A Miniaturized, Fuel-Free, Self-Propelled, Bio-Inspired Soft Actuator for Copper Ion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Working Principles
2.3. Method of Fabrication
2.4. Material Characterization
2.5. Locomotion Characterization
2.6. Characterization of Copper Ion Absorption
3. Results and Discussion
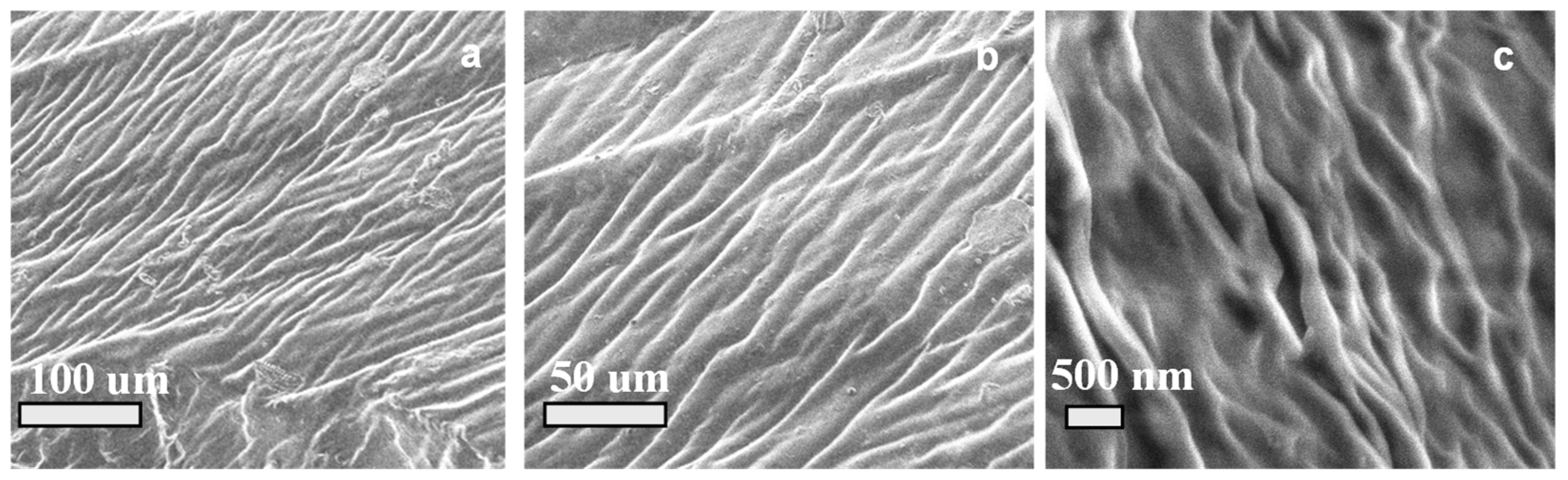
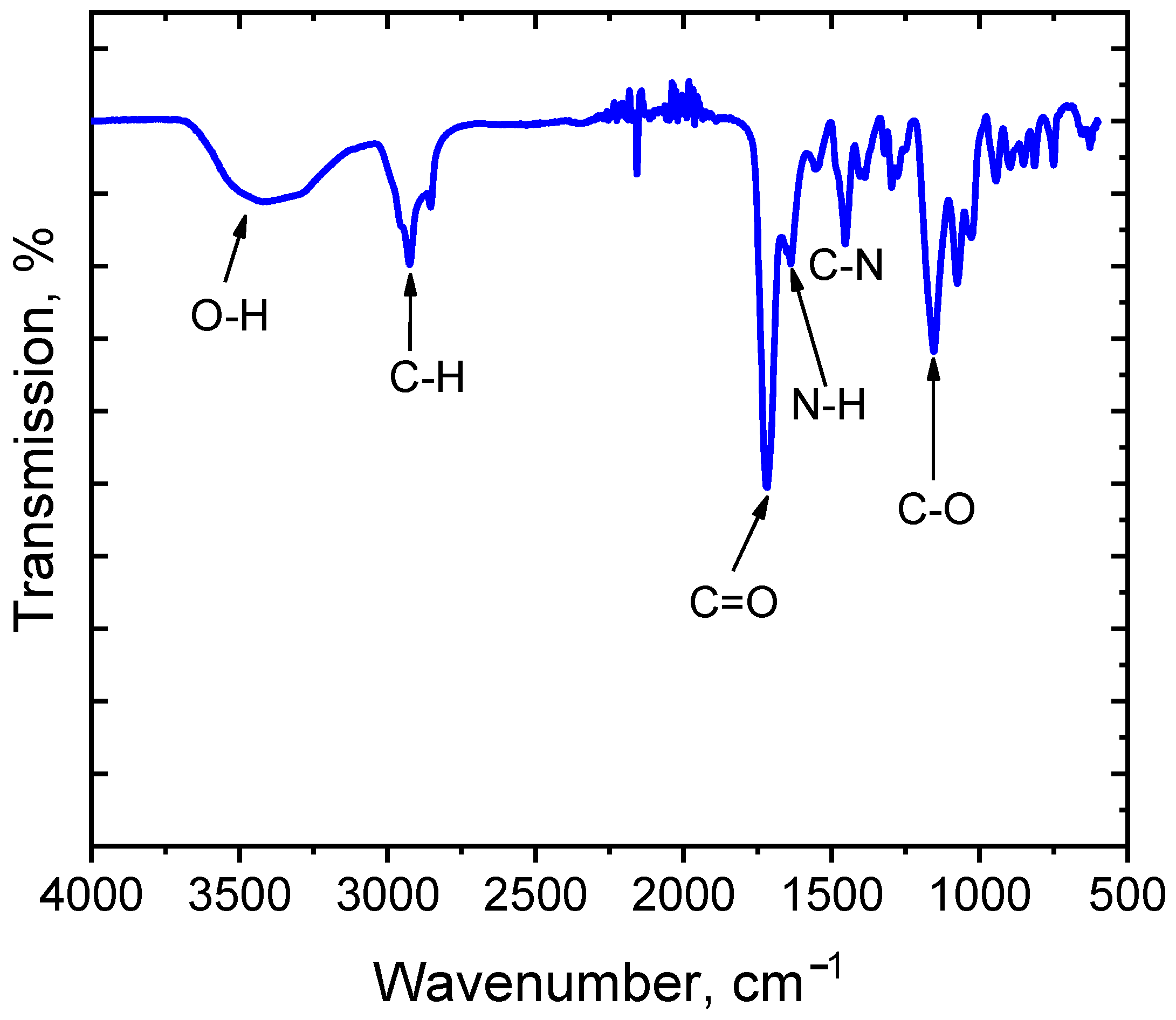
3.1. Morphological and Chemical Characterization
3.2. Chemical Aspect of Locomotion
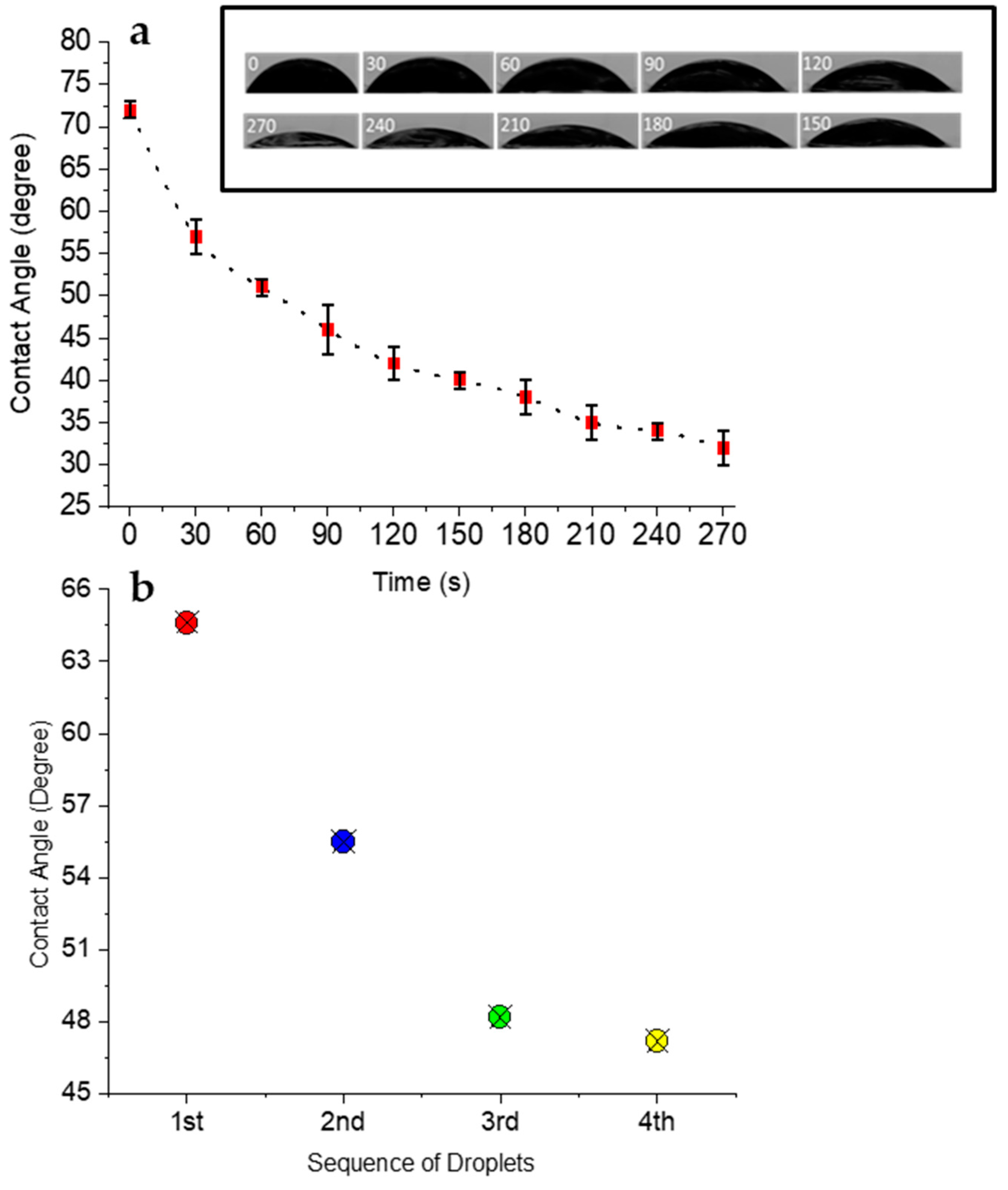
3.3. The Physical Aspect of Locomotion
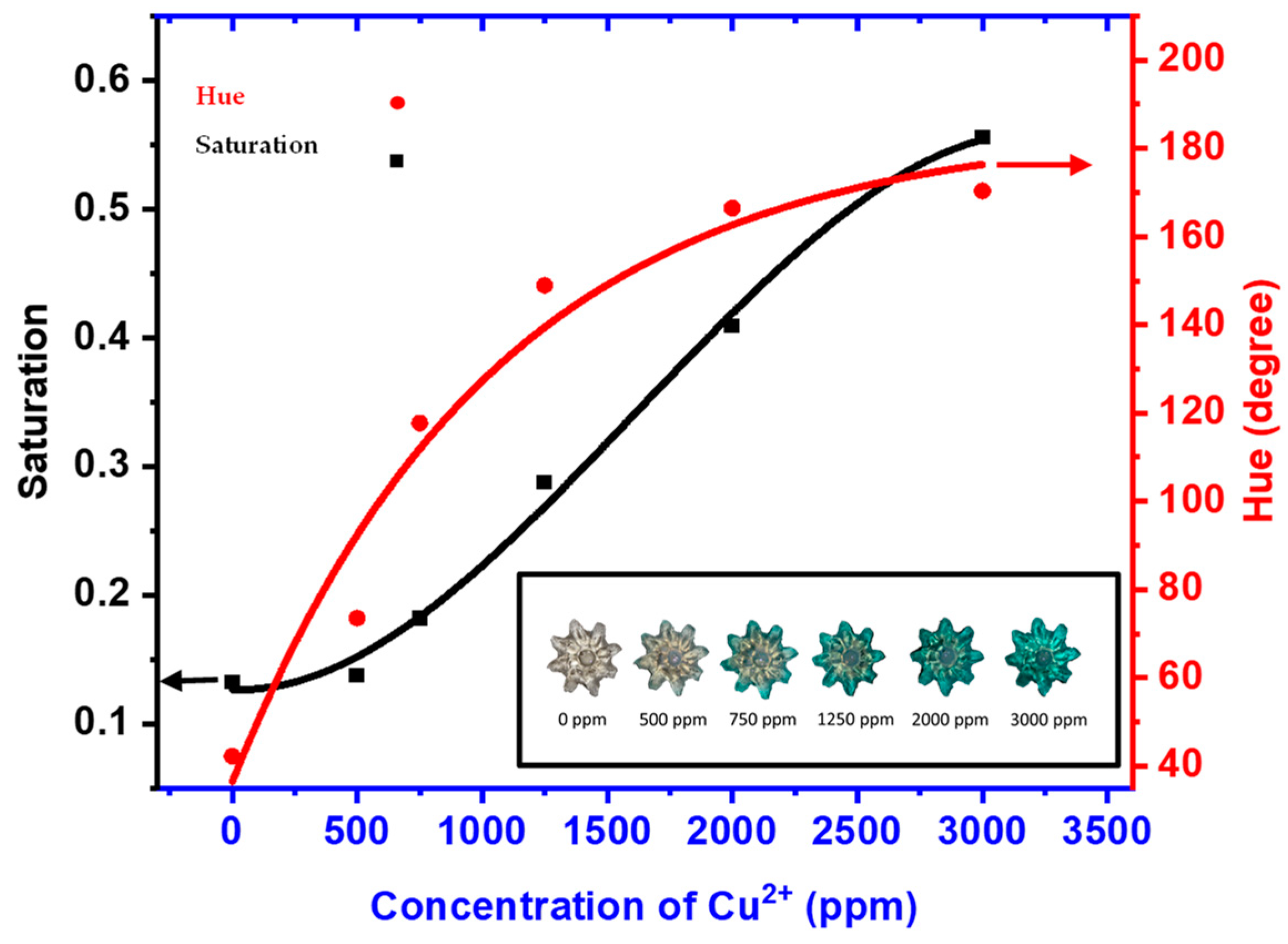
3.4. Copper Ion Absorption Capacity
3.5. Adsorption Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Borjian, P.; Chimerad, M.; Pathak, P.; Childs, A.; Rajaraman, S.; Cho, H.J. Electrochemical Sensors for Lead Ion Detection Using Sodium Alginate Crosslinked with 2-Acrylamido-2-Methyl Propane Sulfonic Acid and Aluminum Microparticles. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Soni, R.; Suyal, D.C.; Morales-Oyervides, L.; Chauhan, J.S. Current Status of Fresh Water Microbiology; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.; Jaafar, J.; Ismail, A. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kouhkord, A.; Hassani, F.; Amirmahani, M.; Golshani, A.; Naserifar, N.; Moghanlou, F.S.; Beris, A.T. Controllable Microfluidic System through Intelligent Framework: Data-Driven Modeling, Machine Learning Energy Analysis, Comparative Multiobjective Optimization, and Experimental Study. Ind. Eng. Chem. Res. 2024, 63, 13326–13344. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, X.; Yang, L.; Chang, Z.; Luo, S. Progress toward hydrogels in removing heavy metals from water: Problems and solutions—A review. ACS EST Water 2021, 1, 1098–1116. [Google Scholar] [CrossRef]
- Chimerad, M.; Barazesh, A.; Zandi, M.; Zarkesh, I.; Moghaddam, A.; Borjian, P.; Chimehrad, R.; Asghari, A.; Akbarnejad, Z.; Khonakdar, H.A. Tissue engineered scaffold fabrication methods for medical applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1455–1479. [Google Scholar] [CrossRef]
- Moghaddam, A.; Bahrami, M.; Mirzadeh, M.; Khatami, M.; Simorgh, S.; Chimehrad, M.; Kruppke, B.; Bagher, Z.; Mehrabani, D.; Khonakdar, H.A. Recent trends in bone tissue engineering: A review of materials, methods, and structures. Biomed. Mater. 2024, 19, 042007. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, S.; Luo, Y. Adsorption mechanisms of hydrogels for heavy metal and organic dyes removal: A short review. J. Agric. Food Res. 2023, 12, 100552. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Pathak, P.; Borjian, P.; Chimerad, M.; Cho, H.J. A ppt level PFOS (Perfluorooctanesulfonic Acid) sensor based on an eco-friendly chitosan biopolymer. In Proceedings of the 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kyoto, Japan, 25–29 June 2023. [Google Scholar]
