Rapid Production of Nanoscale Liposomes Using a 3D-Printed Reactor-In-A-Centrifuge: Formulation, Characterisation, and Super-Resolution Imaging
Abstract
:1. Introduction
2. Materials and Methods
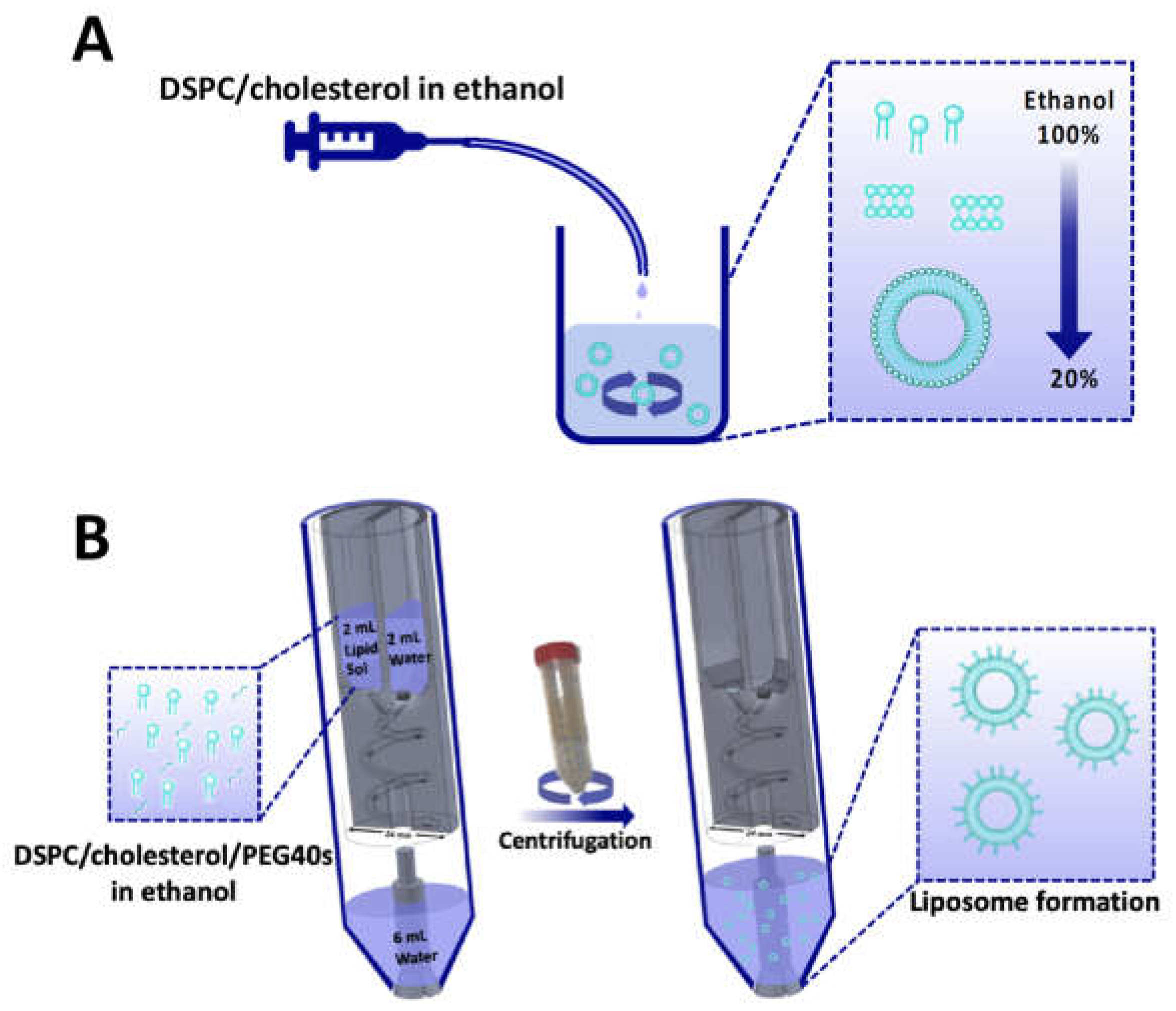
2.1. Liposome Production via Ethanol Injection
2.2. 3D-Printed Reactor-In-A-Centrifuge (RIAC)
2.3. Liposome Production Using the RIAC
2.4. Measurement of Liposome Size and Charge through a Dynamic Light Scattering Apparatus
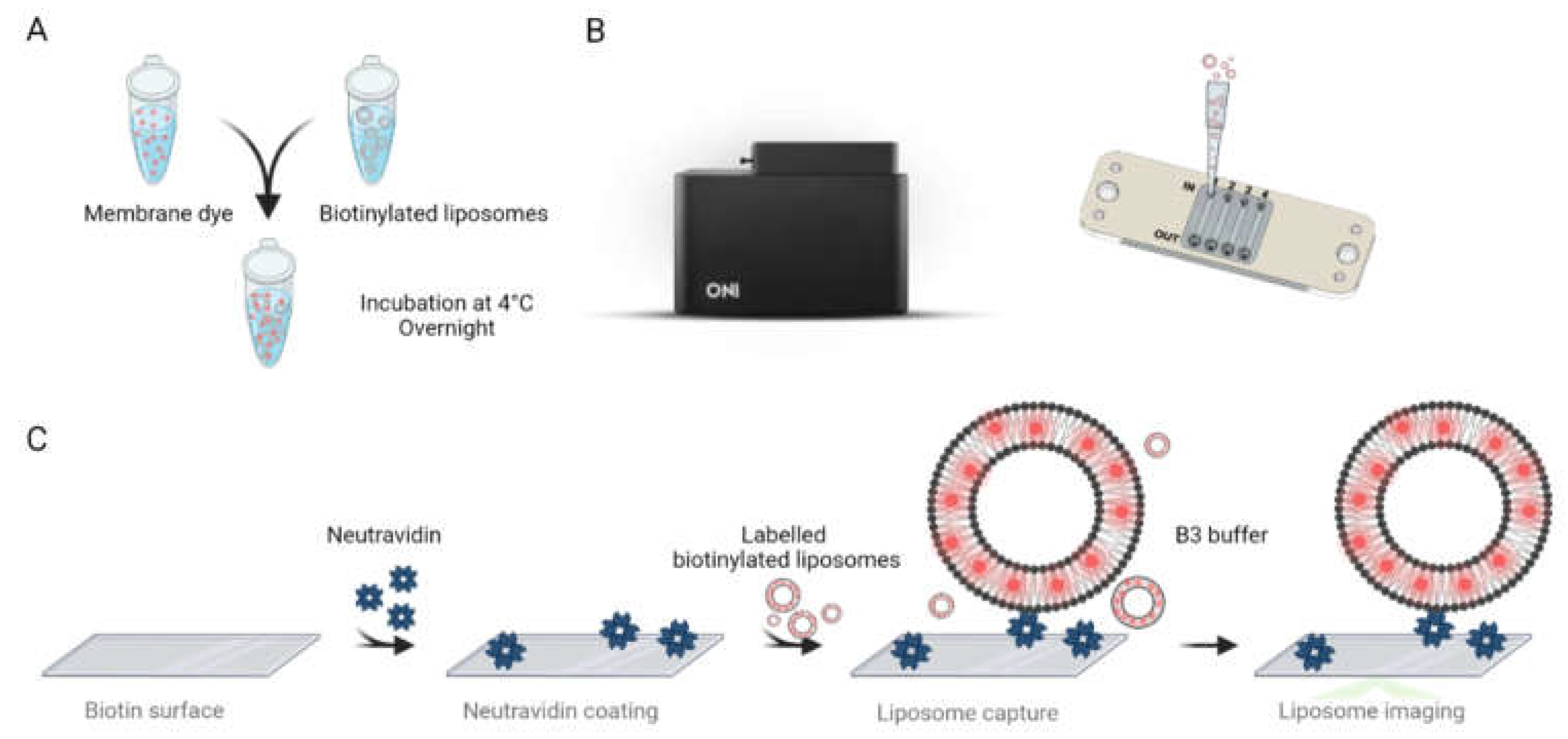
2.5. Super-Resolution Imaging of Liposomes
2.6. Statistical Analysis
3. Results and Discussion
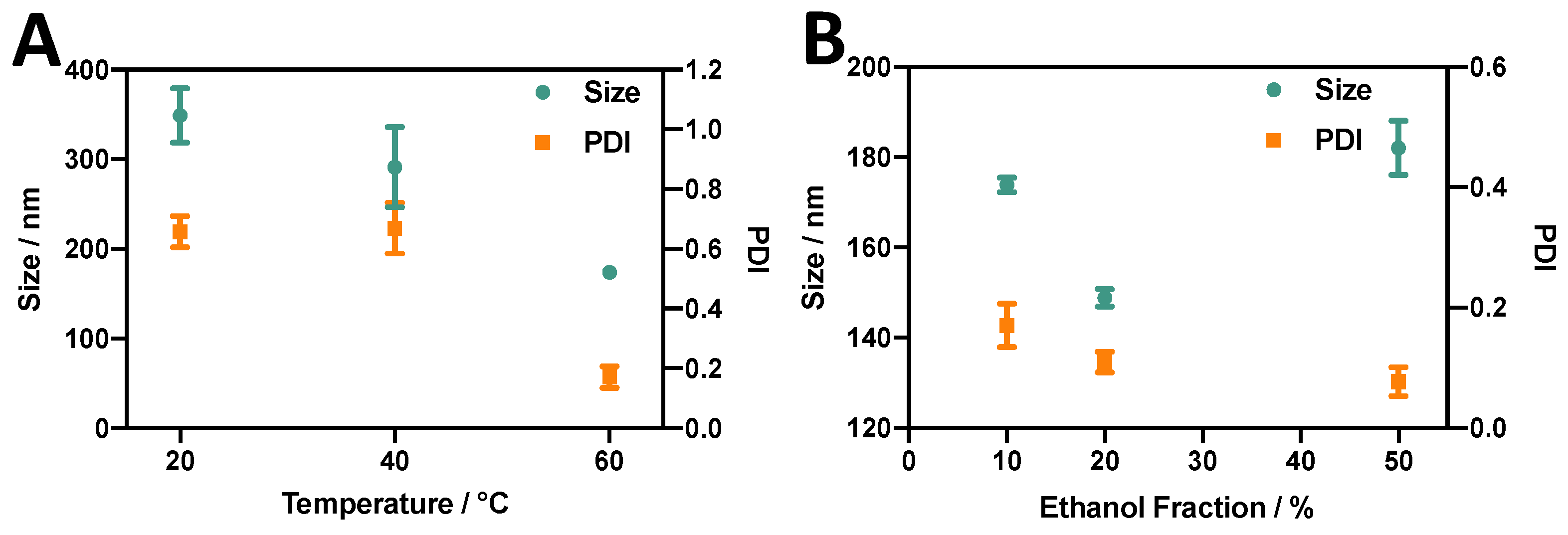
3.1. Effect of Temperature and Ethanol Fraction on Liposomes Produced via the Ethanol Injection (EI) Method
3.2. Liposome Production Using the RIAC: Identification of Suitable Experimental Parameters
3.2.1. Effect of Temperature
3.2.2. Effect of Inclusion of a PEG Moiety
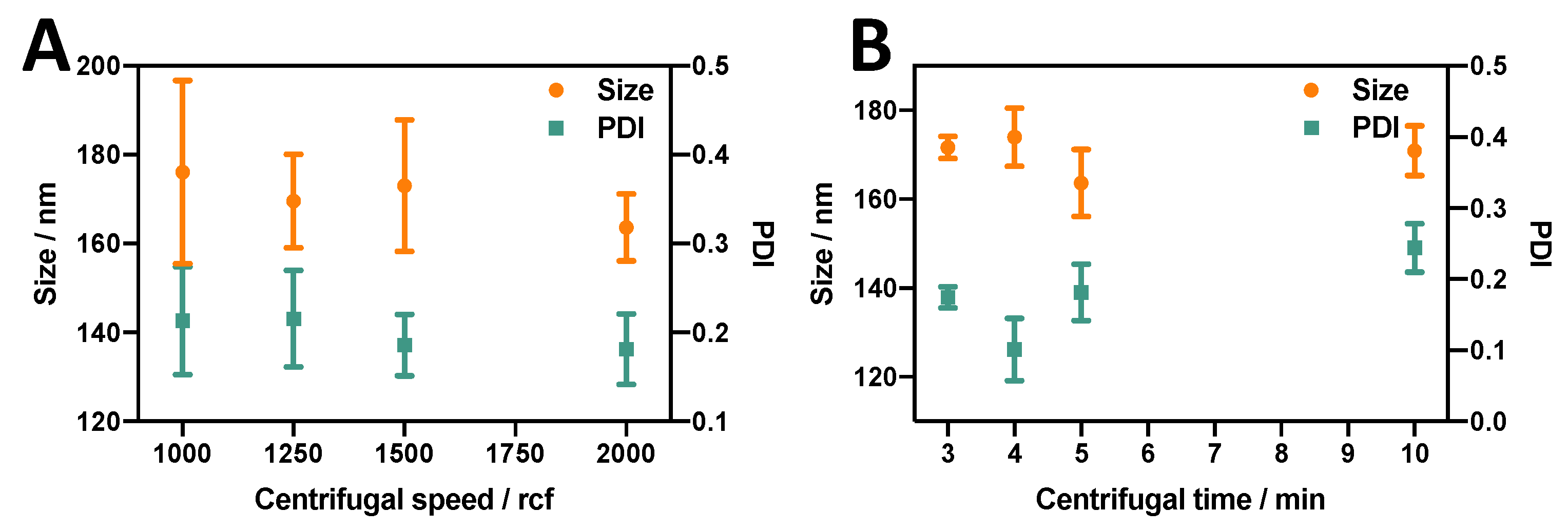
3.2.3. Effect of Centrifugation Parameters
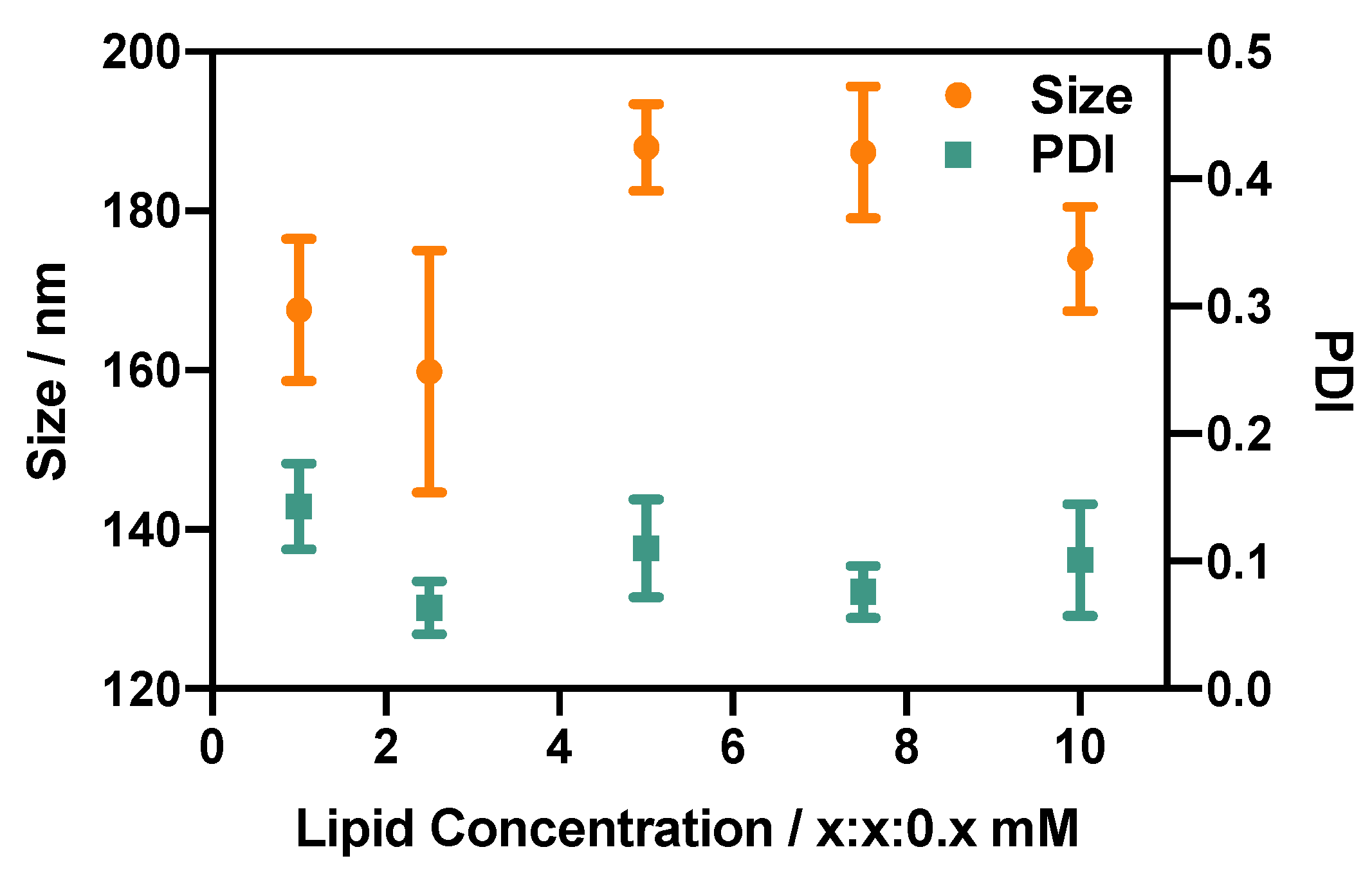
3.2.4. Effect of Lipid Concentration
3.3. Production of Functionalised Liposomes Using the RIAC
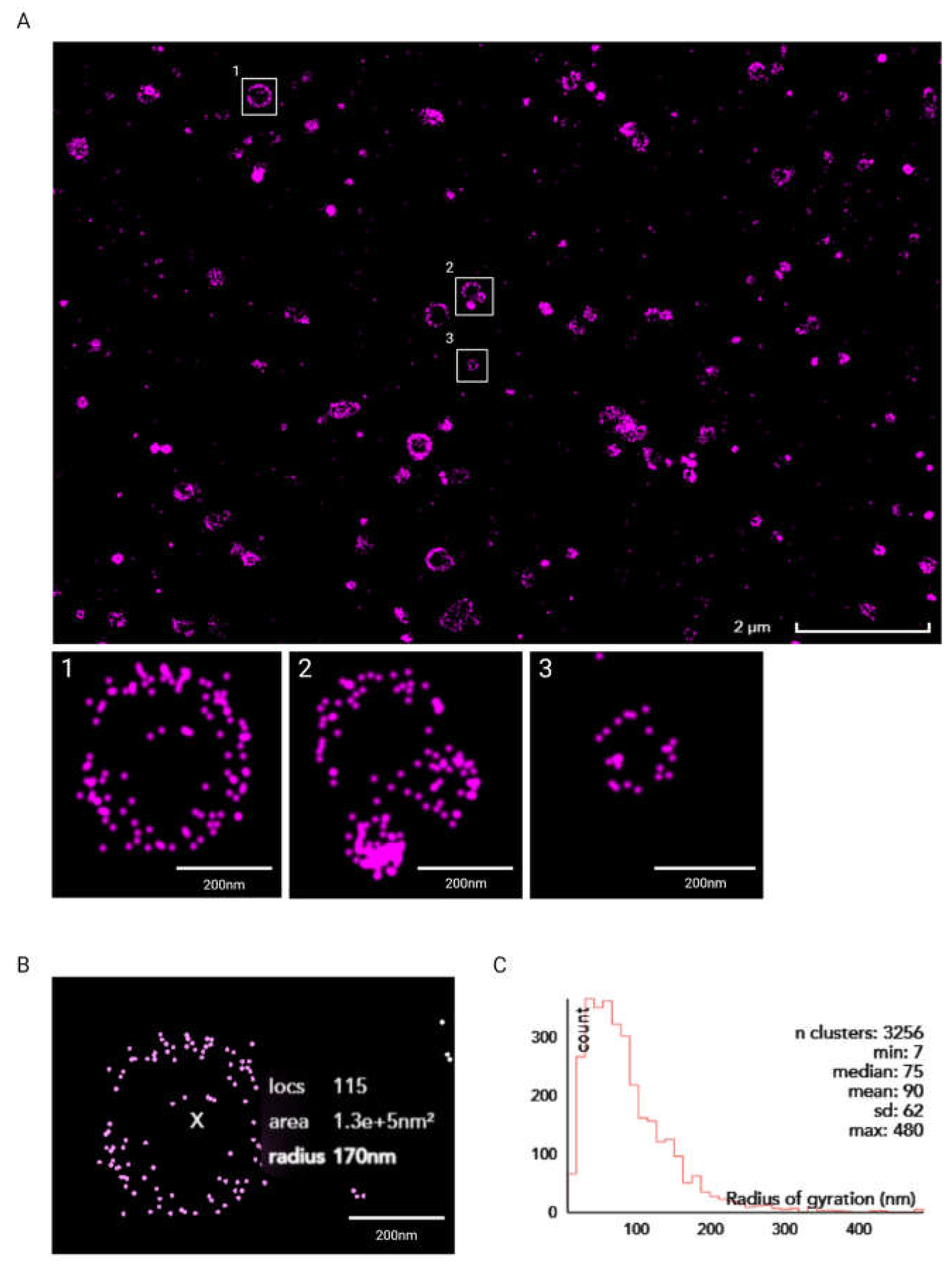
3.4. Super-Resolution Imaging of Liposomes Produced Using the RIAC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. Appl. Nanotechnol. Drug Deliv. 2014, 1, 1–100. [Google Scholar]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Sudimack, J.J.; Guo, W.; Tjarks, W.; Lee, R.J. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochim. Biophys. Acta 2002, 1564, 31–37. [Google Scholar] [CrossRef]
