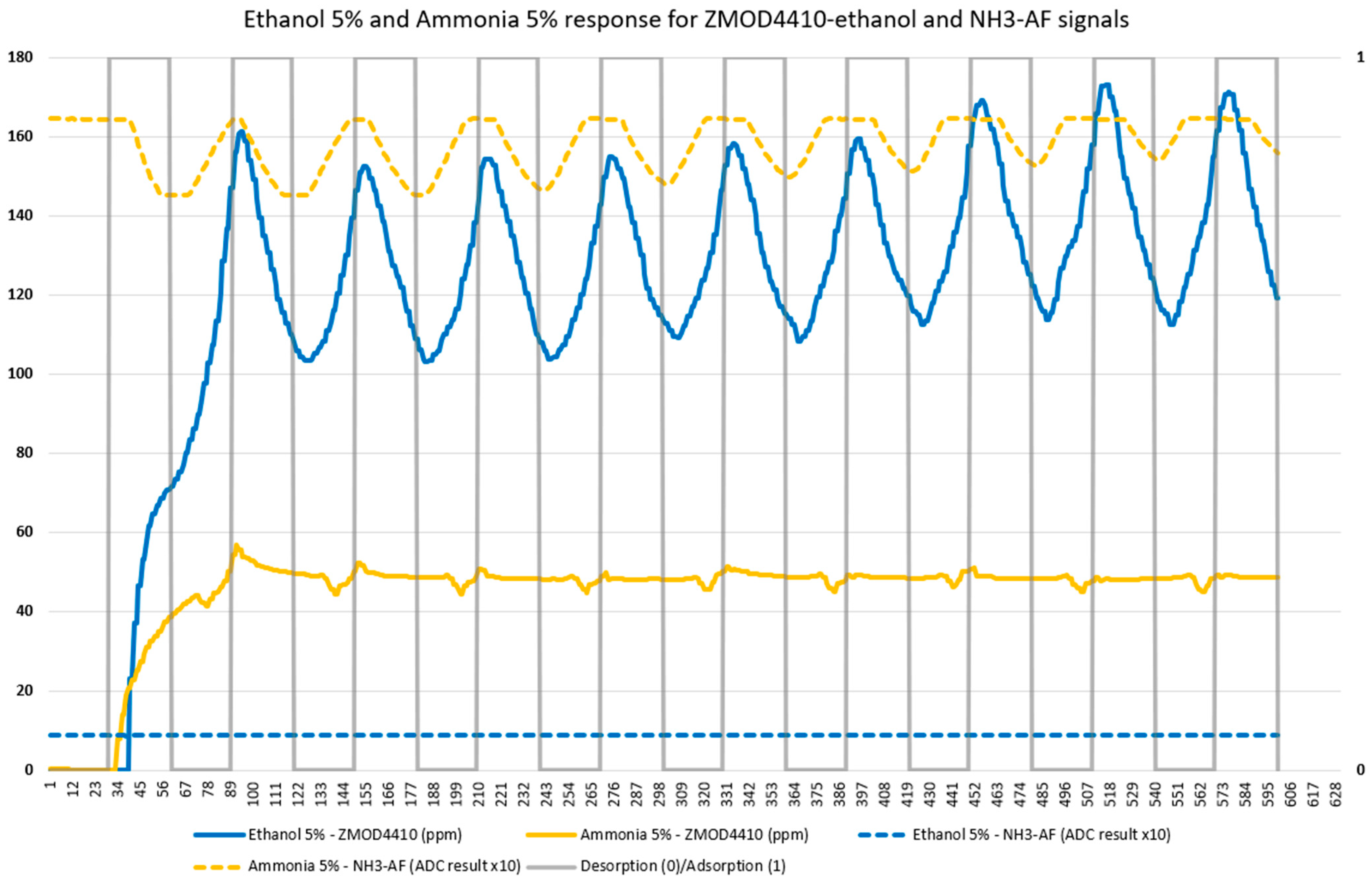
3.1. Laboratory Test Results
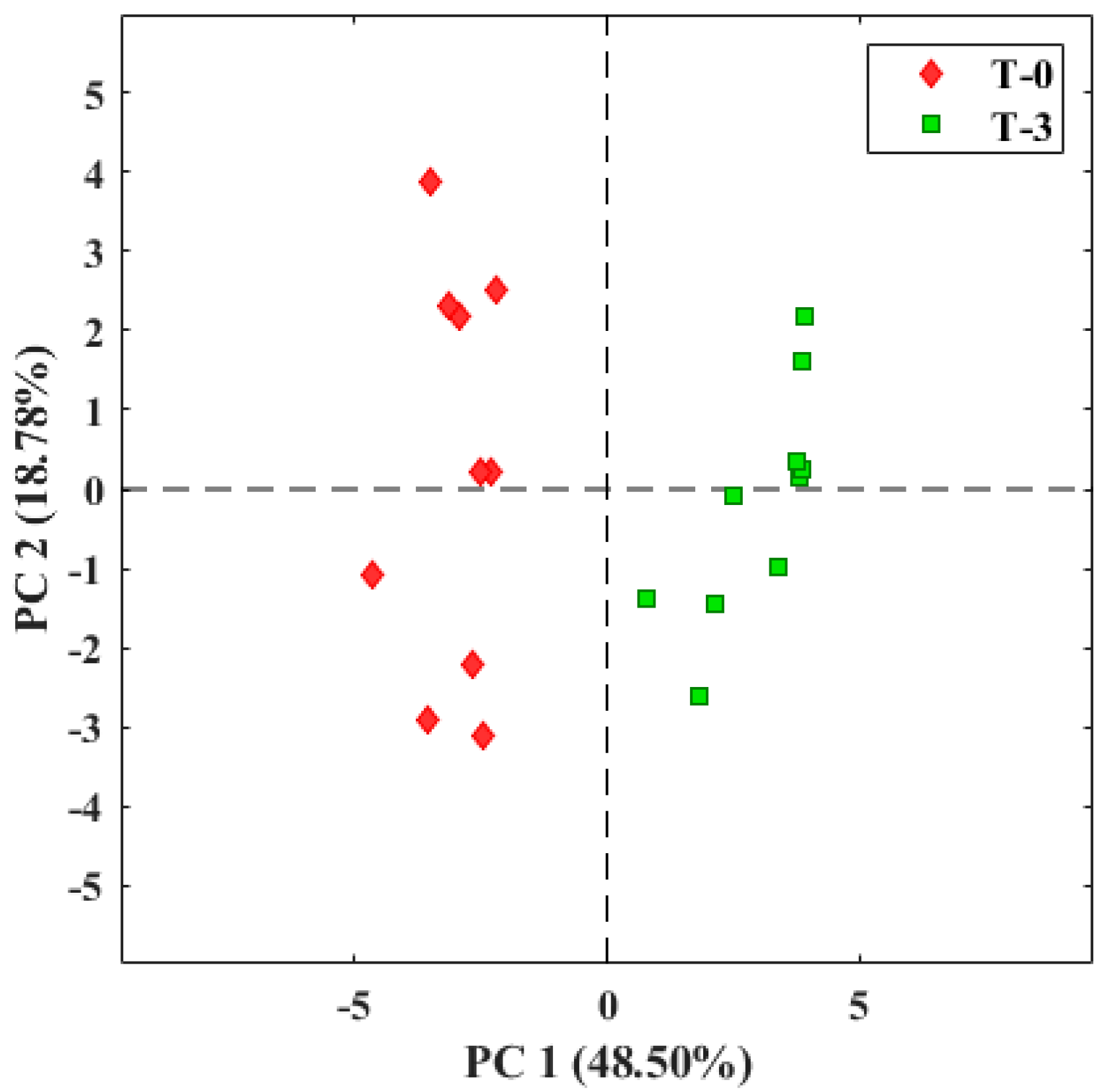
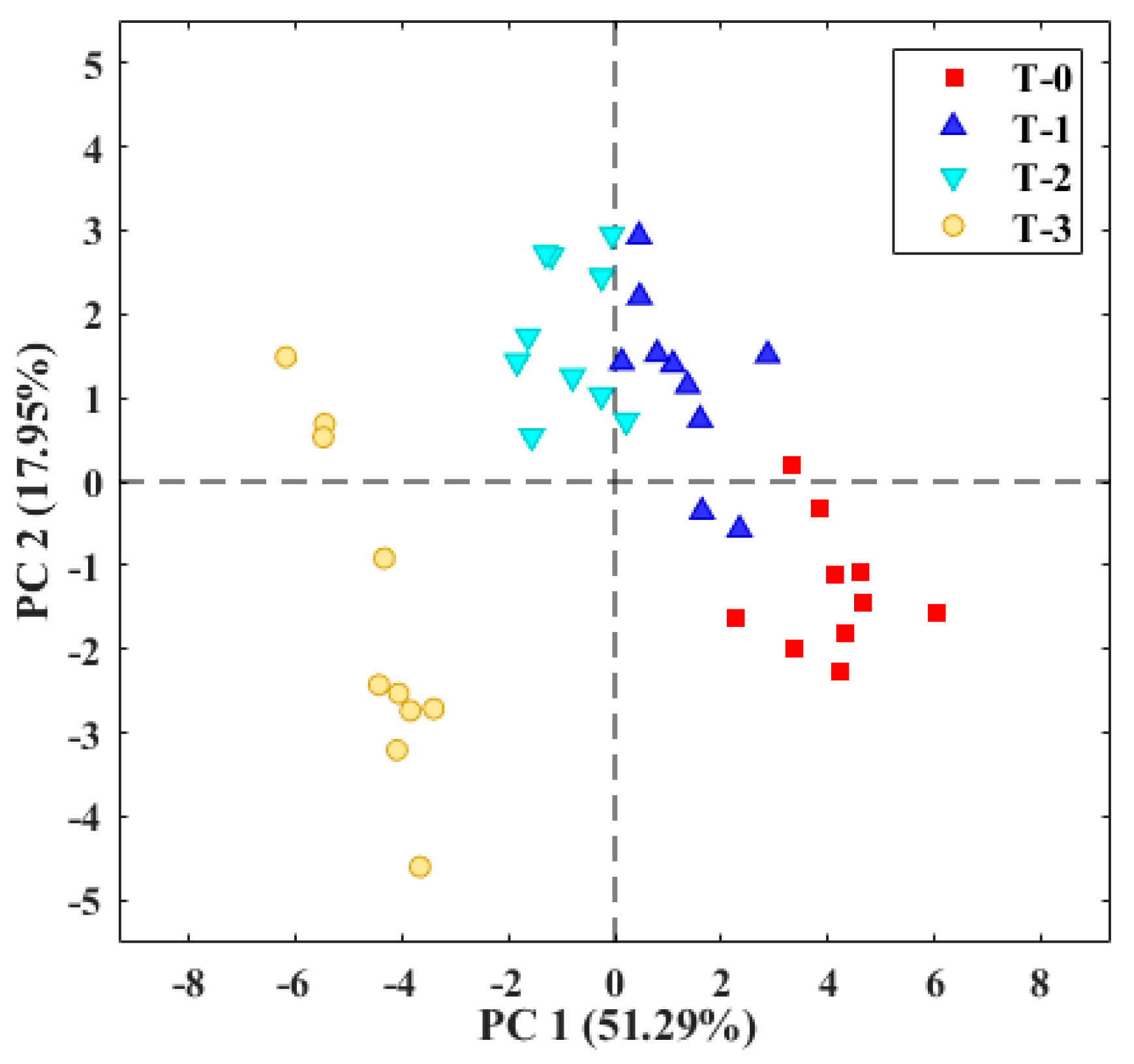
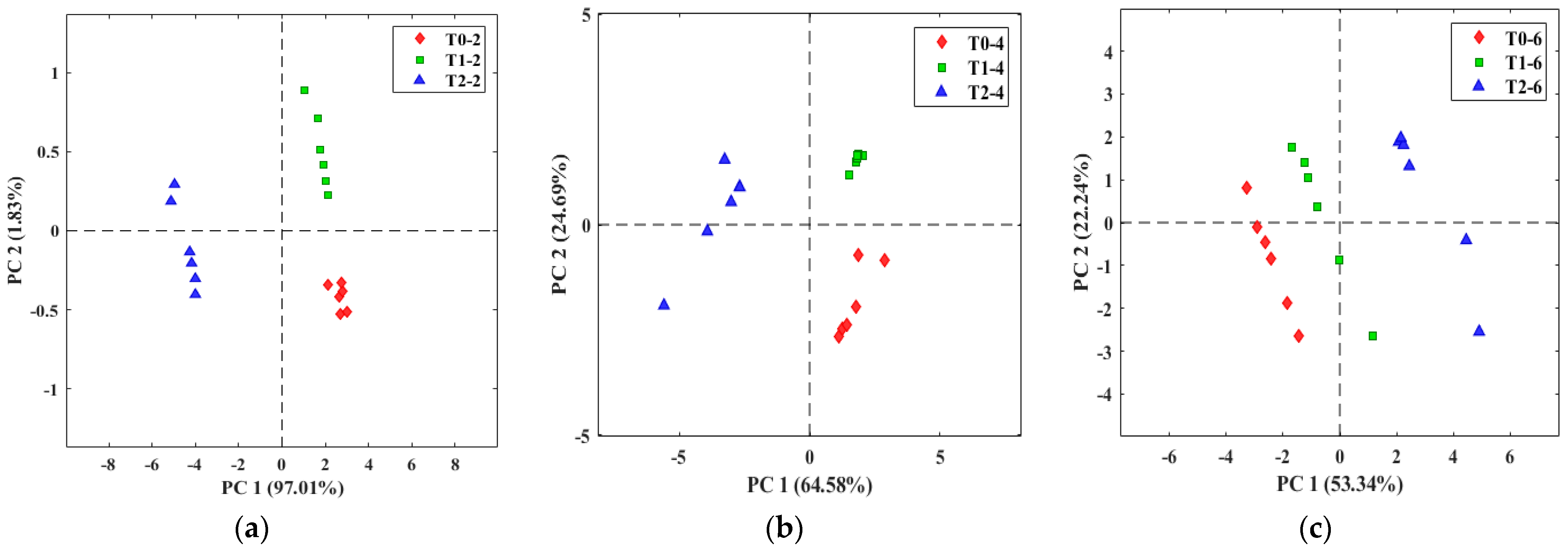
3.2. Evaluation of Tomato Olfactory Pattern with TOMATO-NOSE
3.3. Botrytis Cinerea Infection
3.4. Lateral Flow Test Device
3.4.1. Commercial Lateral Flow Test Selection
3.4.2. Establishment of LFD Cut-Off Thresholds
3.4.3. Vision Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| J | P (Peak Values) | Ground Truth | Image | |||||
|---|---|---|---|---|---|---|---|---|