- Zhang, J.; Chen, Z.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Self-propelling micro-/nano-motors: Mechanisms, applications, and challenges in drug delivery. Int. J. Pharm. 2021, 596, 120275. [Google Scholar] [CrossRef]
- Wu, Y.; Boymelgreen, A.; Yossifon, G. Micromotor-mediated label-free cargo manipulation. Curr. Opin. Colloid Interface Sci. 2022, 61, 101611. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, S.H.; Kim, T.Y.; Yi, J.; Hahn, S.K. Bioinspired urease-powered micromotor as an active oral drug delivery carrier in stomach. Bioact. Mater. 2022, 9, 54–62. [Google Scholar] [CrossRef]
- Lin, R.; Yu, W.; Chen, X.; Gao, H. Self-Propelled Micro/Nanomotors for Tumor Targeting Delivery and Therapy. Adv. Healthc. Mater. 2021, 10, 2001212. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, E.; Cui, H.; Hu, Z.; Wang, H. Bioinspired Self-Propelled Micromotors with Improved Transport Efficiency in the Subsurface Environment for Soil Decontamination. Adv. Funct. Mater. 2023, 33, 2307632. [Google Scholar] [CrossRef]
- Hassani, F.; Sadegh Moghanlou, F.; Minaei, A.; Vajdi, M.; Golshani, A.; Kouhkord, A.; Dehghani, T. An efficient framework for controllable micromixer design through the fusion of data-driven modeling and machine learning insights: Numerical and experimental analysis. Phys. Fluids 2024, 36, 033606. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, Y.; Jiang, T.; Xia, H.; Gu, X.; Chen, H. Recent progress of biomimetic motions—From microscopic micro/nanomotors to macroscopic actuators and soft robotics. RSC Adv. 2021, 11, 27406–27419. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Zarei, M. Self-propelled micro/nanomotors for sensing and environmental remediation. Small 2018, 14, 1800912. [Google Scholar] [CrossRef]
- Hassani, F.; Kouhkord, A.; Golshani, A.; Amirmahani, M.; Moghanlou, F.S.; Naserifar, N.; Beris, A.T. Micro-electro-mechanical acoustofluidic mixing system: A response surface-metaheuristic machine learning fusion framework. Expert Syst. Appl. 2024, 249, 123638. [Google Scholar] [CrossRef]
- Archer, R.; Kubodera, Y.; Ebbens, S.; Matsuo, M.; Shin-ichiro, M.N. Active mixing by self-propelled Janus sponge Marangoni motors with self-maintaining surface tension gradients. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Luo, C.; Li, H.; Qiao, L.; Liu, X. Development of surface tension-driven microboats and microflotillas. Microsyst. Technol. 2012, 18, 1525–1541. [Google Scholar] [CrossRef]
- Lin, C.-H.; Kinane, C.; Zhang, Z.; Pena-Francesch, A. Functional Chemical Motor Coatings for Modular Powering of Self-Propelled Particles. ACS Appl. Mater. Interfaces 2022, 14, 39332–39342. [Google Scholar] [CrossRef]