- Chen, M.; Song, F.; Liu, Y.; Tian, J.; Liu, C.; Li, R.; Zhang, Q. A dual pH-sensitive liposomal system with charge-reversal and NO generation for overcoming multidrug resistance in cancer. Nanoscale 2019, 11, 3814–3826. [Google Scholar] [CrossRef]
- Maples, D.; McLean, K.; Sahoo, K.; Newhardt, R.; Venkatesan, P.; Wood, B.; Ranjan, A. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int. J. Hyperth. 2015, 31, 674–685. [Google Scholar] [CrossRef]
- Wen, C.J.; Zhang, L.W.; Al-Suwayeh, S.A.; Yen, T.C.; Fang, J.Y. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int. J. Nanomed. 2012, 7, 1599–1611. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y. Multi-functional liposome: A powerful theranostic nano-platform enhancing photodynamic therapy. Adv. Sci. 2021, 8, 2100876. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.; Lasic, D.D. An overview of liposome scaled-up production and quality control. In Handbook of Nonmedical Applications of Liposomes; CRC Press: Boca Raton, FL, USA, 2018; pp. 23–30. [Google Scholar]
- Zhang, G.; Sun, J. Lipid in chips: A brief review of liposomes formation by microfluidics. Int. J. Nanomed. 2021, 16, 7391–7416. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Golden, J.P.; Justin, G.A.; Nasir, M.; Ligler, F.S. Hydrodynamic focusing—A versatile tool. Anal. Bioanal. Chem. 2012, 402, 325–335. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.-S.; Jung, H.-S. Precise control of liposome size using characteristic time depends on solvent type and membrane properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef]
- Chen, Z.; Han, J.Y.; Shumate, L.; Fedak, R.; DeVoe, D.L. High Throughput Nanoliposome Formation Using 3D Printed Microfluidic Flow Focusing Chips. Adv. Mater. Technol. 2019, 4, 1800511. [Google Scholar] [CrossRef]
- Andrew Brown, A.T.; Ordobadi, M.; Ip, S.; Heuck, G.; Ramsay, E. Using Formulation Parameters to Tune Size on the NanoAssemblr® Benchtop. Available online: https://www.precisionnanosystems.com/docs/default-source/pni-files/app-notes/pni-app-bt-012.pdf (accessed on 29 August 2023).
- Han, J.Y.; Chen, Z.; Devoe, D.L. Scalable Liposome Synthesis by High Aspect Ratio Microfluidic Flow Focusing. In Liposomes: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 87–93. [Google Scholar]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab A Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.D.; Labanca, A.; Donal Pottinger, T.; Owen, J.; Stulz, E.; Zhang, X.; Carugo, D. 3D printed reactor-in-a-centrifuge (RIAC): Making flow-synthesis of nanoparticles pump-free and cost-effective. Chem. Eng. J. 2021, 425, 130656. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Sun, J.; Xianyu, Y.; Li, M.; Liu, W.; Zhang, L.; Liu, D.; Liu, C.; Hu, G.; Jiang, X. A microfluidic origami chip for synthesis of functionalized polymeric nanoparticles. Nanoscale 2013, 5, 5262–5265. [Google Scholar] [CrossRef]
- Aghaei, H.; Solaimany Nazar, A.R. Continuous production of the nanoscale liposome in a double flow-focusing microfluidic device. Ind. Eng. Chem. Res. 2019, 58, 23032–23045. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- De Grandi, D.; Meghdadi, A.; LuTheryn, G.; Carugo, D. Facile production of quercetin nanoparticles using 3D printed centrifugal flow reactors. RSC Adv. 2022, 12, 20696–20713. [Google Scholar] [CrossRef]
- Delama, A.; Teixeira, M.I.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Microfluidic encapsulation method to produce stable liposomes containing iohexol. J. Drug Deliv. Sci. Technol. 2019, 54, 101340. [Google Scholar] [CrossRef]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef]
- Yanar, F.; Mosayyebi, A.; Nastruzzi, C.; Carugo, D.; Zhang, X. Continuous-Flow Production of Liposomes with a Millireactor under Varying Fluidic Conditions. Pharmaceutics 2020, 12, 1001. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Javadi, H.; Darvishi, M.H. Study of Structural stability and formation mechanisms in DSPC and DPSM liposomes: A coarse-grained molecular dynamics simulation. Sci. Rep. 