| Picture | Min | Max | Test | Control | Test | Control | Class | T C |
| 010730.PNG | 0 | 10 | 5.1710 | 8.0450 | Yes | Yes | Positive |  |
| 012623.PNG | 0 | 14 | 0.3822 | 10.3418 | No | Yes | Negative |  |
| 012735.PNG | 0 | 15 | 3.9090 | 11.6835 | Yes | Yes | Positive |  |
| 012842.PNG | 0 | 14 | 0.2562 | 10.5070 | No | Yes | Negative |  |
| 013013.PNG | 0 | 10 | 4.8370 | 4.8320 | Yes | Yes | Positive |  |
| 013126.PNG | 0 | 3 | 0.0252 | 0.1860 | No | No | Null |  |
| 013225.PNG | 0 | 14 | 8.5512 | 10.5056 | Yes | Yes | Positive |  |
| 013409.PNG | 0 | 12 | 8.7432 | 10.0104 | Yes | Yes | Positive |  |
| 013457.PNG | 0 | 8 | 4.0768 | 0.0456 | Yes | No | Null |  |
| 014230.PNG | 0 | 14 | 8.9292 | 10.0310 | Yes | Yes | Positive |  |
| 014415.PNG | 0 | 16 | 12.3680 | 2.5504 | Yes | Yes | Positive |  |
| 014923.PNG | 0 | 9 | 5.0652 | 0.0000 | Yes | No | Null |  |
| 015023.PNG | 0 | 14 | 0.6020 | 10.5000 | No | Yes | Negative |  |
| 015144.PNG | 0 | 8 | 0.0200 | 3.9904 | No | Yes | Negative |  |
| 015300.PNG | 0 | 12 | 9.3672 | 0.0612 | Yes | No | Null |  |
| 015437.PNG | 0 | 8 | 4.8976 | 2.1120 | Yes | Yes | Positive |  |
| 015537.PNG | 0 | 5 | 0.0000 | 2.3170 | No | Yes | Negative |  |
References
- Burton-Freeman, B.; Reimers, K. Tomato Consumption and Health: Emerging Benefits. Am. J. Lifestyle Med. 2011, 5, 182–191. [Google Scholar] [CrossRef]
- Grandillo, S.; Zamir, D.; Tanksley, S.D. Genetic Improvement of Processing Tomatoes: A 20 Years Perspective. Euphytica 1999, 110, 85–97. [Google Scholar] [CrossRef]
- Straits Research Tomato Market: Information by Product (Sauces, Paste, Canned Tomatoes, Ketchup, Juice) and Regions-Forecast Till 2031. 2022. Available online: https://straitsresearch.com/report/tomato-market (accessed on 22 April 2023).
- Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Post-harvest Factors. Agronomy 2022, 12, 376. [Google Scholar] [CrossRef]
- Cantwell, M. Optimum Procedures for Ripening Tomatoes. Manag. Fruit Ripening 2000, 9, 80–88. [Google Scholar]
- Tikunov, Y.; Lommen, A.; De Vos, C.H.R.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A Novel Approach for Nontargeted Data Analysis for Metabolomics. Large-Scale Profiling of Tomato Fruit Volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef]
- Zewdie, T.; Desaiegn, A.; Olijira, G.; Amare, H.; Fetene, K.; Gebrie, L. Review on Effects of Pre and Post-Harvest Factors Affecting the Quality and Shelf Life of Tomato (Lycopersicon esculentum Mill.). South Asian J. Agric. Sci. 2021, 1, 51–56. [Google Scholar]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science (1979) 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic Mechanisms and Control Strategies of Botrytis cinerea Causing Post-Harvest Decay in Fruits and Vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis spp. and Diseases They Cause in Agricultural Systems—An Introduction. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 1–8. [Google Scholar]
- Dik, A.J.; Wubben, J.P. Epidemiology of Botrytis Cinerea Diseases in Greenhouses. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Fillinger, S.; Walker, A.S. Chemical Control and Resistance Management of Botrytis Diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty Years of Post-harvest Biocontrol Research: Is It Time for a New Paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Lee, J.; Nazki, H.; Baek, J.; Hong, Y.; Lee, M. Artificial Intelligence Approach for Tomato Detection and Mass Estimation in Precision Agriculture. Sustainability 2020, 12, 9138. [Google Scholar] [CrossRef]
- Mohana Saranya, S.; Rajalaxmi, R.R.; Prabavathi, R.; Suganya, T.; Mohanapriya, S.; Tamilselvi, T. Deep Learning Techniques in Tomato Plant—A Review. J. Phys. Conf. Ser. 2021, 1767, 012010. [Google Scholar] [CrossRef]
- de Luna, R.G.; Dadios, E.P.; Bandala, A.A.; Vicerra, R.R.P. Tomato Growth Stage Monitoring for Smart Farm Using Deep Transfer Learning with Machine Learning-Based Maturity Grading. Agrivita 2020, 42, 24–36. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; Moustafa, K.; Tran, L.-S.P. ‘Omics’ and Plant Responses to Botrytis Cinerea. Front. Plant Sci. 2016, 7, 1658. [Google Scholar] [CrossRef] [PubMed]
- Berna, A.Z.; Lammertryn, J.; Saevels, S.; Di Natale, C.; Nicolaï, B.M. Electronic Nose Systems to Study Shelf Life and Cultivar Effect on Tomato Aroma Profile. Sens. Actuators B Chem. 2004, 97, 324–333. [Google Scholar] [CrossRef]
- Brezmes, J.; Llobet, E. Electronic Noses for Monitoring the Quality of Fruit. In Electronic Noses and Tongues in Food Science; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early Detection and Classification of Pathogenic Fungal Disease in Post-Harvest Strawberry Fruit by Electronic Nose and Gas Chromatography-Mass Spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Lee, G.; Hossain, O.; Jamalzadegan, S.; Liu, Y.; Wang, H.; Saville, A.C.; Shymanovich, T.; Paul, R.; Rotenberg, D.; Whitfield, A.E.; et al. Abaxial Leaf Surface-Mounted Multimodal Wearable Sensor for Continuous Plant Physiology Monitoring. Sci. Adv. 2023, 9, eade2232. [Google Scholar] [CrossRef]
- Duan, Y.B.; Ge, C.Y.; Zhang, X.K.; Wang, J.X.; Zhou, M.G. Development and Evaluation of a Novel and Rapid Detection Assay for Botrytis cinerea based on Loop-Mediated Isothermal Amplification. PLoS ONE 2014, 9, e111094. [Google Scholar] [CrossRef]
- Obanor, F.O.; Walter, M.; Waipara, N.W.; Cernusko, R. Rapid Method for the Detection and Quantification of Botrytis Cinerea in Plant Tissues. N. Z. Plant Prot. 2002, 55, 150–153. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, I.S.; Ju, H.J.; Jeong, R.D. Rapid and Visual Detection of Tomato Spotted Wilt Virus Using Recombinase Polymerase Amplification Combined with Lateral Flow Strips. Mol. Cell. Probes 2021, 57, 101727. [Google Scholar] [CrossRef]