- Billard, G.; Bruyant, C. Sur un mode particulier de locomotion de certains stenus. CR Soc. Biol. 1905, 59, 102. [Google Scholar]
- Velarde, M.G.; Zeytounian, R.K. Interfacial Phenomena and the Marangoni Effect; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Lang, C.; Seifert, K.; Dettner, K. Skimming behaviour and spreading potential of Stenus species and Dianous coerulescens (Coleoptera: Staphylinidae). Naturwissenschaften 2012, 99, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.; Bishop, K.J.; Grzybowski, B.A. Dynamic self-assembly in ensembles of camphor boats. J. Phys. Chem. B 2008, 112, 10848–10853. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Salvagnini, P.; Scarpellini, A.; Carzino, R.; Beltran, C.; Mele, E.; Murino, V.; Athanassiou, A. Biomimetic locomotion on water of a porous natural polymeric composite. Adv. Mater. Interfaces 2016, 3, 1500854. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Xu, W.; Xin, C.; Wu, T.; Feng, W.; Yu, H.; Chen, C.; Jiang, S.; Zhang, Y.; et al. High-performance Marangoni hydrogel rotors with asymmetric porosity and drag reduction profile. Nat. Commun. 2023, 14, 20. [Google Scholar] [CrossRef]
- Lauga, E.; Davis, A.M. Viscous marangoni propulsion. J. Fluid Mech. 2012, 705, 120–133. [Google Scholar] [CrossRef]
- Pike, J.K.; Ho, T.; Wynne, K.J. Water-induced surface rearrangements of poly (dimethylsiloxane− urea− urethane) segmented block copolymers. Chem. Mater. 1996, 8, 856–860. [Google Scholar] [CrossRef]