2020, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Montanha, M.C.; Pellosi, D.S.; Kimura, E.; Caetano, W.; Hioka, N. Biotin-targeted mixed liposomes: A smart strategy for selective release of a photosensitizer agent in cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109923. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, J.; Lu, Y.; He, W.; Li, X.; Wu, W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine 2014, 10, 167–176. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Phillips, W.T.; Soundararajan, A.; Goins, B.A. Use of avidin/biotin-liposome system for enhanced peritoneal drug delivery in an ovarian cancer model. Int. J. Pharm. 2007, 337, 316–328. [Google Scholar] [CrossRef]
- Wang, L.; MacDonald, R. New strategy for transfection: Mixtures of medium-chain and long-chain cationic lipids synergistically enhance transfection. Gene Ther. 2004, 11, 1358–1362. [Google Scholar] [CrossRef]
- Dai, Y.; Xing, H.; Song, F.; Yang, Y.; Qiu, Z.; Lu, X.; Liu, Q.; Ren, S.; Chen, X.; Li, N. Biotin-Conjugated Multilayer Poly [D,L-lactide-co-glycolide]-Lecithin-Polyethylene Glycol Nanoparticles for Targeted Delivery of Doxorubicin. J. Pharm. Sci. 2016, 105, 2949–2958. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, J.; Kitamura, H.; Otake, Y.; Fuse, S.; Nakamura, H. Size-Controllable and Scalable Production of Liposomes Using a V-Shaped Mixer Micro-Flow Reactor. Org. Process Res. Dev. 2020, 24, 2122–2127. [Google Scholar] [CrossRef]