- Cai, P.; Wang, R.; Ling, S.; Wang, S. Rapid and Sensitive Detection of Tenuazonic Acid in Agricultural By-Products Based on Gold Nano-Flower Lateral Flow. Front. Bioeng. Biotechnol. 2022, 10, 1021758. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A Brief History of Electronic Noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Chi, Z.-T.; Liu, J.; Li, D.-H.; Sun, X.-J.; Yan, C.; Wang, Y.-C.; Li, H.; Wang, X.-D.; Xie, W.-F. Enhanced Gas Sensing Performance Based on P-NiS/n-In2O3 Heterojunction Nanocomposites. Sens. Actuators B Chem. 2020, 304, 127305. [Google Scholar] [CrossRef]
- Gai, L.-Y.; Lai, R.-P.; Dong, X.-H.; Wu, X.; Luan, Q.-T.; Wang, J.; Lin, H.-F.; Ding, W.-H.; Wu, G.-L.; Xie, W.-F. Recent Advances in Ethanol Gas Sensors Based on Metal Oxide Semiconductor Heterojunctions. Rare Metals 2022, 41, 1818–1842. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.Z.; Kang, Y.R.; Sun, Z.S.; Wang, X.D.; Meng, Y.; Hong, M.H.; Xie, W.F. Cauliflower-Shaped Bi2O3–ZnO Heterojunction with Superior Sensing Performance towards Ethanol. J. Alloys Compd. 2021, 854, 157152. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef]
- Esteves, C.H.A.; Iglesias, B.A.; Ogawa, T.; Araki, K.; Hoehne, L.; Gruber, J. Identification of Tobacco Types and Cigarette Brands Using an Electronic Nose Based on Conductive Polymer/Porphyrin Composite Sensors. ACS Omega 2018, 3, 6476–6482. [Google Scholar] [CrossRef]
- Roto, R.; Rianjanu, A.; Rahmawati, A.; Fatyadi, I.A.; Yulianto, N.; Majid, N.; Syamsu, I.; Wasisto, H.S.; Triyana, K. Quartz Crystal Microbalances Functionalized with Citric Acid-Doped Polyvinyl Acetate Nanofibers for Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 5687–5697. [Google Scholar] [CrossRef]
- Länge, K. Bulk and Surface Acoustic Wave Sensor Arrays for Multi-Analyte Detection: A Review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef]
- Palacín, J.; Rubies, E.; Clotet, E.; Martínez, D. Classification of Two Volatiles Using an ENose Composed by an Array of 16 Single-Type Miniature Micro-Machined Metal-Oxide Gas Sensors. Sensors 2022, 22, 1120. [Google Scholar] [CrossRef]
- Buratti, S.; Malegori, C.; Benedetti, S.; Oliveri, P.; Giovanelli, G. E-Nose, e-Tongue and e-Eye for Edible Olive Oil Characterization and Shelf Life Assessment: A Powerful Data Fusion Approach. Talanta 2018, 182, 131–141. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Adv. Mater. Technol. 2019, 4, 1800488. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, G.; Mishra, H.N. Fuzzy Controller Based E-Nose Classification of Sitophilus Oryzae Infestation in Stored Rice Grain. Food Chem. 2019, 283, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Zaim, O.; Saidi, T.; El Bari, N.; Bouchikhi, B. Assessment of ”Breath Print” in Patients With Chronic Kidney Disease during Dialysis by Non-Invasive Breath Screening of Exhaled Volatile Compounds Using an Electronic Nose. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose Discrimination of Abnormal Fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.L.; Chou, T.I.; Yang, S.R.; Lin, Y.J.; Ye, Z.K.; Chiu, S.W.; Lee, S.W.; Tang, K.T. Development of a Non-Destructive Moldy Coffee Beans Detection System Based on Electronic Nose. IEEE Sens. Lett. 2023, 7, 6001204. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Hernandez-Brenes, C. Smart Detection of Faults in Beers Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Artificial Intelligence. Fermentation 2021, 7, 117. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A Novel Wearable Electronic Nose for Healthcare Based on Flexible Printed Chemical Sensor Array. Sensors 2014, 14, 9700. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic Nose with Digital Gas Sensors Connected via Bluetooth to a Smartphone for Air Quality Measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef]
- Meléndez, F.; Arroyo, P.; Gómez-Suárez, J.; Palomeque-Mangut, S.; Suárez, J.I.; Lozano, J. Portable Electronic Nose Based on Digital and Analog Chemical Sensors for 2,4,6-Trichloroanisole Discrimination. Sensors 2022, 22, 3453. [Google Scholar] [CrossRef]