- Senshu, K.; Yamashita, S.; Mori, H.; Ito, M.; Hirao, A.; Nakahama, S. Time-resolved surface rearrangements of poly (2-hydroxyethyl methacrylate-block-isoprene) in response to environmental changes. Langmuir 1999, 15, 1754–1762. [Google Scholar] [CrossRef]
- Lewis, K.B.; Ratner, B.D. Observation of surface rearrangement of polymers using ESCA. J. Colloid Interface Sci. 1993, 159, 77–85. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, B.; Wang, Y.; Pan, X.; Qu, Z.; Mei, Y. Self-powered locomotion of a hydrogel water strider. Sci. Robot. 2021, 6, eabe7925. [Google Scholar] [CrossRef]
- Young, T. III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimerad, M.; Borjian, P.; Pathak, P.; Fasano, J.; Cho, H.J. A Miniaturized, Fuel-Free, Self-Propelled, Bio-Inspired Soft Actuator for Copper Ion Removal. Micromachines 2024, 15, 1208. https://doi.org/10.3390/mi15101208
Chimerad M, Borjian P, Pathak P, Fasano J, Cho HJ. A Miniaturized, Fuel-Free, Self-Propelled, Bio-Inspired Soft Actuator for Copper Ion Removal. Micromachines. 2024; 15(10):1208. https://doi.org/10.3390/mi15101208
Chicago/Turabian StyleChimerad, Mohammadreza, Pouya Borjian, Pawan Pathak, Jack Fasano, and Hyoung J. Cho. 2024. "A Miniaturized, Fuel-Free, Self-Propelled, Bio-Inspired Soft Actuator for Copper Ion Removal" Micromachines 15, no. 10: 1208. https://doi.org/10.3390/mi15101208