- Lim, S.W.Z.; Wong, Y.S.; Czarny, B.; Venkatraman, S. Microfluidic-directed self-assembly of liposomes: Role of interdigitation. J. Colloid. Interface Sci. 2020, 578, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sommonte, F.; Weaver, E.; Mathew, E.; Denora, N.; Lamprou, D.A. In-house innovative “diamond shaped” 3D printed microfluidic devices for lysozyme-loaded liposomes. Pharmaceutics 2022, 14, 2484. [Google Scholar] [CrossRef]
- Gentine, P.; Bourel-Bonnet, L.; Frisch, B. Modified and derived ethanol injection toward liposomes: Development of the process. J. Liposome Res. 2013, 23, 11–19. [Google Scholar] [CrossRef]
- Maitani, Y.; Soeda, H.; Junping, W.; Takayama, K. Modified ethanol injection method for liposomes containing beta-sitosterol beta-D-glucoside. J. Liposome Res. 2001, 11, 115–125. [Google Scholar] [CrossRef]
- Pons, M.; Foradada, M.; Estelrich, J. Liposomes obtained by the ethanol injection method. Int. J. Pharm. 1993, 95, 51–56. [Google Scholar] [CrossRef]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Al-Jamal, W.T. Sterically stabilized liposomes production using staggered herringbone micromixer: Effect of lipid composition and PEG-lipid content. Int. J. Pharm. 