- Li, C.; Krewer, G.W.; Ji, P.; Scherm, H.; Kays, S.J. Gas Sensor Array for Blueberry Fruit Disease Detection and Classification. Postharvest Biol. Technol. 2010, 55, 144–149. [Google Scholar] [CrossRef]
- Pallottino, F.; Costa, C.; Antonucci, F.; Strano, M.C.; Calandra, M.; Solaini, S.; Menesatti, P. Electronic Nose Application for Determination of Penicillium Digitatum in Valencia Oranges. J. Sci. Food Agric. 2012, 92, 2008–2012. [Google Scholar] [CrossRef]
- Haghbin, N.; Bakhshipour, A.; Mousanejad, S.; Zareiforoush, H. Monitoring Botrytis cinerea Infection in Kiwifruit Using Electronic Nose and Machine Learning Techniques. Food Bioprocess Technol. 2023, 16, 749–767. [Google Scholar] [CrossRef]
- Tyagi, P.; Semwal, R.; Sharma, A.; Tiwary, U.S.; Varadwaj, P. E-Nose: A Low-Cost Fruit Ripeness Monitoring System. J. Agric. Eng. 2022, 54, 1389. [Google Scholar] [CrossRef]
- Maiolo, L.; Polese, D. Advances in Sensing Technologies for Smart Monitoring in Precise Agriculture. In Proceedings of the SENSORNETS 2021-10th International Conference on Sensor Networks, Online, 9–10 February 2021. [Google Scholar]
- Seesaard, T.; Goel, N.; Kumar, M.; Wongchoosuk, C. Advances in Gas Sensors and Electronic Nose Technologies for Agricultural Cycle Applications. Comput. Electron. Agric. 2022, 193, 106673. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D. Electronic-Nose Applications for Fruit Identification, Ripeness and Quality Grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Lozano, J.; Meléndez, F.; Arroyo, P.; Suárez, J.-I.; Herrero, J.-L.; Carmona, P.; Fernández, J.-Á. Towards the Miniaturization of Electronic Nose as Personal Measurement Systems. Proceedings 2019, 14, 30. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of Sulphur Volatiles and GC-Olfactometry Aroma Profiling in Two Fresh Tomato Cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma Perception of Individual Volatile Compounds in Fresh Tomatoes (Lycopersicon esculentum, Mill.) as Affected by the Medium of Evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Ghaffari, R.; Zhang, F.; Iliescu, D.; Hines, E.; Leeson, M.; Napier, R.; Clarkson, J. Early Detection of Diseases in Tomato Crops: An Electronic Nose and Intelligent Systems Approach. In Proceedings of the International Joint Conference on Neural Networks, Barcelona, Spain, 18–23 July 2010. [Google Scholar]
- Cui, S.; Inocente, E.A.A.; Acosta, N.; Keener, H.M.; Zhu, H.; Ling, P.P. Development of Fast E-Nose System for Early-Stage Diagnosis of Aphid-Stressed Tomato Plants. Sensors 2019, 19, 3480. [Google Scholar] [CrossRef]
- Zhang, F.; Iliescu, D.D.; Hines, E.L.; Leeson, M.S. Tomato Plant Health Monitoring: An Electronic Nose Approach. In Intelligent Systems for Machine Olfaction: Tools and Methodologies; IGI Global: Hershey, PA, USA, 2011. [Google Scholar]
- Laothawornkitkul, J.; Moore, J.P.; Taylor, J.E.; Possell, M.; Gibson, T.D.; Hewitt, C.N.; Paul, N.D. Discrimination of Plant Volatile Signatures by an Electronic Nose: A Potential Technology for Plant Pest and Disease Monitoring. Environ. Sci. Technol. 2008, 42, 8433–8439. [Google Scholar] [CrossRef]
- Feng, H.; Gonzalez Viejo, C.; Vaghefi, N.; Taylor, P.W.J.; Tongson, E.; Fuentes, S. Early Detection of Fusarium oxysporum Infection in Processing Tomatoes (Solanum lycopersicum) and Pathogen–Soil Interactions Using a Low-Cost Portable Electronic Nose and Machine Learning Modeling. Sensors 2022, 22, 8645. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and Growth Tracking of Fungi Contamination in Peaches Using Electronic Nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
- Meyer, U.M.; Dewey, F.M. Efficacy of Different Immunogens for Raising Monoclonal Antibodies to Botrytis cinerea. Mycol. Res. 2000, 104, 979–987. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Cantwell, M. Impact of Ripening & Storage Conditions. 2008. Available online: https://iifiir.org/en/fridoc/impact-of-ripening-and-storage-conditions-on-ripe-tomato-quality-26403 (accessed on 25 April 2023).