2019, 566, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the role and impact of poly (ethylene glycol)(PEG) on nanoparticle formulation: Implications for COVID-19 vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Bru, M.; Thompson, D.H.; Szleifer, I. Size and structure of spontaneously forming liposomes in lipid/PEG-lipid mixtures. Biophys. J. 2002, 83, 2419–2439. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab A Chip 2006, 6, 74–82. [Google Scholar] [CrossRef]
- Massing, U.; Cicko, S.; Ziroli, V. Dual asymmetric centrifugation (DAC)—A new technique for liposome preparation. J. Control Release 2008, 125, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Choi, T.H.; Lee, J.C.; Cheon, G.J.; Kim, K.Y.; Park, M.; Kim, M. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol. Ther. 2007, 15, 1145–1152. [Google Scholar] [CrossRef]
- Zempleni, J. Uptake, localization, and noncarboxylase roles of biotin. Annu. Rev. Nutr. 2005, 25, 175–196. [Google Scholar] [CrossRef]

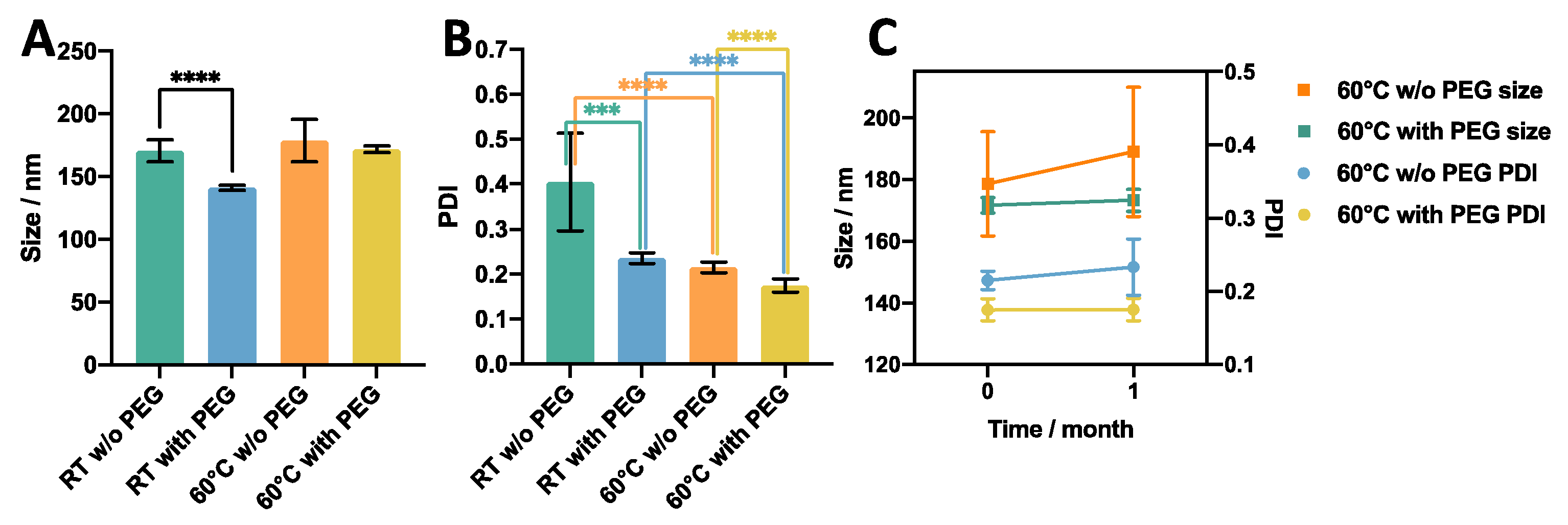
| Liposome Production Method | Liposome Formulations Evaluated |
|---|---|
| Ethanol injection |
|
| Reactor-in-a-centrifuge (RIAC) |
|
| |
| |
|
| Liposome Properties | Cationic Liposome | Biotinylated Liposome |
|---|---|---|
| Composition (mM) | DSPC:chol:EPC:PEG40s (1:2:1:0.2) | DSPC:chol:PEG40s:DSPE-PEG-biotin (2:2:0.1:0.1) 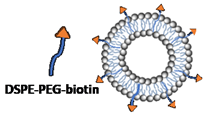 |
| Size (nm) | 120.1 ± 0.6 | 118.9 ± 1.6 |
| PDI | 0.17 ± 0.01 | 0.17 ± 0.02 |
| Zeta potential (mV) | 81.43 ± 4.48 | NA |