- Fu, Q.; Lu, K.; Li, N.; Dong, Z. Advances in the Development of MOS-Based Sensors for Detection of Ethanol: A Review. Mater. Res. Bull. 2023, 168, 112457. [Google Scholar] [CrossRef]
- Cämmerer, M.; Mayer, T.; Penzel, S.; Rudolph, M.; Borsdorf, H. Application of Low-Cost Electrochemical Sensors to Aqueous Systems to Allow Automated Determination of NH3 and H2S in Water. Sensors 2020, 20, 2814. [Google Scholar] [CrossRef]
- Sinesio, F.; Di Natale, C.; Quaglia, G.B.; Bucarelli, F.M.; Moneta, E.; Macagnano, A.; Paolesse, R.; D’Amico, A. Use of Electronic Nose and Trained Sensory Panel in the Evaluation of Tomato Quality. J. Sci. Food. Agric. 2000, 80, 63–71. [Google Scholar] [CrossRef]
- Gómez, A.H.; Hu, G.; Wang, J.; Pereira, A.G. Evaluation of Tomato Maturity by Electronic Nose. Comput. Electron. Agric. 2006, 54, 44–52. [Google Scholar] [CrossRef]
- Concina, I.; Falasconi, M.; Gobbi, E.; Bianchi, F.; Musci, M.; Mattarozzi, M.; Pardo, M.; Mangia, A.; Careri, M.; Sberveglieri, G. Early Detection of Microbial Contamination in Processed Tomatoes by Electronic Nose. Food Control 2009, 20, 873–880. [Google Scholar] [CrossRef]
- Mota, I.; Teixeira-Santos, R.; Cavaleiro Rufo, J. Detection and Identification of Fungal Species by Electronic Nose Technology: A Systematic Review. Fungal Biol. Rev. 2021, 37, 59–70. [Google Scholar] [CrossRef]


| Board | Sensor | Manufacturer | Type | Signals |
|---|---|---|---|---|
| Main Board | BME680 | Bosch Sensortech GmbH, Germany | Metal Oxide (MOS) | Temperature, Relative humidity, Pressure, Resistance value |
| SGP30 | Sensirion AG, Switzerland | MOS | CO2, TVOCs 1, H2 (raw signal 2), Ethanol (raw signal) | |
| SGP40 | Sensirion | MOS | Resistance value | |
| ZMOD4410 | Renesas Electronic Corporation, Japan | MOS | Ethanol (raw signal), Resistance value, CO2, TVOCs, IAQ 3 | |
| CCS811 | ScioSense B.V., The Netherlands | MOS | CO2, TVOCs, Resistance value | |
| SHT21 | Sensirion | MOS | Temperature, Relative humidity | |
| SCD40 | Sensirion | Photoacoustic | CO2, Temperature, Resistance value | |
| Secondary Board | IRM-AT | Alphasense Ltd., UK | Non-Dispersive Infra-Red (NDIR) | Reference electrode, Active electrode, thermistor output |
| PID-AH2 | Alphasense | Photo Ionization Detector (PID) | Raw output | |
| NH3-AF | Alphasense | Electrochemical (EC) | Work electrode, Active electrode | |
| ETO-A1 | Alphasense | EC | Work electrode, Active electrode | |
| HCN-A1 | Alphasense | EC | Work electrode, Active electrode | |
| SO2-AF | Alphasense | EC | Work electrode, Active electrode |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez, F.; Sánchez, R.; Fernández, J.Á.; Belacortu, Y.; Bermúdez, F.; Arroyo, P.; Martín-Vertedor, D.; Lozano, J. Design of a Multisensory Device for Tomato Volatile Compound Detection Based on a Mixed Metal Oxide—Electrochemical Sensor Array and Optical Reader. Micromachines 2023, 14, 1761. https://doi.org/10.3390/mi14091761
Meléndez F, Sánchez R, Fernández JÁ, Belacortu Y, Bermúdez F, Arroyo P, Martín-Vertedor D, Lozano J. Design of a Multisensory Device for Tomato Volatile Compound Detection Based on a Mixed Metal Oxide—Electrochemical Sensor Array and Optical Reader. Micromachines. 2023; 14(9):1761. https://doi.org/10.3390/mi14091761
Chicago/Turabian StyleMeléndez, Félix, Ramiro Sánchez, Juan Álvaro Fernández, Yaiza Belacortu, Francisco Bermúdez, Patricia Arroyo, Daniel Martín-Vertedor, and Jesús Lozano. 2023. "Design of a Multisensory Device for Tomato Volatile Compound Detection Based on a Mixed Metal Oxide—Electrochemical Sensor Array and Optical Reader" Micromachines 14, no. 9: 1761. https://doi.org/10.3390/mi14091761
APA StyleMeléndez, F., Sánchez, R., Fernández, J. Á., Belacortu, Y., Bermúdez, F., Arroyo, P., Martín-Vertedor, D., & Lozano, J. (2023). Design of a Multisensory Device for Tomato Volatile Compound Detection Based on a Mixed Metal Oxide—Electrochemical Sensor Array and Optical Reader. Micromachines, 14(9), 1761. https://doi.org/10.3390/mi14091761