| Concentration | 9.62 × 109 ± 5.09 × 108 | 2.12 × 1010 ± 4.04 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Grandi, D.D.; Chandradoss, S.; LuTheryn, G.; Cidonio, G.; Nunes Bastos, R.; Pereno, V.; Carugo, D. Rapid Production of Nanoscale Liposomes Using a 3D-Printed Reactor-In-A-Centrifuge: Formulation, Characterisation, and Super-Resolution Imaging. Micromachines 2023, 14, 1763. https://doi.org/10.3390/mi14091763
He Y, Grandi DD, Chandradoss S, LuTheryn G, Cidonio G, Nunes Bastos R, Pereno V, Carugo D. Rapid Production of Nanoscale Liposomes Using a 3D-Printed Reactor-In-A-Centrifuge: Formulation, Characterisation, and Super-Resolution Imaging. Micromachines. 2023; 14(9):1763. https://doi.org/10.3390/mi14091763
Chicago/Turabian StyleHe, Yongqing, Davide De Grandi, Stanley Chandradoss, Gareth LuTheryn, Gianluca Cidonio, Ricardo Nunes Bastos, Valerio Pereno, and Dario Carugo. 2023. "Rapid Production of Nanoscale Liposomes Using a 3D-Printed Reactor-In-A-Centrifuge: Formulation, Characterisation, and Super-Resolution Imaging" Micromachines 14, no. 9: 1763. https://doi.org/10.3390/mi14091763
APA StyleHe, Y., Grandi, D. D., Chandradoss, S., LuTheryn, G., Cidonio, G., Nunes Bastos, R., Pereno, V., & Carugo, D. (2023). Rapid Production of Nanoscale Liposomes Using a 3D-Printed Reactor-In-A-Centrifuge: Formulation, Characterisation, and Super-Resolution Imaging. Micromachines, 14(9), 1763. https://doi.org/10.3390/mi14091763








